


The article primarily focuses on outlining the essential elements that contribute to a successful pharmaceutical market access strategy. It highlights the significance of:
These components are crucial for navigating the complexities of market entry and ensuring the financial viability of pharmaceutical products.
Understanding these elements is vital for professionals in the pharmaceutical industry. Regulatory approval serves as the gateway to market entry, while reimbursement planning ensures that products are financially accessible. Engaging stakeholders effectively can facilitate smoother processes and foster collaboration. Moreover, integrating real-world evidence strengthens the case for product efficacy and safety, making it a pivotal aspect of market access strategies.
In conclusion, the interplay of these factors underscores the importance of a comprehensive approach to market access. By prioritizing collaboration and strategic planning, pharmaceutical companies can enhance their chances of success in a competitive landscape.
The pharmaceutical landscape is marked by rapid advancements and intense competition, making effective market access strategies more crucial than ever. Companies looking to successfully launch their products must navigate a complex web of regulatory approvals, reimbursement frameworks, and stakeholder engagement. This article explores the ten essential elements that form the foundation of a successful pharma market access strategy, providing insights into how organizations can boost their chances of commercial success. What challenges lie ahead for businesses as they strive to align these strategies with the ever-evolving market dynamics?
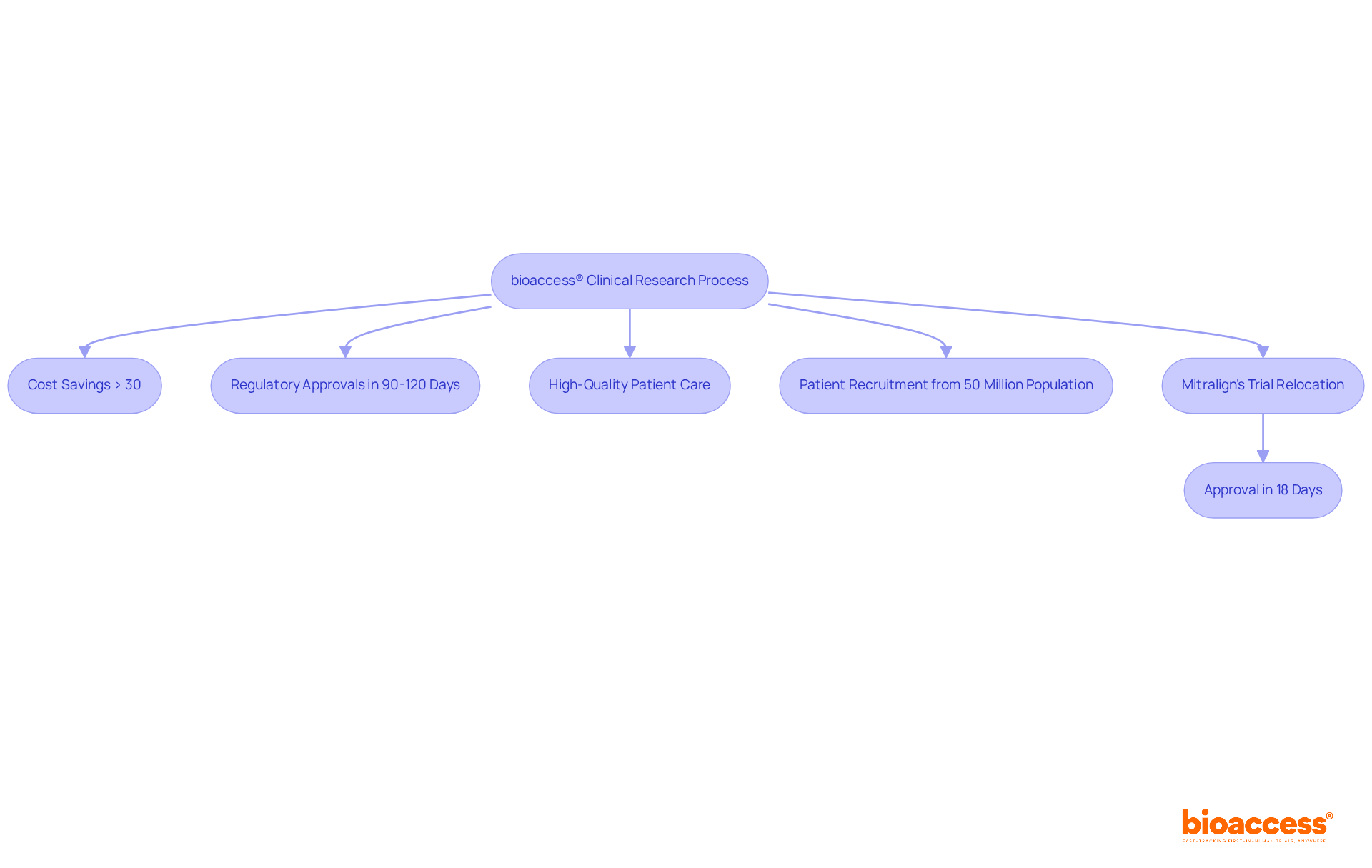
bioaccess® leverages its extensive expertise in early-phase clinical research to facilitate swift entry into the commercial realm for Medtech, Biopharma, and Radiopharma organizations. By harnessing Colombia's competitive advantages—such as cost savings exceeding 30% compared to North America and Western Europe, alongside a regulatory process that permits ethical approvals in as little as 90-120 days—bioaccess® significantly boosts the efficiency of clinical trials. The country’s healthcare system, ranked among the top five globally, guarantees high-quality patient care, while a population of over 50 million, with 95% covered by universal healthcare, offers a robust pool for patient recruitment.
For example, Mitralign successfully relocated its trial to Colombia, securing ethical approval in just 18 days and enrolling patients far more quickly than in the EU. This agility is crucial for companies aiming to launch innovative products in a competitive landscape, as industry leaders underscore the vital role of a pharma market access strategy and regulatory speed in clinical trials. With over 20 years of experience managing various study types and having completed more than 159 regulatory submissions for over 75 medical device trials, bioaccess® is exceptionally positioned to guide clients through the complexities of clinical research, ultimately enabling a successful pharma market access strategy for their products.
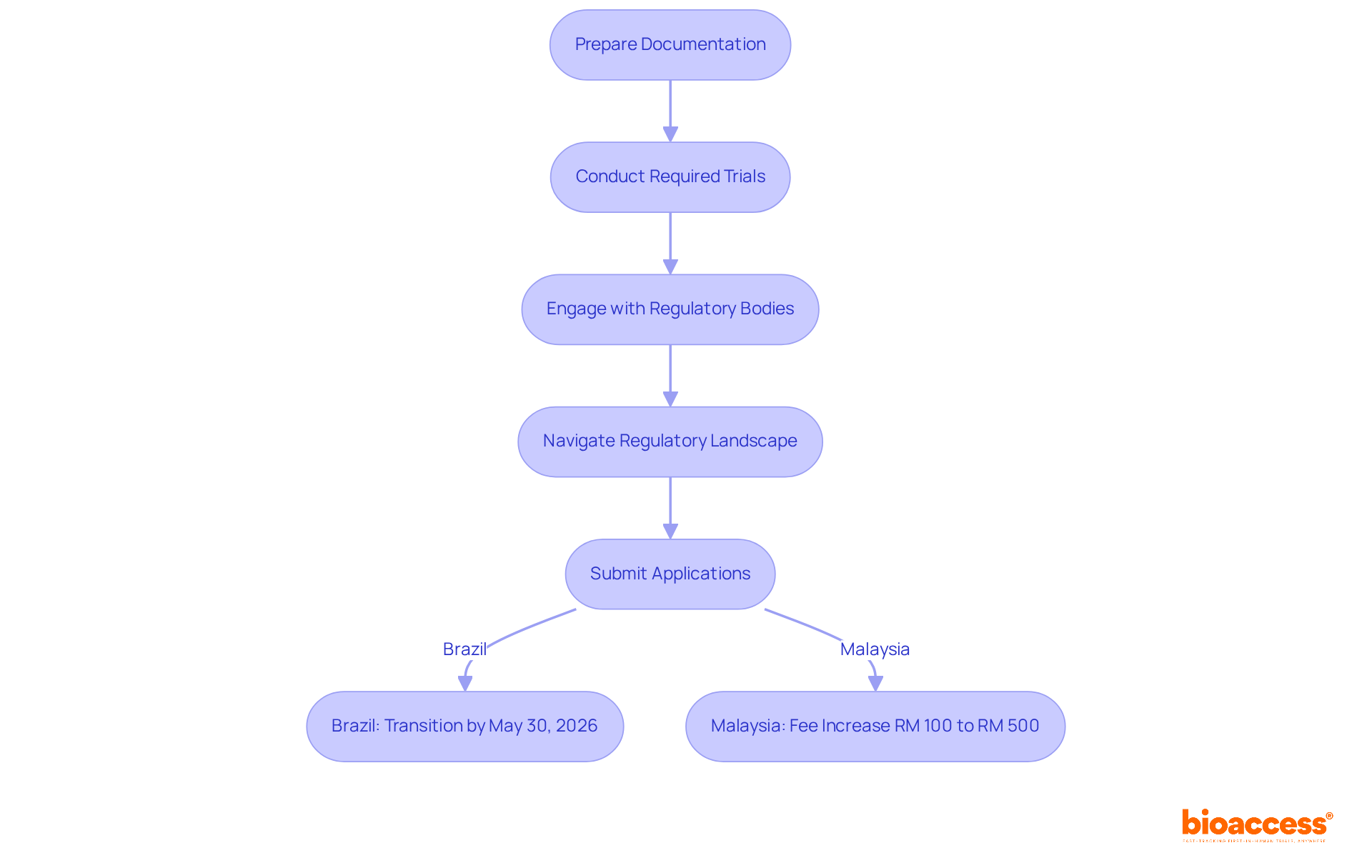
Obtaining regulatory approval is a crucial initial step in any access strategy. Companies must navigate a complex landscape of regulations that vary by region, necessitating thorough preparation of documentation, execution of required trials, and strict adherence to local laws. Engaging with regulatory bodies early in the process is essential; it not only streamlines approvals but also helps identify potential hurdles before they escalate into significant challenges.
Recent statistics reveal that around 70% of businesses encounter regulatory obstacles that can delay their market entry. For instance, Brazil's ANVISA has recently reclassified sodium-chloride saline solutions as Class IV medical devices, requiring organizations to transition their registrations by May 30, 2026. This regulation includes specific transition conditions, GMP applicability, and stock-depletion policies, underscoring the importance of staying informed about evolving regulations.
Moreover, Malaysia's upcoming fee increases for Class A medical device applications—raising the application fee from RM 100 to RM 500 and introducing a RM 750 registration fee effective January 1, 2026—highlight the necessity for timely submissions to avoid higher costs. By proactively addressing these regulatory requirements and leveraging expert services like those offered by bioaccess®, which include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, companies can enhance their pharma market access strategy and capitalize on the opportunities within the Medtech and Biopharma sectors.
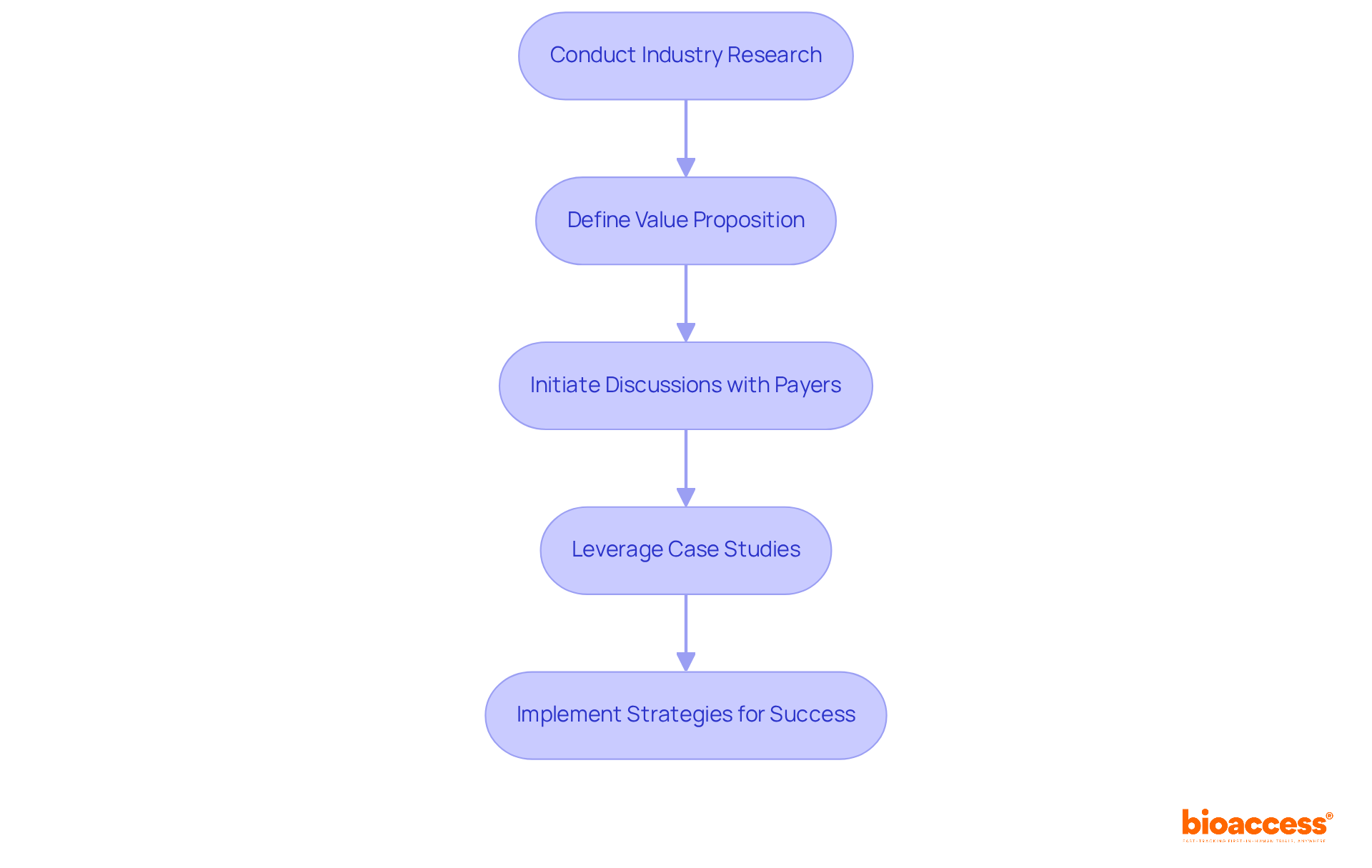
Creating a robust reimbursement plan is essential for ensuring that innovative products remain financially viable. Understanding the reimbursement landscape and the criteria payers use to evaluate products is a critical first step in formulating a pharma market access strategy. Companies should engage with payers early as part of their pharma market access strategy to discuss pricing, value propositions, and potential reimbursement pathways. Statistics reveal that organizations proactively engaging payers see a significant increase in successful reimbursement outcomes. For example, those that have established clear communication channels with payers report a 30% higher success rate in securing reimbursement for new medical devices.
To develop an effective reimbursement plan, companies should focus on several key elements of their pharma market access strategy:
By implementing these strategies, companies can significantly enhance their chances of achieving commercial success.
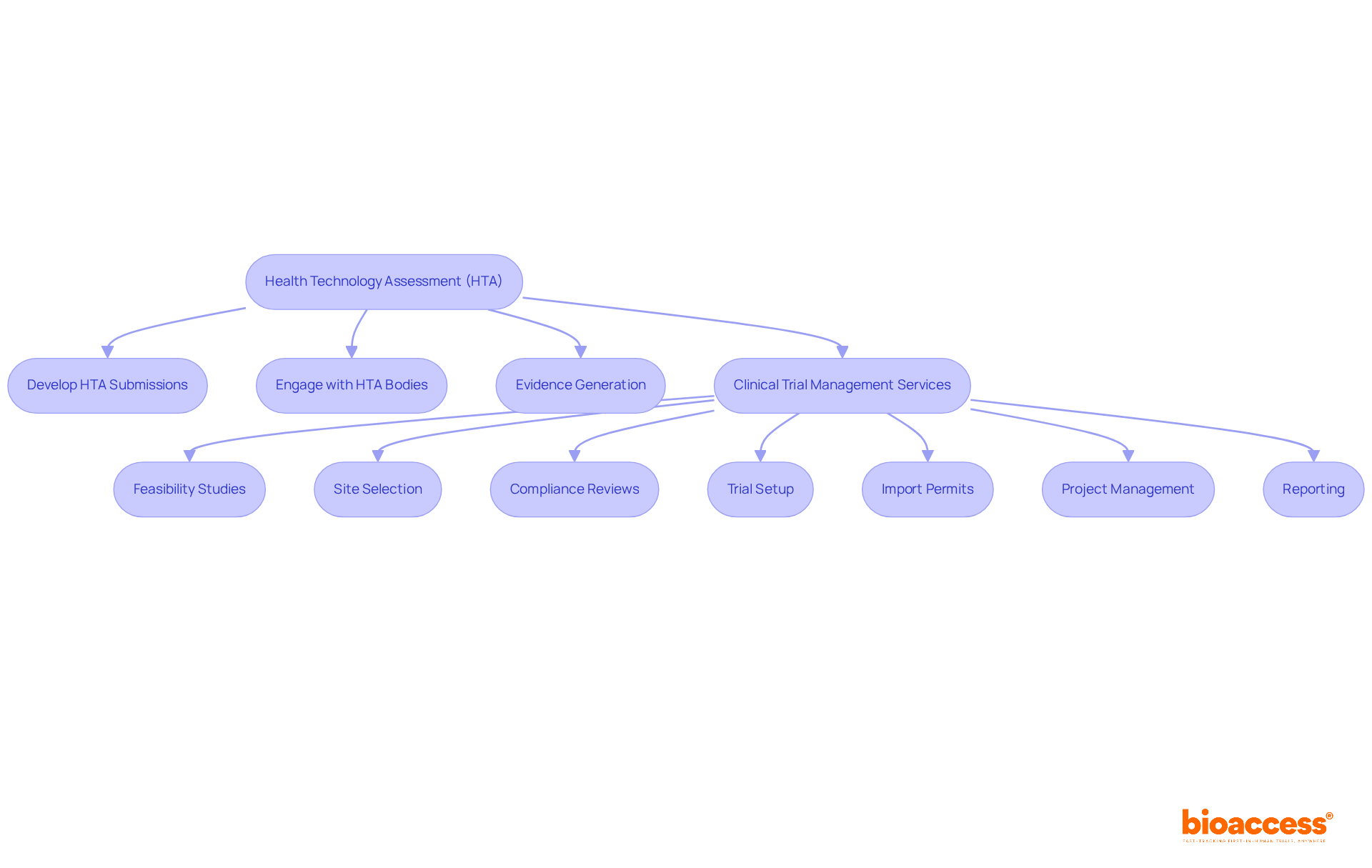
Health Technology Assessment (HTA) plays a crucial role in evaluating the clinical and economic value of new medical products. Companies must develop comprehensive HTA submissions that effectively showcase their products' advantages over existing alternatives, emphasizing clinical efficacy, cost-effectiveness, and overall patient outcomes. Engaging with HTA bodies early in the process can significantly enhance submission quality, ensuring alignment with specific evaluation criteria.
Recent developments underscore the importance of HTA in market entry, particularly with the EU HTA Regulation set to impact new oncology drugs and advanced therapy medicinal products (ATMPs) starting January 12, 2025. This regulation highlights the necessity for robust evidence generation strategies, as demonstrated in successful case studies where clients received tailored recommendations for optimizing their data packages based on anticipated Patient, Intervention, Comparison, and Outcome (PICO) frameworks.
For example, a client preparing for their first Joint Clinical Assessment (JCA) submission in 2025 benefited from a thorough EU HTA readiness assessment, which identified gaps and established new operational methods to meet JCA requirements. Such proactive measures not only streamline the submission process but also enhance the likelihood of positive evaluations, ultimately facilitating faster entry for innovative medical technologies.
In this context, bioaccess provides comprehensive clinical trial management services, including:
These services are essential for ensuring that clinical trials adhere to regulatory standards and generate the necessary evidence for HTA submissions, thereby supporting the successful market access of new pharmaceutical products.
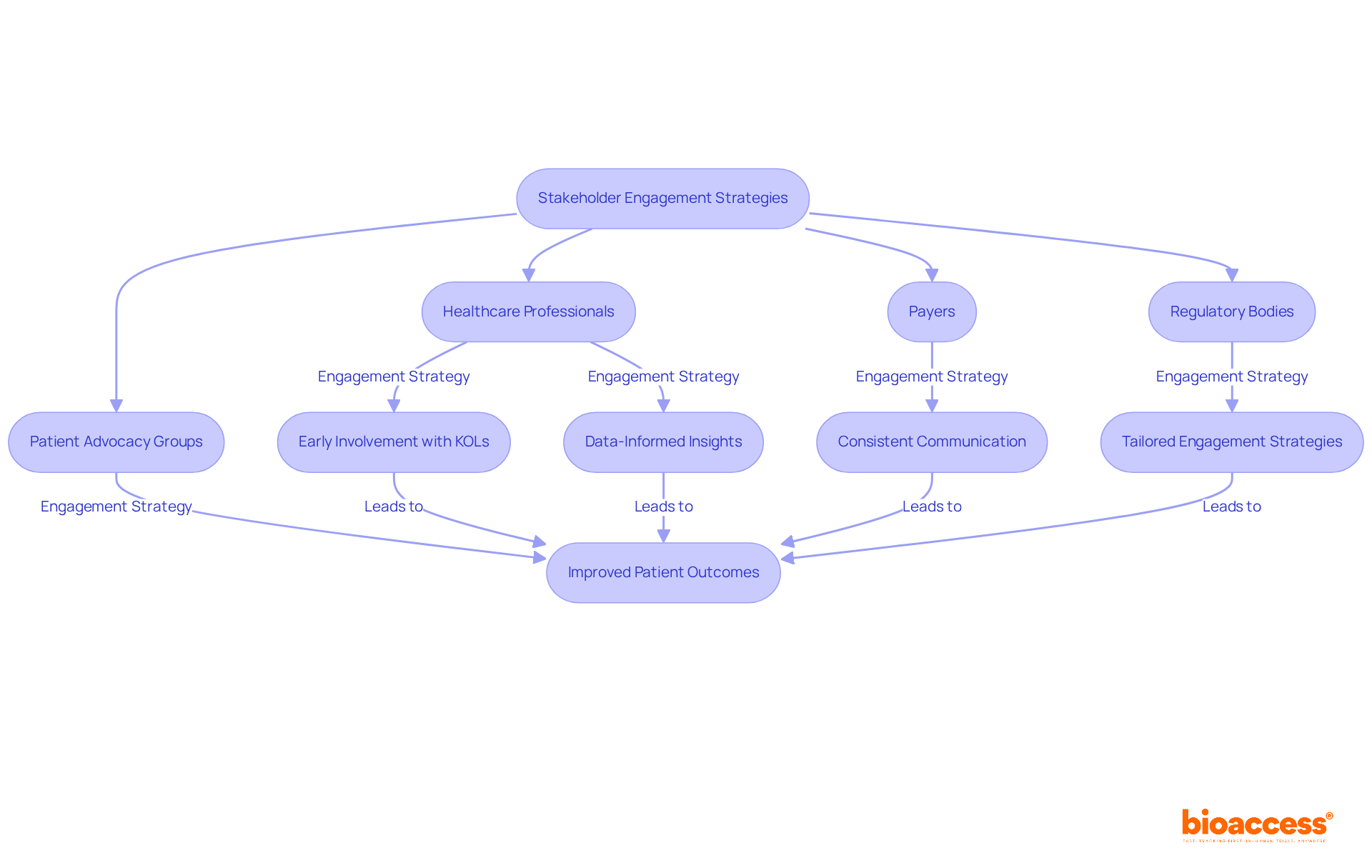
Effective stakeholder involvement is crucial for a successful pharma market access strategy, especially in the pharmaceutical sector. Building strong relationships with healthcare professionals, payers, regulatory bodies, and patient advocacy groups allows companies to understand the unique needs and concerns of these stakeholders. For instance, organizations that emphasize early involvement with key opinion leaders (KOLs) can significantly enhance their entry strategies. Data shows that:
This underscores the essential nature of these connections.
Consistent communication and cooperation with healthcare experts can lead to more beneficial outcomes during the market entry process. Successful case studies illustrate how tailored engagement strategies within Integrated Delivery Networks (IDNs) improve service delivery and client outcomes. Pharmaceutical firms that incorporate a pharma market access strategy in aligning their product offerings with IDN objectives have experienced smoother entry to formulary placements, thereby increasing the likelihood of adoption among medical providers.
Healthcare experts emphasize the importance of teamwork in achieving industry entry. As one expert noted, "Your credibility is your currency," highlighting the significance of trust and ongoing dialogue in these relationships. Another specialist remarked, "Doing so can help anticipate nuances that a one-size-fits-all method cannot," further stressing the necessity for customized approaches. Moreover, 91% of patients expect timely responses from their healthcare providers, indicating that responsiveness is a critical factor in building trust and loyalty.
Integrating data-informed insights into outreach strategies can also enhance interactions with healthcare professionals. By leveraging analytics, companies can identify key trends and patient needs, allowing for more effective communication and collaboration. Ultimately, nurturing robust connections with stakeholders not only supports a successful pharma market access strategy but also improves patient outcomes and satisfaction.
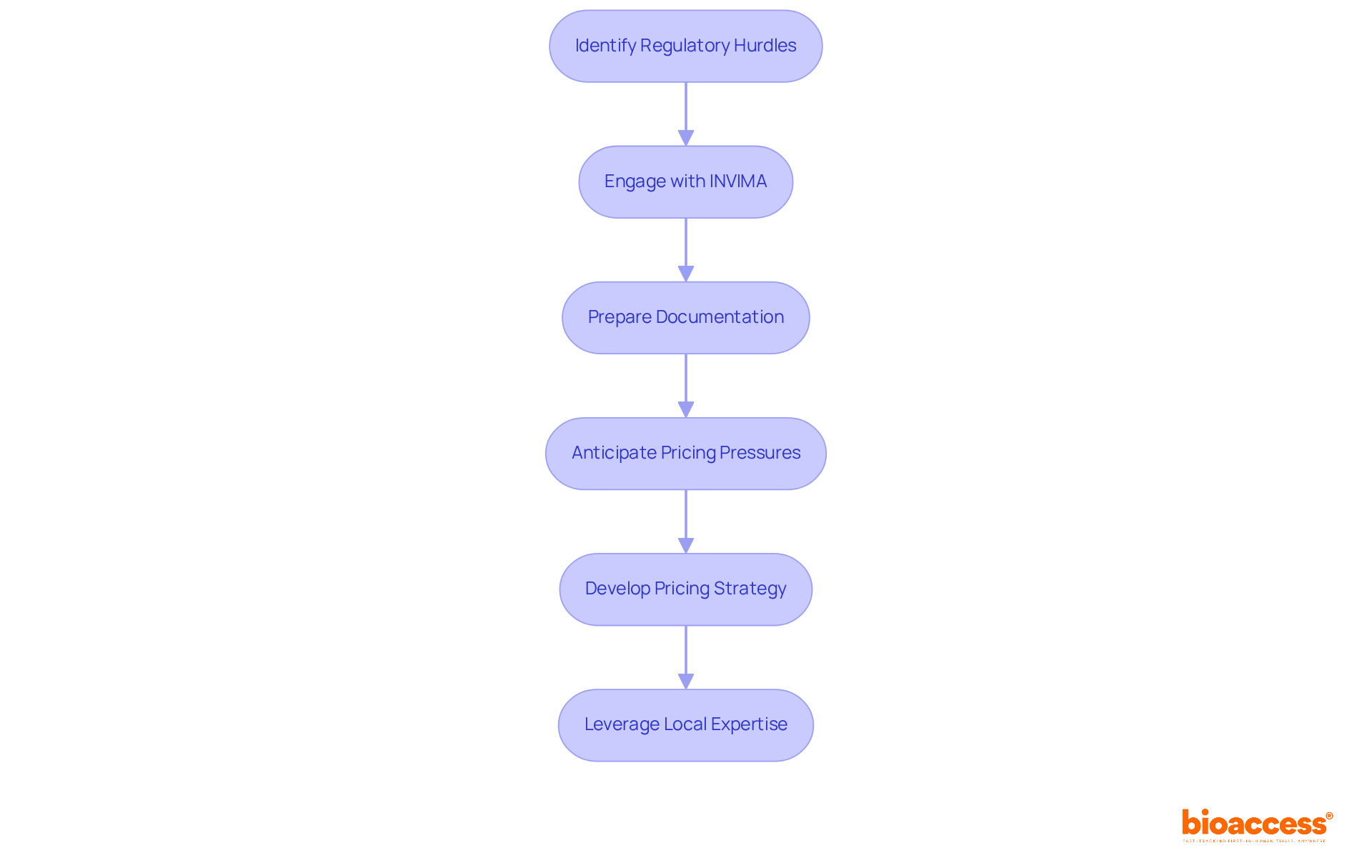
Navigating the complexities of market access demands a pharma market access strategy to effectively overcome regulatory hurdles and pricing pressures. Companies encounter significant challenges, including potential delays in approvals, evolving regulations, and the need for comprehensive documentation. In Colombia, the INVIMA (Colombia National Food and Drug Surveillance Institute) plays a pivotal role in this landscape, overseeing the marketing and manufacturing of health products while ensuring compliance with health standards. Recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA's stringent review processes shape the regulatory environment, often resulting in extended timelines for product approvals.
Pricing strategies must not only remain competitive but also clearly communicate the product's value to payers. As pharmaceutical firms contend with pricing pressures—especially amid rising healthcare costs and the demand for cost-effective solutions—a proactive pharma market access strategy becomes essential. Industry leaders emphasize the importance of anticipating entry-related challenges and adjusting the pharma market access strategy accordingly. By fostering a culture of flexibility and comprehensive readiness, organizations can significantly enhance their capacity to navigate these challenges and achieve successful market entry. Moreover, leveraging local expertise, such as that of Katherine Ruiz in regulatory affairs for medical devices and in vitro diagnostics, offers invaluable insights into the nuances of the Latin American Medtech landscape.
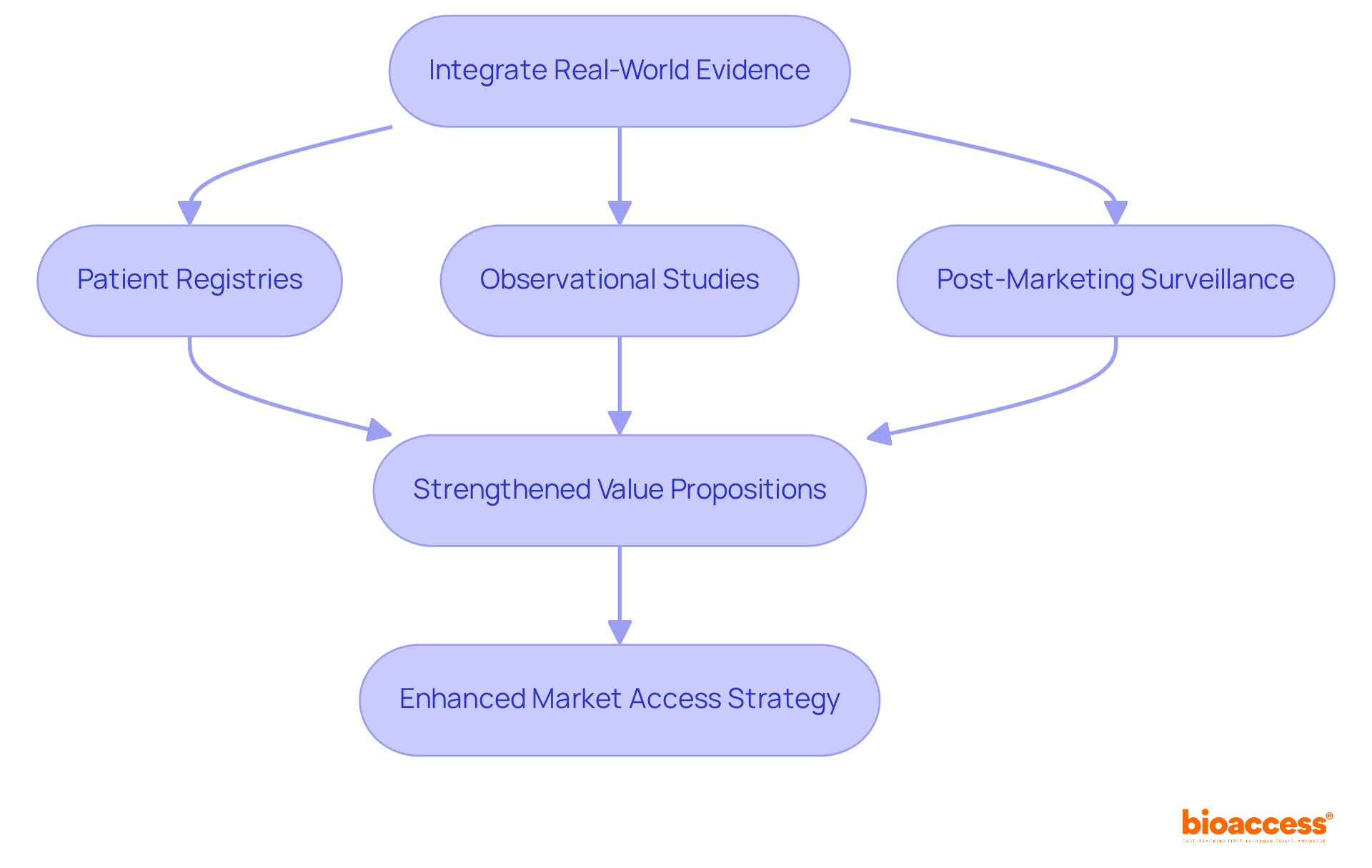
Real-world evidence (RWE) is increasingly recognized as a vital element in commercialization strategies, offering crucial insights into product performance within everyday clinical environments. By complementing traditional clinical trial data, RWE deepens the understanding of a product's effectiveness and safety across diverse patient populations. To strengthen their value propositions, organizations must actively incorporate RWE into their commercial dossiers. This integration can draw from various data sources, such as:
Collectively showcasing how products perform in real-world scenarios.
Statistics reveal that health technology assessments (HTAs) are becoming more open to integrating RWE into cost-effectiveness models, validating the relevance of trial findings across different demographics. By leveraging RWE, pharmaceutical companies can enhance their entry documents and align their pharma market access strategy with payer expectations, ultimately facilitating smoother reimbursement processes and broader patient access. To effectively weave RWE into commercial entry strategies, companies should prioritize diversity in clinical trials, ensuring that the data reflects the varied demographics of the patient population. This approach not only bolsters the credibility of the evidence but also meets the growing demand from patients for affordability and access.
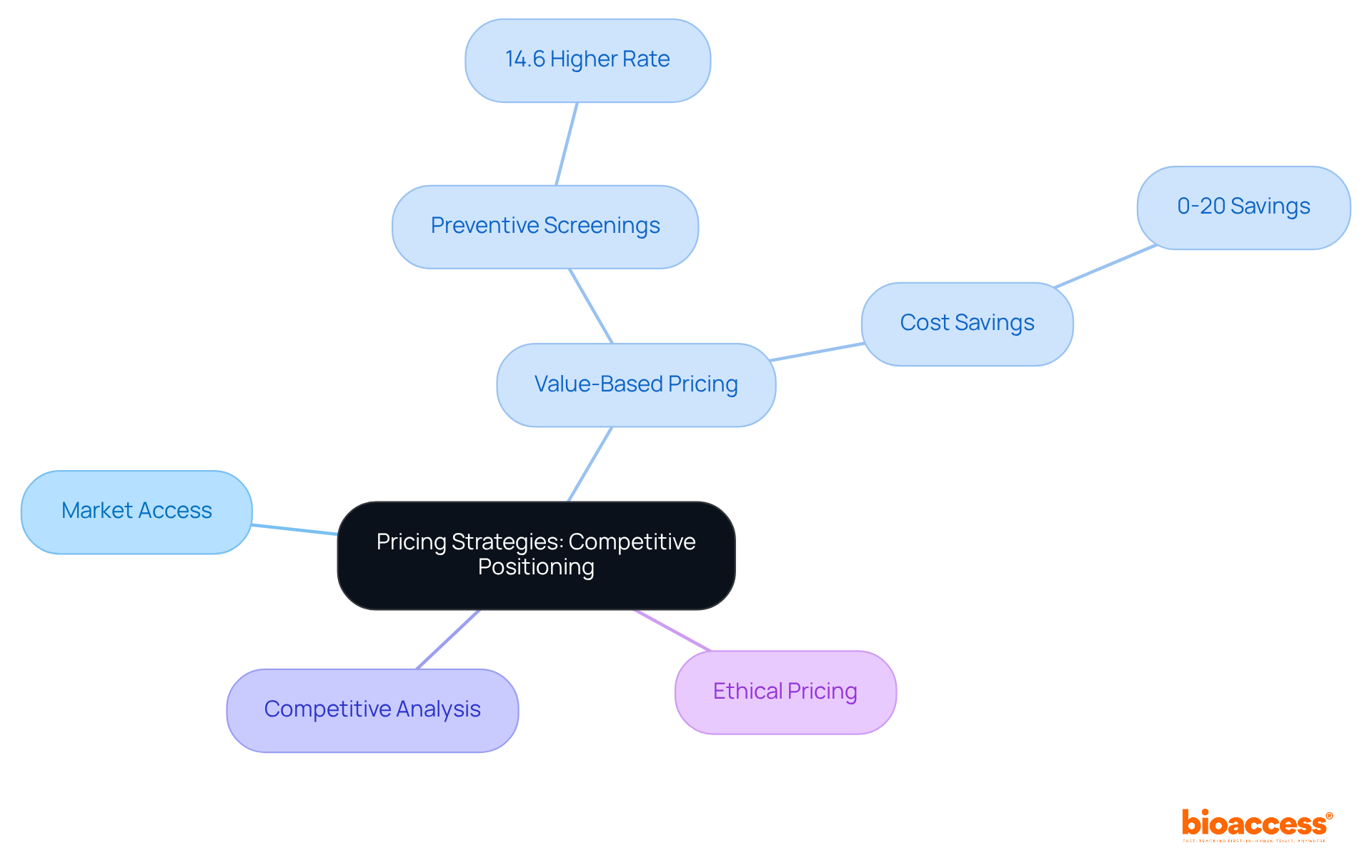
Effective pricing strategies are crucial for gaining a competitive edge through a pharma market access strategy in the pharmaceutical industry. Companies must conduct thorough analyses of market trends and competitor pricing to identify optimal price points that reflect the perceived value of their products. The adoption of flexible pricing models, especially value-based pricing, is becoming increasingly vital. This approach aligns the product's cost with its demonstrated benefits for patients and healthcare systems, fostering greater acceptance and availability.
For instance, value-based care models have shown significant advantages, such as a 14.6% higher rate of preventive screenings among patients compared to traditional models. This underscores the effectiveness of aligning pricing with health outcomes. Furthermore, these models can lead to savings ranging from 0% to as much as 20% in high-touch primary care groups, highlighting the financial benefits associated with this pricing strategy.
As the healthcare landscape evolves, implementing a pharma market access strategy that includes innovative pricing approaches will be essential for navigating the complexities of market entry and ensuring sustainable growth in the Medtech industry. Tim Cook emphasizes that ethical pricing practices contribute to sustainability and customer trust, further underscoring the importance of responsible pricing strategies.
How can your organization adapt to these changes and leverage pricing strategies to enhance value?
Examining the competitive environment is crucial for formulating a successful pharma market access strategy in the pharmaceutical sector. Companies must engage in comprehensive industry research to identify key competitors, evaluate their strengths and weaknesses, and recognize prevailing trends. This tactical insight not only informs product placement but also shapes pricing and promotional strategies.
For instance, insights from industry researchers can reveal essential competitive dynamics, enabling businesses to effectively differentiate their offerings. Recent trends underscore a growing focus on customer experience research, which aids in understanding key purchase drivers and refining product messaging.
By leveraging these insights, businesses can enhance their pharma market access strategy, ultimately increasing their chances of success in a competitive healthcare landscape.
The commercial entry environment in healthcare is rapidly evolving, driven by technological advancements and changing dynamics. A significant trend is the increasing adoption of digital health technologies, which are revolutionizing how companies approach market entry. For instance, the global digital health sector is projected to grow from $389.18 billion in 2024 to an astounding $1,921.38 billion by 2031, reflecting a compound annual growth rate (CAGR) of 25.7% from 2025 to 2031. This remarkable growth underscores the critical need for integrating digital solutions into pharma market access strategy.
Artificial intelligence (AI) is also crucial in data analysis, empowering companies to extract actionable insights from extensive datasets. By harnessing AI, organizations can refine patient engagement strategies, effectively addressing the diverse needs of various populations. For example, telehealth services have experienced a surge, with their value expected to rise from $613 billion in 2021 to $3.42 trillion by 2028, highlighting the urgent demand for accessible healthcare solutions.
Thought leaders stress the importance of adapting to these innovations. As one expert noted, "Digital health technologies have the potential to improve healthcare accessibility and equity by providing remote care," illustrating the transformative impact of these tools on entering the healthcare sector. Companies that embrace these advancements can streamline their operations, enhance patient outcomes, and refine their pharma market access strategy more efficiently, positioning themselves for success in an increasingly competitive landscape.

The success of a pharmaceutical market access strategy relies on a multifaceted approach that integrates several critical elements. By understanding and implementing strategies related to:
companies can navigate the complexities of market entry more effectively. This article emphasizes the importance of a well-rounded strategy that not only accelerates product launch but also ensures long-term commercial viability.
Key arguments highlight the necessity of:
Additionally, recognizing the competitive landscape and adapting to emerging technologies are crucial for staying ahead in the rapidly evolving healthcare environment.
As the pharmaceutical industry faces ongoing challenges, embracing a comprehensive market access strategy becomes essential for achieving success. Organizations must remain agile and informed, leveraging expert insights and innovative solutions to overcome barriers and capitalize on opportunities. By prioritizing these key elements, companies can enhance their market access strategies while contributing to improved patient outcomes and greater accessibility to essential healthcare solutions.
What is bioaccess® and how does it support clinical research?
bioaccess® is an organization that leverages its expertise in early-phase clinical research to facilitate faster market entry for Medtech, Biopharma, and Radiopharma companies. It utilizes Colombia's advantages, including significant cost savings and a quick regulatory approval process, to enhance the efficiency of clinical trials.
What are the competitive advantages of conducting clinical trials in Colombia?
Colombia offers cost savings of over 30% compared to North America and Western Europe, a healthcare system ranked among the top five globally, and a regulatory process that allows for ethical approvals in as little as 90-120 days. Additionally, the country has a population of over 50 million, with 95% covered by universal healthcare, providing a robust patient recruitment pool.
Can you provide an example of a successful clinical trial in Colombia?
Mitralign successfully relocated its trial to Colombia, obtaining ethical approval in just 18 days and enrolling patients significantly faster than in the EU, showcasing the agility and efficiency of conducting trials in the country.
Why is regulatory approval important in the market access strategy?
Regulatory approval is a crucial first step in any market access strategy as it involves navigating complex regulations that vary by region. Early engagement with regulatory bodies helps streamline approvals and identify potential hurdles, which can prevent significant challenges later on.
What challenges do businesses face in obtaining regulatory approval?
Approximately 70% of businesses encounter regulatory obstacles that can delay market entry. For instance, changes in classification by regulatory bodies, such as Brazil's ANVISA reclassifying saline solutions, require companies to adapt their registrations accordingly.
What are some upcoming regulatory changes that companies should be aware of?
Companies should note that Malaysia will increase fees for Class A medical device applications, raising the application fee from RM 100 to RM 500 and introducing a RM 750 registration fee effective January 1, 2026. Staying informed about such changes is vital to avoid higher costs.
How can companies enhance their pharma market access strategy?
Companies can enhance their market access strategy by proactively addressing regulatory requirements, conducting feasibility studies, selecting sites, performing compliance reviews, and managing projects effectively. Services like those offered by bioaccess® can assist in these areas.
What is the significance of a reimbursement strategy in market access?
A robust reimbursement plan is essential for ensuring the financial viability of innovative products. Understanding the reimbursement landscape and engaging with payers early can significantly improve the chances of securing reimbursement for new medical devices.
What steps should companies take to develop an effective reimbursement plan?
Companies should conduct thorough industry research to understand payer expectations, clearly define the product's value proposition, initiate early discussions with payers about pricing and reimbursement, and leverage successful case studies from similar products.
How does early engagement with payers impact reimbursement outcomes?
Organizations that proactively engage with payers report a 30% higher success rate in securing reimbursement for new medical devices, highlighting the importance of establishing clear communication channels early in the process.