


The article titled "10 Key Differences Between EMA and FDA for Clinical Research Directors" addresses the distinct regulatory frameworks and approval processes of the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) in the context of clinical research. It asserts that the EMA typically necessitates more extensive evidence and longer review times than the FDA. This reality compels clinical research directors to develop tailored strategies for compliance and successful submissions. Understanding these differences is crucial for navigating the regulatory environments effectively, thereby enhancing the prospects for clinical research success.
Understanding the regulatory landscapes of the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) is essential for clinical research directors aiming to navigate the complexities of drug approval processes. Each agency operates under distinct frameworks that influence timelines, data requirements, and compliance strategies, ultimately affecting the speed at which innovative therapies reach patients.
With the stakes high, how can research directors effectively align their strategies to meet the unique demands of both agencies while ensuring successful product introductions? This article delves into the ten key differences between the EMA and FDA, offering insights and strategies to optimize clinical research efforts in a rapidly evolving regulatory environment.
bioaccess® specializes in accelerating clinical research in Latin America while ensuring compliance with the regulations set by EMA and FDA. By harnessing Colombia's competitive advantages—cost reductions exceeding 30% compared to North America and Western Europe, along with a review process that lasts only 90-120 days—bioaccess® enables ethical approvals in a mere 4-6 weeks, significantly faster than conventional markets. Colombia's healthcare system ranks among the top globally, offering high-quality care and a diverse patient pool for recruitment, with a population exceeding 50 million and 95% coverage under universal healthcare.
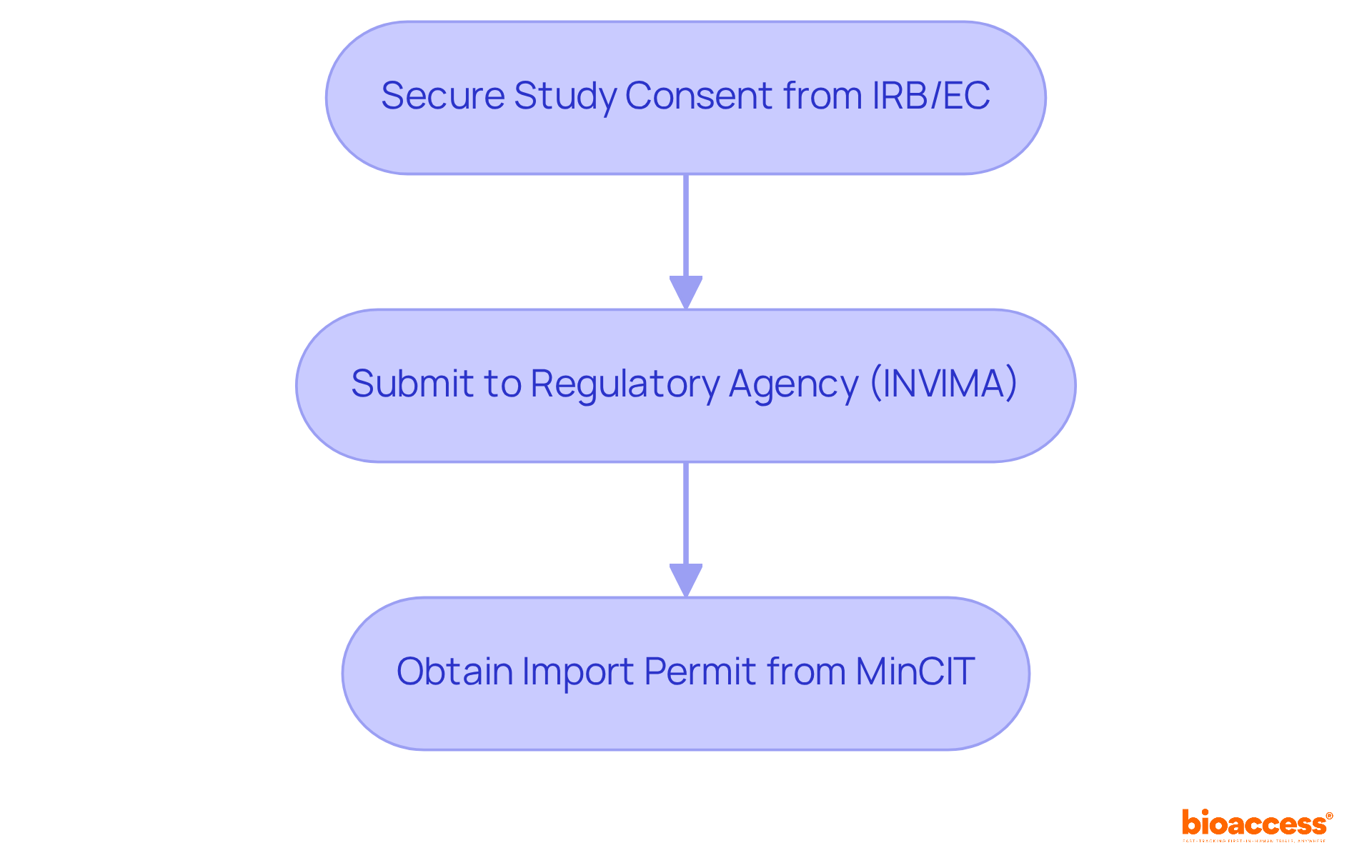
This agility is crucial for Medtech, Biopharma, and Radiopharma innovators who need to navigate intricate compliance environments efficiently. They can also benefit from R&D tax incentives that provide substantial deductions and grants. The process for obtaining trial approval in Colombia involves:
Collaborating with bioaccess® positions organizations to leverage these advantages, enhancing their clinical research initiatives.
The EMA and FDA operate within a decentralized framework, collaborating with national authorities across EU member states. In contrast, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) function as centralized agencies overseeing drug approvals throughout the United States. This fundamental structural difference significantly impacts how each agency assesses medical data. The EMA often mandates more extensive evidence due to its multi-national scope, complicating the approval process for innovative therapies. For instance, the FDA's median review time for new therapeutics has dramatically improved, averaging around 10 months, compared to the EMA's process, which typically spans 12 to 15 months. This efficiency is vital for research directors aiming to expedite product development and market entry.
Moreover, comprehending these governing structures is essential for aligning research strategies with the expectations of both organizations. Regulatory experts emphasize that the distinct approaches of the EMA and FDA can lead to variations in data evaluation, necessitating tailored strategies to meet the specific requirements of the EMA and FDA. By utilizing insights from both regulatory environments, research directors can optimize their development pathways and enhance the chances of successful approvals.

The approval process by the EMA and FDA is significantly faster, utilizing expedited pathways such as Fast Track and Breakthrough Therapy designations to streamline timelines. In contrast, the approval process of the EMA and FDA is characterized by its rigor, mandating extensive medical data and longer review periods.
For example, the median review time for the EMA is approximately 426 days, which is markedly longer than the FDA's median of 200 days. This disparity can profoundly impact trial timelines, as the stringent requirements of the EMA and FDA demand meticulous planning and resource allocation. Clinical research directors must adeptly navigate these complexities to ensure compliance and facilitate timely market access.
As one director noted, 'The expedited pathways offered by the EMA and FDA can dramatically shorten the time to market, allowing us to bring innovative therapies to patients more swiftly.' This underscores the critical need for strategic planning in the face of varying regulatory landscapes.
Bioaccess® offers extensive trial management services, including:
These services effectively navigate the complexities of regulatory approval to ensure adherence and facilitate timely market access.
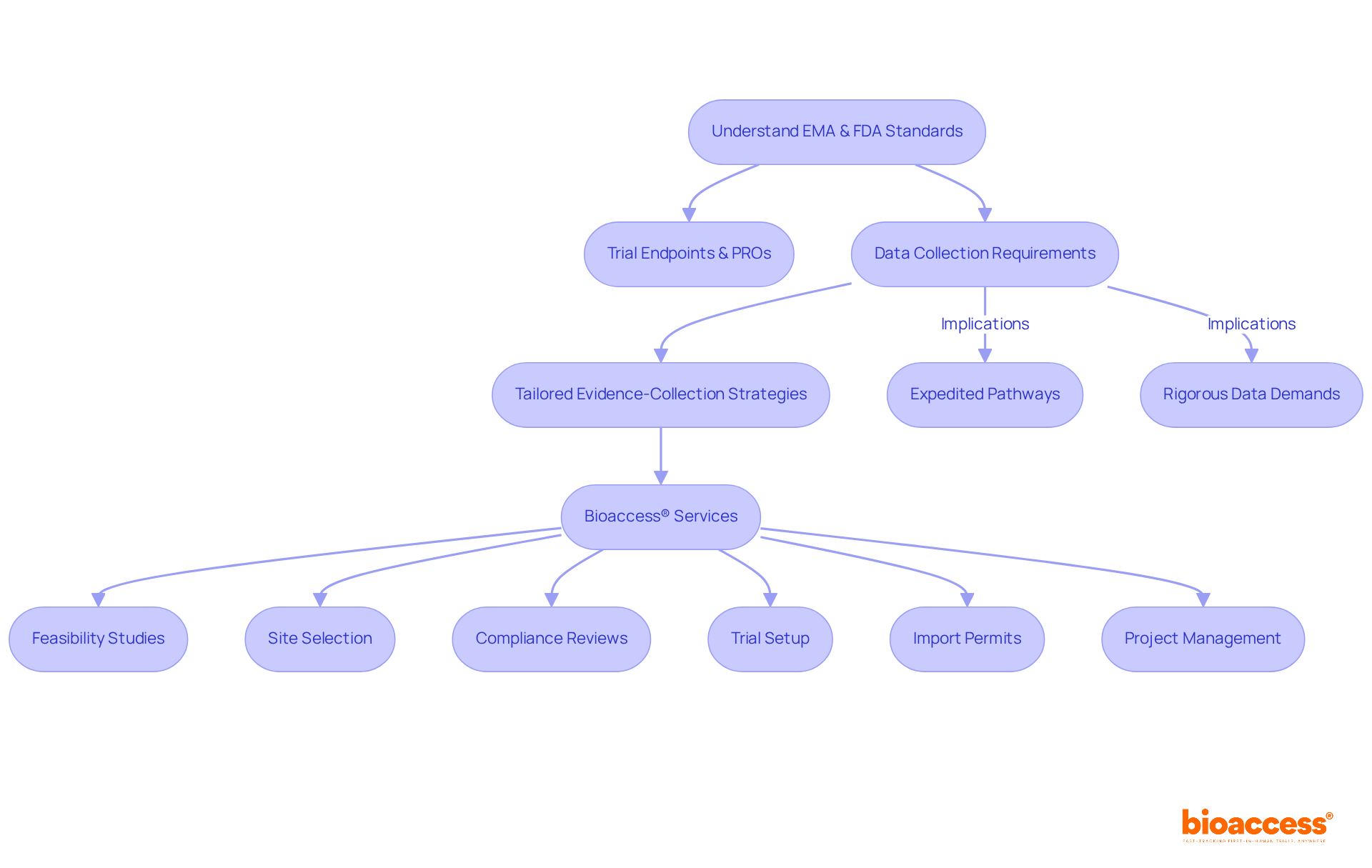
The EMA and FDA place a strong emphasis on trial endpoints and patient-reported outcomes (PROs), which allows for greater flexibility in study designs. This flexibility enables researchers to integrate real-world insights that significantly influence treatment choices and compliance submissions. In contrast, the EMA and FDA typically mandate more extensive data collection, including long-term efficacy studies and larger patient populations, reflecting their commitment to high patient safety standards. Therefore, research directors must develop tailored evidence-collection strategies that align with the unique requirements of each oversight organization.
For instance, the expedited pathways of the FDA can facilitate quicker access to therapies, while the EMA and FDA's rigorous data demands may result in longer review times but enhance the credibility of approved medications. Understanding these differences is essential for refining research approaches and ensuring compliance with regulatory expectations from EMA and FDA. Bioaccess® enhances this process by offering comprehensive trial management services, including:
This approach not only accelerates patient enrollment—achieving 50% faster enrollment for cardiology and neurology cohorts—but also results in significant cost savings of $25K per patient with FDA-ready data, thereby minimizing rework and delays. Additionally, by optimizing trials, Bioaccess® supports local economies through job creation and improved healthcare outcomes, making it imperative for research directors to leverage these advantages in their strategies.
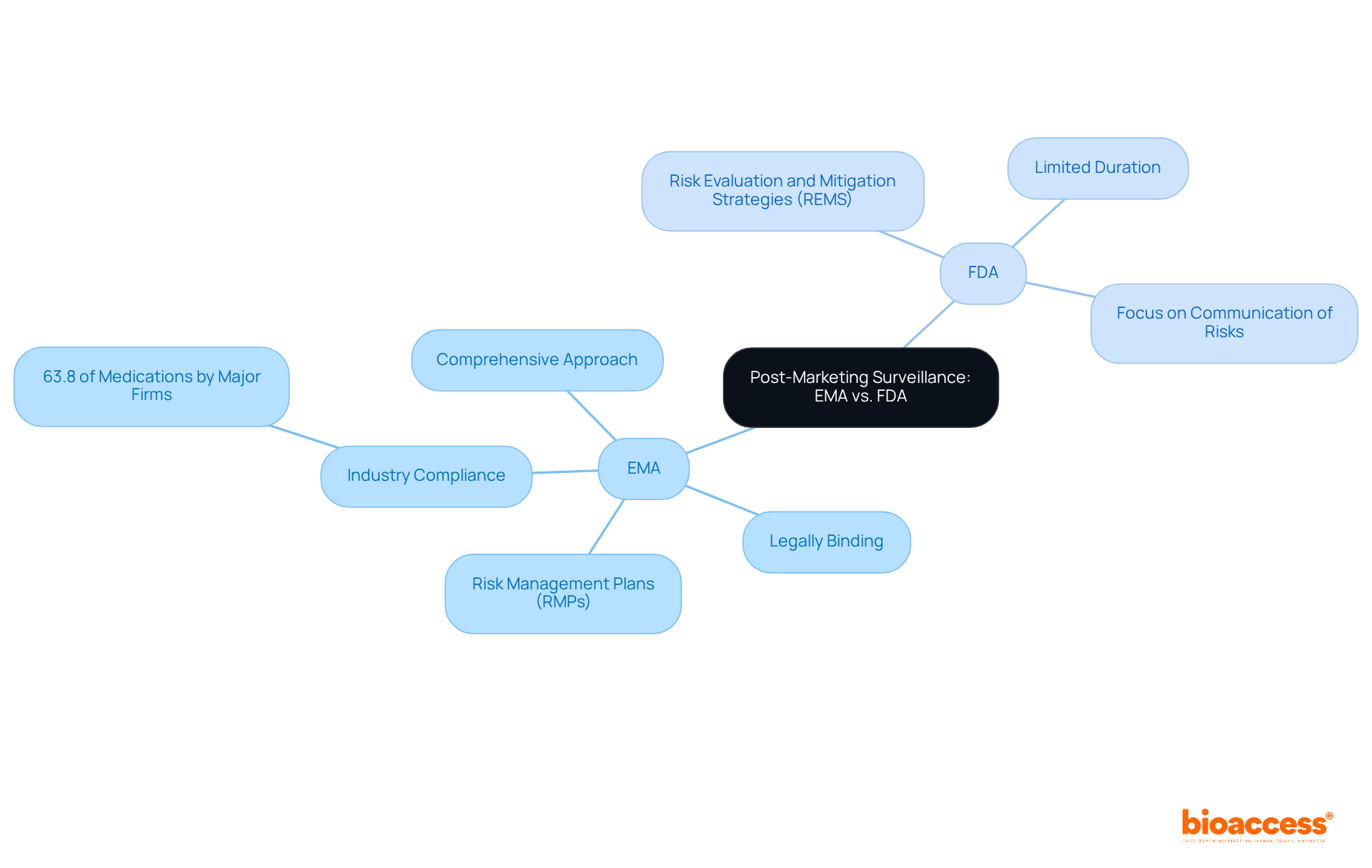
Post-marketing surveillance practices exhibit notable differences between the EMA and FDA. The FDA utilizes Risk Evaluation and Mitigation Strategies (REMS) to monitor drug safety, which are only required for certain medications and can be limited in duration. In contrast, the EMA mandates Risk Management Plans (RMPs) for all new medicines, ensuring a comprehensive approach to ongoing risk assessment throughout the drug lifecycle. This broader scope of RMPs necessitates that clinical research directors implement robust monitoring systems to ensure compliance and effectively address any safety concerns that may arise post-approval.
Recent data shows that 63.8% of medications authorized by the EMA are produced by major pharmaceutical firms, emphasizing the important role of the industry in complying with these standards. Furthermore, the EMA and FDA have different approaches, as the EMA's RMPs are legally binding and encompass a wider range of safety measures compared to the FDA's REMS, which are primarily focused on communication of risks. This distinction highlights the significance of customized post-marketing strategies that are consistent with the guidelines of each agency.
Specialists in regulatory compliance stress the importance for research directors to remain aware of these differences. As one observed, 'The changing environment of drug safety monitoring necessitates a proactive strategy to guarantee that both REMS and RMPs are successfully incorporated into medical practice.' This proactive stance is essential for navigating the complexities of post-marketing surveillance and ensuring patient safety.
The expedited pathways provided by the EMA and FDA are designed to accelerate patient access to innovative therapies. The designations from the EMA and FDA, including Fast Track and Breakthrough Therapy, facilitate rolling submissions and enhance interactions with regulators, significantly impacting the development timeline for critical treatments. For instance, drugs granted Breakthrough Therapy designation benefit from dedicated guidance from senior FDA leadership, expediting their journey to market.
In contrast, the EMA and FDA's Accelerated Assessment can shorten review times for products deemed of major public health interest, thus reducing the standard review period from 210 days to a maximum of 150 days. This strategic evaluation of expedited pathways is essential for research directors seeking to optimize development timelines and enhance patient access to life-saving therapies. Recent updates from the EMA indicate ongoing efforts to refine these processes, ensuring that innovative treatments reach patients more swiftly while maintaining rigorous safety and efficacy standards.
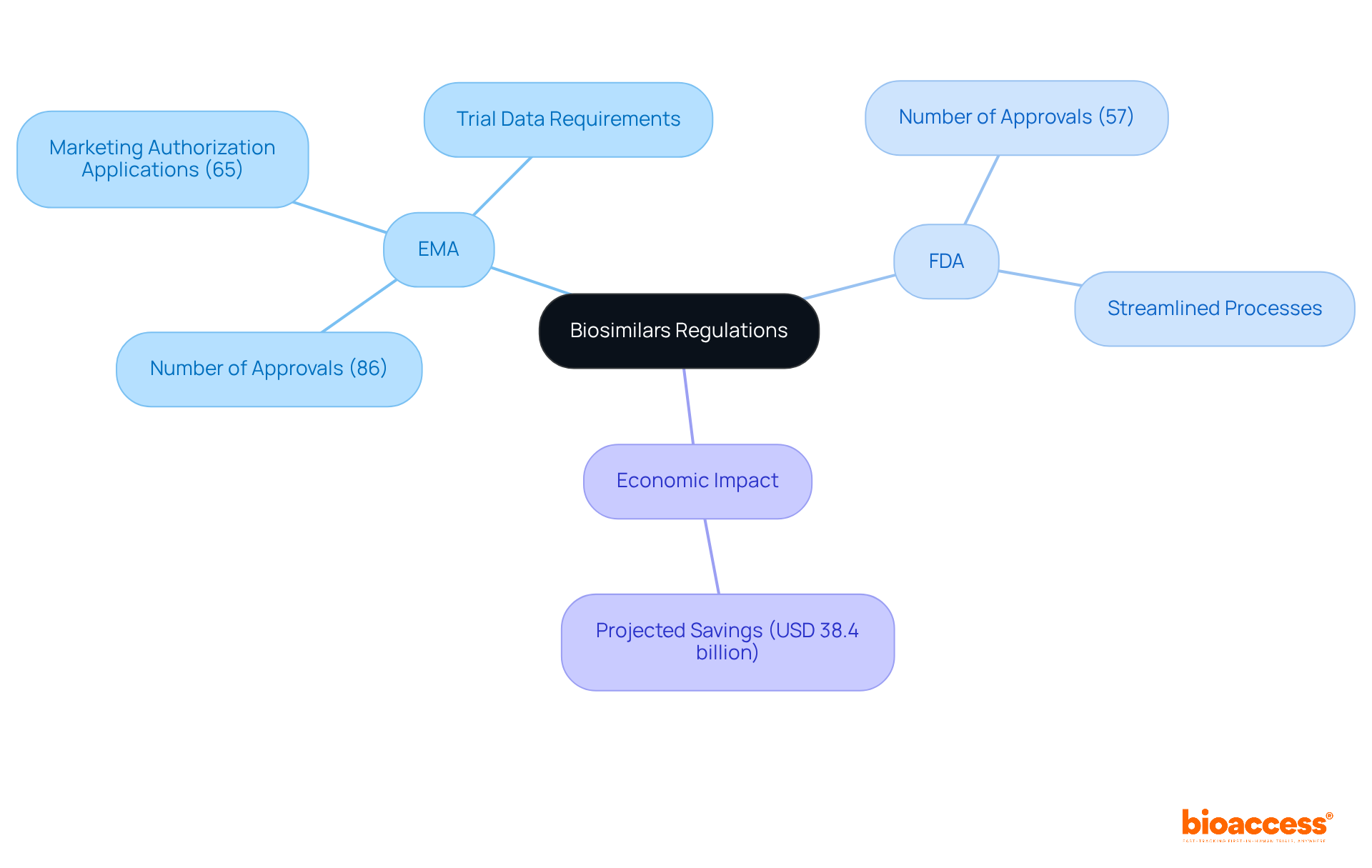
The EMA and FDA have established themselves as leaders in biosimilars regulation, creating a robust framework since 2004. This pioneering approach has enabled the EMA to approve 86 biosimilars, receiving 65 marketing authorization applications (MAAs) and granting 55, underscoring its commitment to patient safety and efficacy. In contrast, the EMA and FDA, which introduced its biosimilar pathway later, has made significant strides in streamlining their approval processes, with the FDA having approved 57 biosimilars by 2022.
While both the EMA and FDA agencies require a rigorous demonstration of biosimilarity, the EMA typically mandates more extensive trial data, including comparative efficacy testing (CCETs) for certain products. This difference can impact the development timeline and costs associated with bringing a biosimilar to market.
Notably, biosimilars are projected to save the U.S. healthcare system approximately USD 38.4 billion between 2021 and 2025. Experts emphasize that understanding these compliance nuances is crucial for clinical research directors to navigate the complexities of biosimilar development effectively. Successful examples of biosimilar development under both frameworks illustrate the importance of strategic planning and compliance to achieve timely market entry.
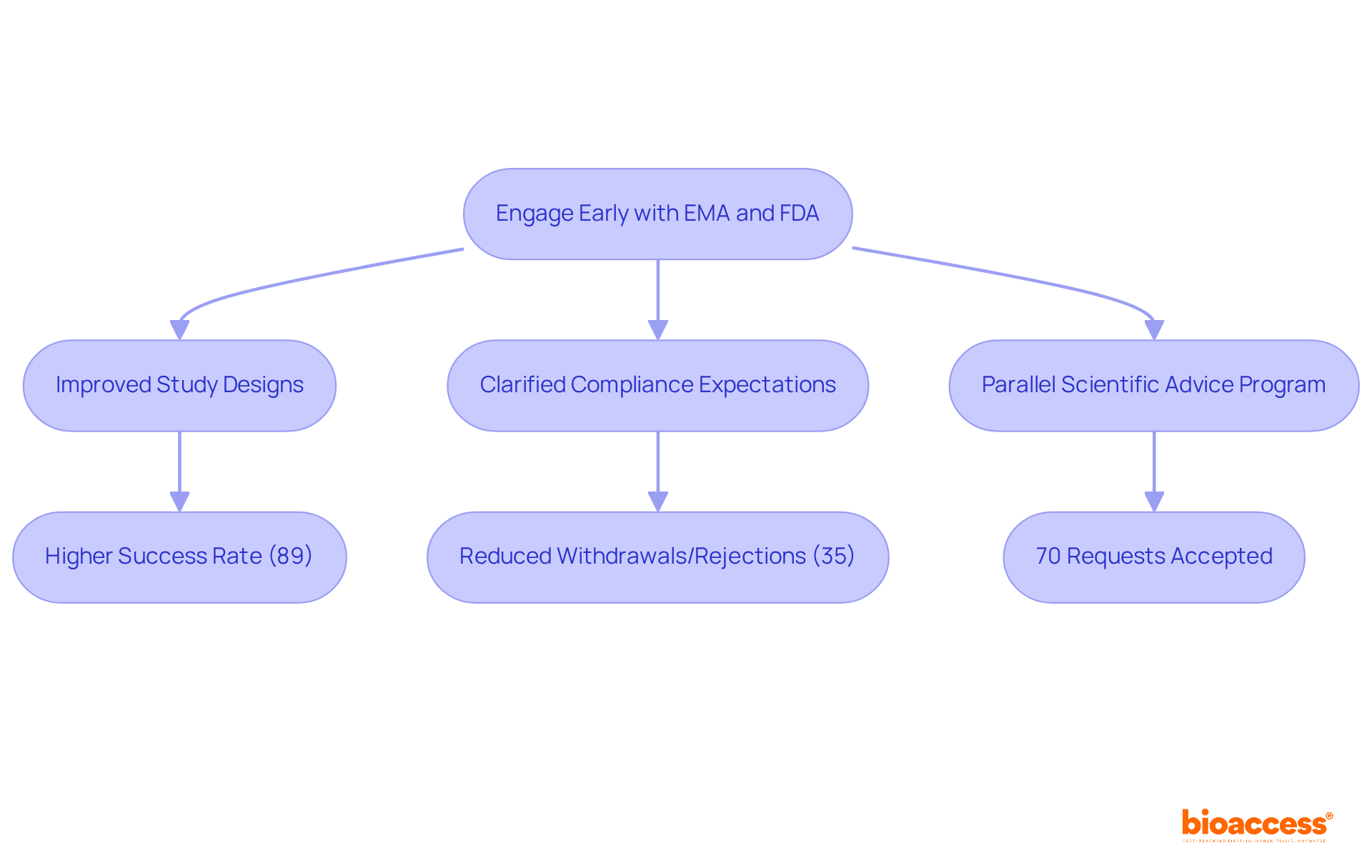
Engaging with the EMA and FDA early in the development process is crucial for improving the chances of successful submissions. Both agencies offer scientific guidance and consultation services that allow sponsors to clarify compliance expectations and enhance study designs. For instance, data indicates that 63% of applications without prior consultation were withdrawn or rejected, compared to only 35% for those that engaged early. This underscores the value of proactive communication, as large companies achieved an impressive 89% success rate in submissions following early engagement from 2004 to 2007.
Clinical research directors should actively seek these opportunities to align their strategies with compliance demands and reduce potential challenges. The Parallel Scientific Advice (PSA) program, established to facilitate concurrent discussions between EMA and FDA, has proven effective, with 70% of requests accepted from 2017 to 2021, showcasing the collaboration between EMA and FDA. This program not only optimizes product development but also helps avoid unnecessary testing, ultimately benefiting public health.
Furthermore, bioaccess® provides a thorough range of services aimed at speeding up trials for Medtech, Biopharma, and Radiopharma startups. By offering feasibility evaluations, trial preparation, investigator selection, compliance assistance, and project oversight, bioaccess guarantees that clients can manage the intricacies of research trials effectively. Statements from industry authorities highlight the significance of this method:
Such insights emphasize the essential role of early involvement in navigating the complexities of compliance environments and achieving timely market access. To maximize the chances of success, consider engaging with bioaccess® early in your trial planning.
Navigating the distinct oversight pathways of the EMA and FDA presents significant strategic complexities for clinical research directors. The differences in approval timelines are notable; for instance, the median review time for drugs approved by the FDA is approximately 121.5 days shorter than that of the EMA. This discrepancy necessitates meticulous planning and resource allocation to ensure compliance while optimizing time-to-market for innovative products.
Companies must develop tailored strategies that address the unique data requirements and post-marketing obligations of each agency. While the FDA has assigned 81% of first-in-class medications to expedited programs, only 30% obtained comparable designations from the EMA and FDA, highlighting the necessity for different strategies in submissions. Clinical research leaders stress the significance of grasping these differing compliance requirements to effectively maneuver through the intricacies of both pathways, ultimately improving the chances of successful product introductions in both markets.

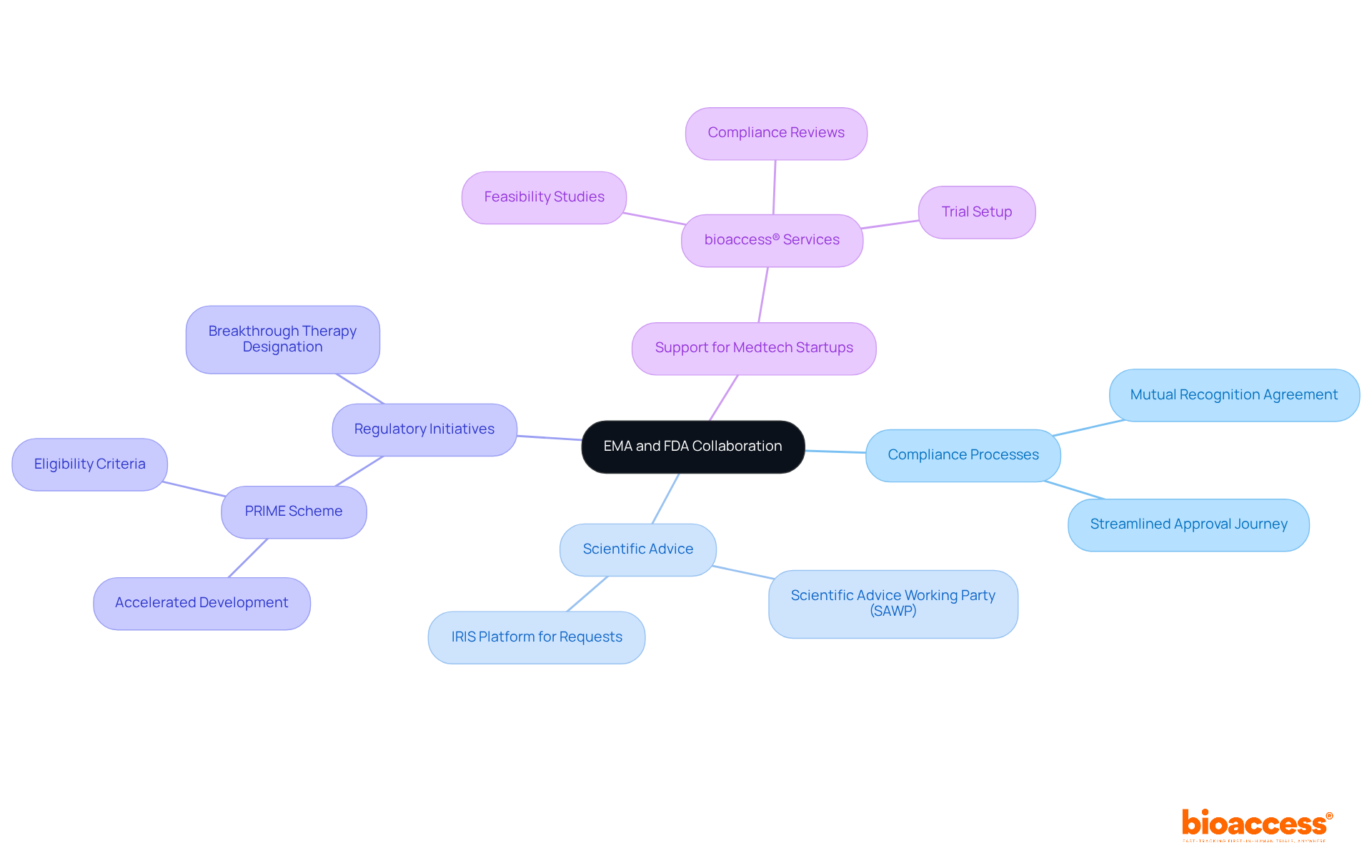
Recent initiatives underscore a significant partnership between the EMA and FDA, focusing on the alignment of compliance processes and enhancing operational efficiency. Their joint efforts in scientific advice and expedited pathways, exemplified by the mutual recognition agreement that allows reliance on each other's inspections, reflect a strong commitment to improving patient access to innovative therapies, supported by the EMA and FDA. For instance, the EMA and FDA have aligned their initiatives, with the EMA's PRIME scheme prioritizing medicines that address unmet medical needs and the FDA's Breakthrough Therapy designation facilitating accelerated development and approval timelines.
The Scientific Advice Working Party (SAWP) plays a crucial role in consolidating responses to scientific inquiries, further strengthening the oversight process. Regulatory experts assert that these harmonized processes not only streamline the approval journey but also cultivate a more cohesive approach to patient care across jurisdictions. As a leading CRO in Latin America, bioaccess® is exceptionally positioned to assist Medtech startups in navigating these complex compliance landscapes. With a comprehensive suite of services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—bioaccess® delivers tailored solutions and expert support throughout the clinical trial process.
Clinical research directors must remain vigilant regarding these developments, as they are poised to shape future regulatory strategies and operational practices, ultimately advancing medical innovations. The collaboration between regulatory bodies and industry stakeholders exemplifies a forward-thinking approach that promises to enhance the landscape of clinical research.
Navigating the regulatory landscapes of the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) is crucial for clinical research directors aiming to optimize their drug development strategies. Understanding the distinct processes and requirements of each agency not only enhances compliance but also accelerates the path to market for innovative therapies. This article highlights how the EMA's more extensive data demands and longer review times contrast with the FDA's efficiency and expedited pathways, emphasizing the need for tailored strategies in clinical research.
Key insights include the differences in approval timelines, clinical evidence requirements, and post-marketing surveillance approaches between the two agencies. For instance, while the FDA typically offers shorter review times and faster access to therapies, the EMA's rigorous standards ensure high safety and efficacy. Furthermore, the collaboration between EMA and FDA, particularly through initiatives like the Parallel Scientific Advice program, signifies a shift towards harmonized regulatory practices that can benefit research directors navigating these complexities.
As the landscape of clinical research continues to evolve, staying informed about the latest regulatory updates and leveraging the advantages offered by specialized organizations like bioaccess® can significantly impact the success of clinical trials. Embracing these insights and strategies will not only enhance compliance but also ultimately improve patient access to life-saving treatments across diverse markets.
What is bioaccess® and what does it specialize in?
bioaccess® specializes in accelerating clinical research in Latin America while ensuring compliance with the regulations set by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA).
What advantages does Colombia offer for clinical research?
Colombia offers cost reductions exceeding 30% compared to North America and Western Europe, a review process lasting only 90-120 days, and ethical approvals in 4-6 weeks. Additionally, Colombia has a high-quality healthcare system and a diverse patient pool with a population exceeding 50 million and 95% coverage under universal healthcare.
What are the steps involved in obtaining trial approval in Colombia?
The steps include securing study consent from the site's institutional review board (IRB)/ethics committee (EC), obtaining approval from the regulatory agency (INVIMA), and acquiring an import permit from the Ministry of Industry and Commerce (MinCIT).
How do the EMA and FDA differ in their regulatory frameworks?
The EMA operates within a decentralized framework, collaborating with national authorities across EU member states, while the FDA is a centralized agency overseeing drug approvals in the United States. This structural difference affects how each agency assesses medical data.
What is the typical review time for drug approvals by the EMA and FDA?
The FDA's median review time for new therapeutics is around 10 months, while the EMA's process typically spans 12 to 15 months, with a median review time of approximately 426 days.
How do expedited pathways like Fast Track and Breakthrough Therapy designations impact the approval process?
These expedited pathways significantly shorten the time to market for innovative therapies, allowing researchers to bring treatments to patients more swiftly compared to the standard approval processes.
What trial management services does bioaccess® offer?
bioaccess® offers a range of trial management services including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting to navigate the complexities of regulatory approval.