


The article titled "10 Key Elements of Clinical Study Design for Success" primarily aims to delineate the critical components that underpin effective clinical study design. It emphasizes the necessity of:
Each of these elements plays a vital role in enhancing the validity, reliability, and ethical integrity of clinical research, ultimately contributing to successful outcomes.
In the intricate landscape of clinical research, the success of a study hinges on a well-structured design that encompasses a multitude of critical elements. From defining the right target population to ensuring robust statistical analysis, each component plays a pivotal role in shaping the outcomes of clinical trials. This article delves into ten key elements of clinical study design, offering insights into best practices that not only enhance research efficiency but also uphold ethical standards. As the demand for innovative therapies grows, researchers must effectively navigate these complexities to ensure meaningful results and regulatory compliance.
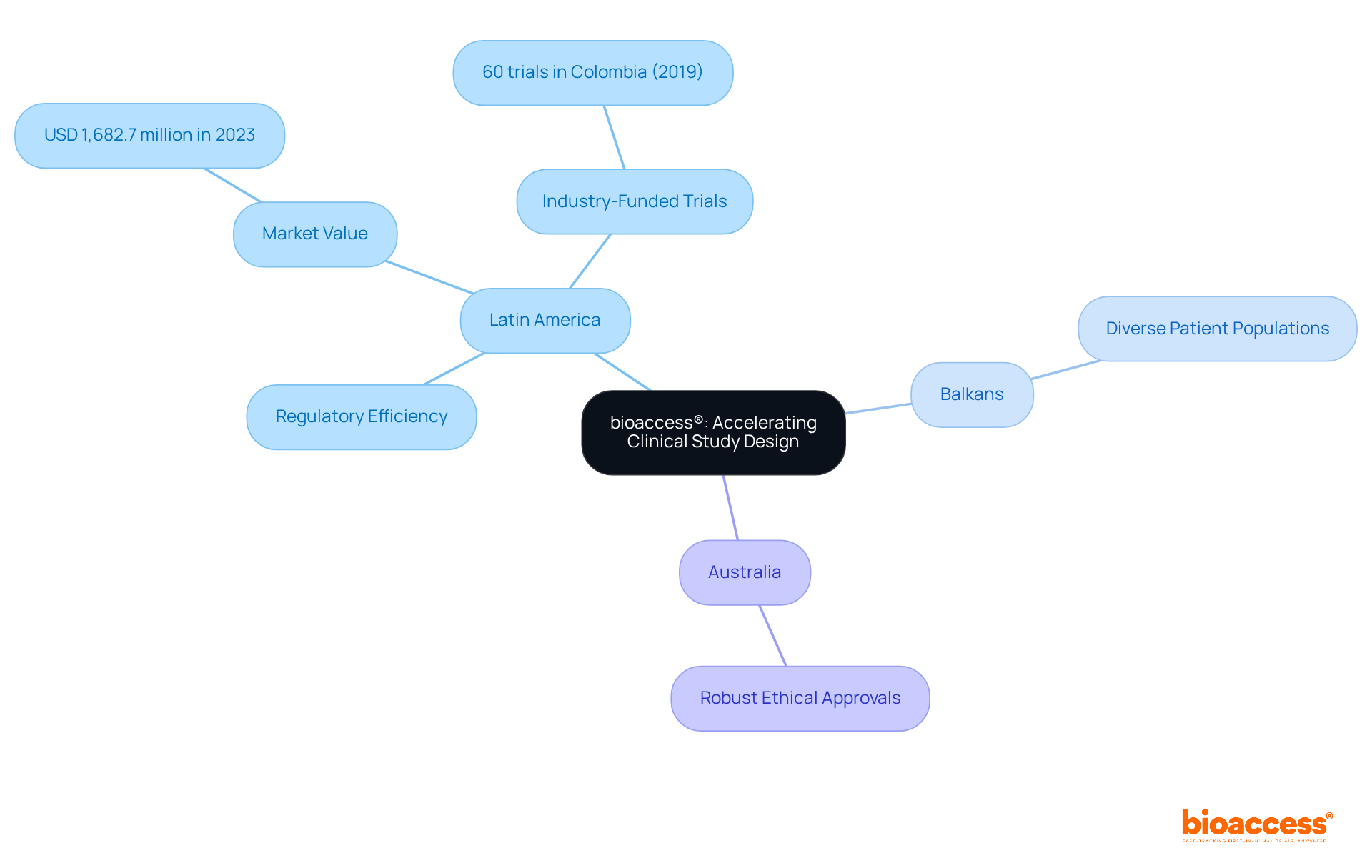
bioaccess® strategically positions itself across Latin America, the Balkans, and Australia, offering unparalleled flexibility in research design. By capitalizing on Latin America's rapid regulatory processes, the diverse patient populations in the Balkans, and Australia's robust ethical approval systems, bioaccess® significantly reduces the time required to initiate and complete research projects compared to traditional markets. This global-first approach not only accelerates timelines but also enhances the overall quality of research outcomes.
Notably, research conducted in Colombia has successfully attracted substantial foreign investment, with the country hosting 60 industry-funded trials in 2019 alone. Additionally, the Latin American clinical trials market generated USD 1,682.7 million in 2023, underscoring its increasing significance in the global arena.
These advancements underscore the critical role of regulatory efficiency in achieving successful research designs, positioning bioaccess® as an essential partner for Medtech and Biopharma innovators in pursuit of timely breakthroughs.
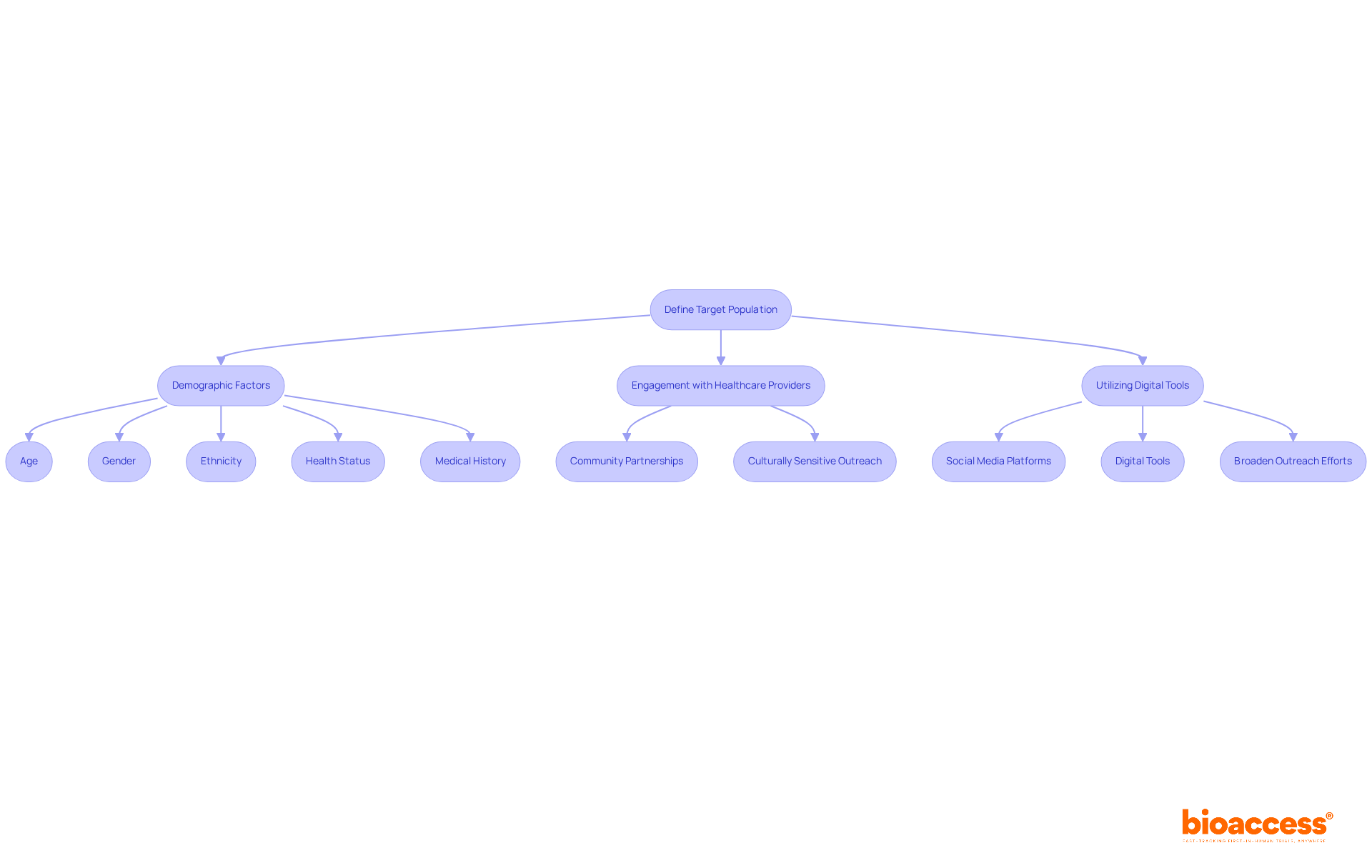
Defining the target population is crucial for aligning participant traits with the research's objectives. This process includes demographic factors such as age, gender, ethnicity, health status, and relevant medical history. In clinical study design, a precisely defined target population not only enhances the relevance of findings but also ensures that results can be generalized to a broader patient demographic.
Engaging local healthcare providers is vital for implementing effective recruitment strategies, particularly in areas with diverse patient populations. For instance, utilizing community partnerships and culturally sensitive outreach can significantly improve recruitment rates. Furthermore, employing digital tools and social media platforms can broaden outreach efforts, facilitating connections with potential participants.
By concentrating on these strategies, researchers can more effectively manage the intricacies of recruiting varied populations within their clinical study design, ultimately resulting in more representative and significant studies.
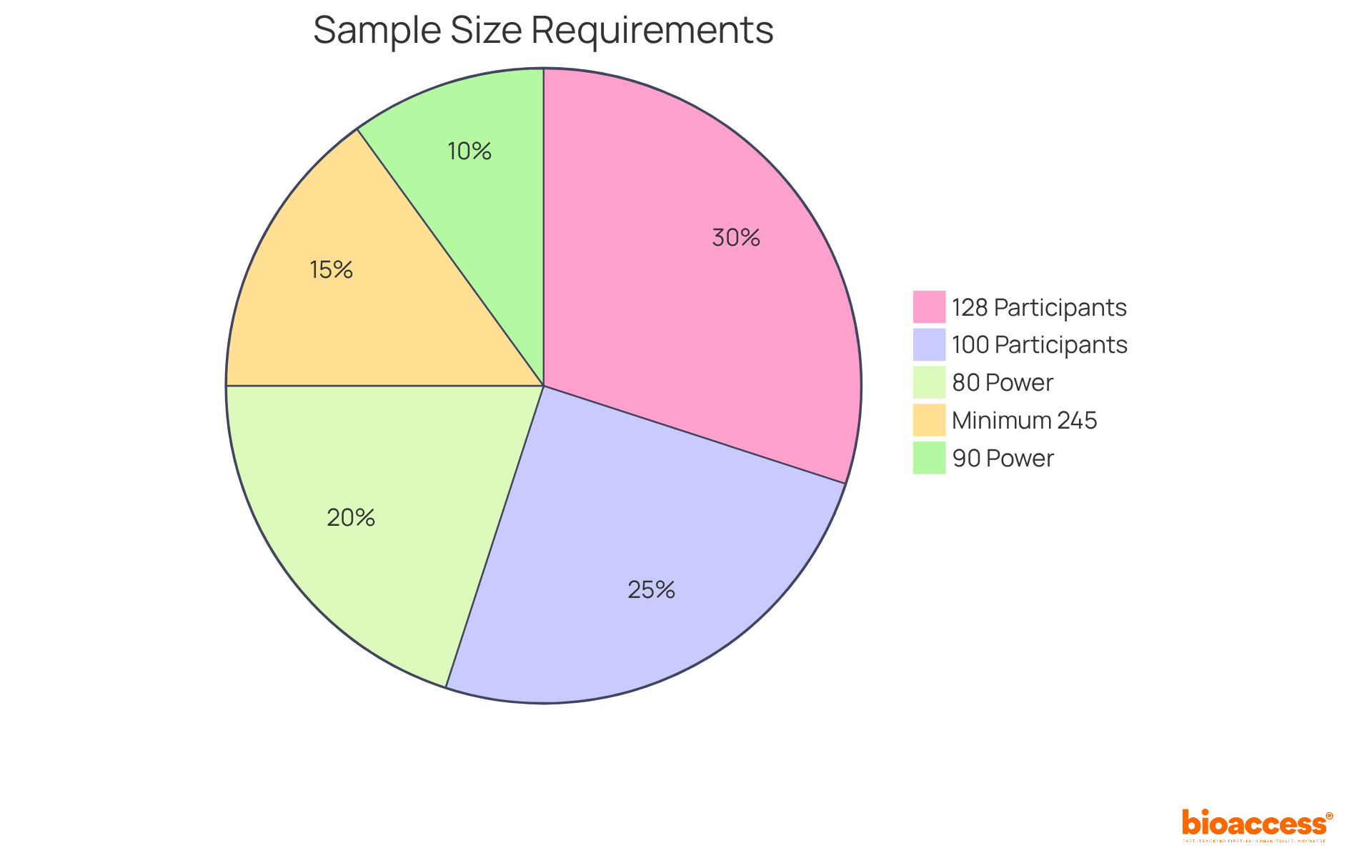
Establishing a suitable sample size is vital in medical research, as it directly affects the research's power—the likelihood of accurately identifying a genuine effect. A larger sample size significantly enhances the likelihood of identifying meaningful differences, while a smaller sample may yield inconclusive results, leading to potential misinterpretations of the data. Research suggests that a minimum sample size of 100 participants per group is frequently advised to guarantee sufficient power, especially in clinical trials where the occurrence of outcomes can differ significantly. Additionally, for a medium effect size, a total sample size of 128 participants is required to achieve 80% power, which is a common standard among regulators.
Statistical power is typically set at 80%, meaning there is an 80% chance of detecting an effect if it exists. This threshold is essential for ensuring that the research can reliably support its hypotheses. The most common alpha level chosen is 0.05, indicating a 5% risk of a Type I error, where a true null hypothesis is incorrectly rejected. In practical terms, researchers must carefully balance the sample size against the expected effect size and the desired power to avoid underpowered analyses that fail to detect significant treatment effects. Alaa Althubaiti emphasizes that determining the necessary sample size should not be viewed as a solution to an inquiry, highlighting the complexities involved in this process.
Utilizing statistical software tools can greatly facilitate this process, allowing researchers to perform complex calculations efficiently. Tools such as G-Power, OpenEpi, PASS, and R offer valuable resources for estimating sample sizes based on different statistical analyses, ensuring that research is sufficiently powered to identify significant effects. Ultimately, a well-calculated sample size not only enhances the validity of findings but also upholds ethical standards by minimizing unnecessary risks to participants. Ethical committees view appropriate sample size calculation as a prerequisite for the approval of research projects, emphasizing its significance in safeguarding participants and maintaining the integrity of the research.
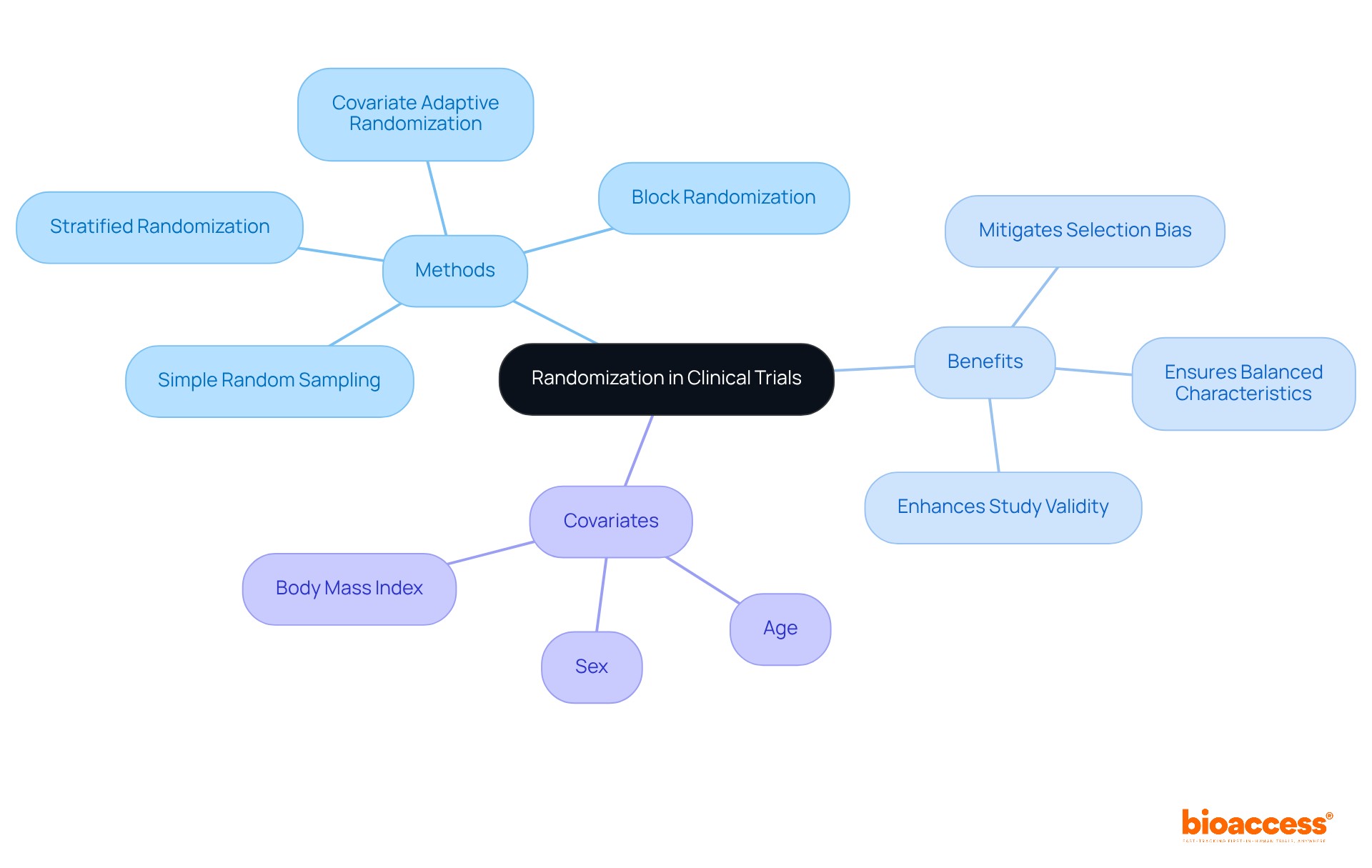
Randomization is a cornerstone in clinical study design, as it crucially ensures that participants are assigned to different groups without bias. This objective can be achieved through a variety of methods, including:
By providing every participant with an equal opportunity for group assignment, researchers effectively mitigate confounding variables, thereby enhancing the validity of the results. For example, stratified randomization facilitates the control of specific covariates, such as:
This ensures balanced characteristics across treatment groups. This approach proves particularly beneficial in larger studies, where the complexity of participant characteristics can lead to imbalances if not properly managed. Evidence indicates that inadequate randomization can result in an overestimation of treatment effects by as much as 40% compared to studies employing proper randomization, underscoring the critical need for robust randomization techniques.
Furthermore, methods like covariate adaptive randomization, which adjusts group assignments based on prior participant characteristics, have demonstrated a capacity to produce less imbalance than traditional methods. Additionally, block randomization guarantees equal sample sizes among treatment groups, further reinforcing the validity of study results. Ultimately, the application of effective randomization practices within clinical study design is indispensable for achieving reliable and interpretable outcomes in health research.
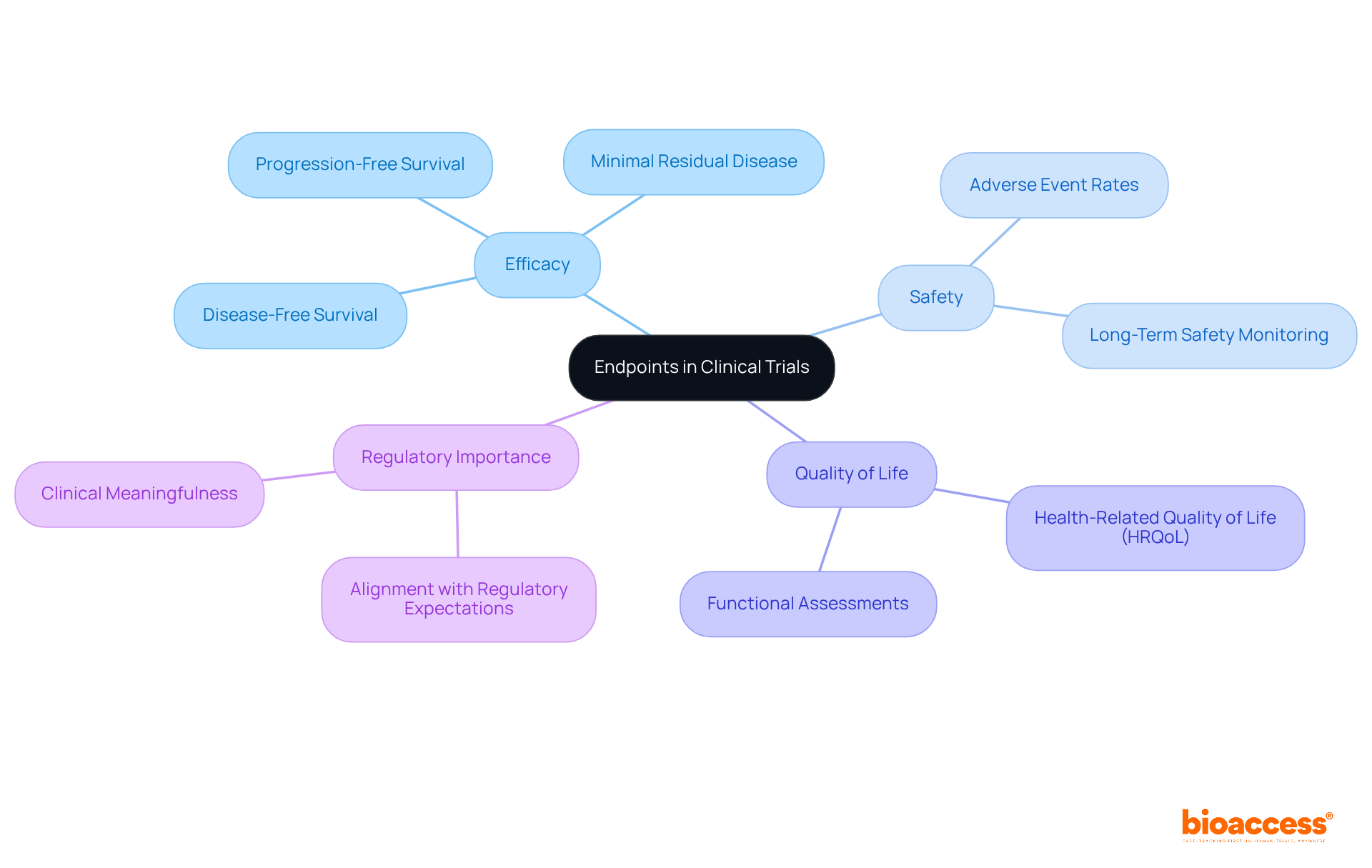
Endpoints represent the specific results that a research study aims to assess, encompassing efficacy, safety, and quality of life. Clearly defining primary and secondary endpoints at the outset is crucial for steering the study's focus and analysis. For instance, successful medical trials often demonstrate clear endpoints that align with regulatory expectations, thereby enhancing their likelihood of approval.
Recent trends indicate a shift toward the development of endpoints, such as disease-free survival and minimal residual disease, which more effectively reflect therapeutic value, especially in complex conditions like Alzheimer's disease, where conventional measures may fall short. As emphasized in various studies, the significance of measurable outcomes cannot be overstated; they not only direct the inquiry process but also inform payer reimbursement decisions.
The FDA underscores that the meaningfulness of research hinges on the relevance of the domains assessed and the magnitude of treatment effects. Consequently, ensuring that endpoints are clinically relevant and measurable is vital, as they ultimately determine the success of the intervention being tested.
With completion rates for Phase III trials reported at 84.9%, the emphasis on robust endpoint definitions is more crucial than ever in navigating the complexities of medical investigations.
Control groups serve as essential standards in medical research, enabling researchers to evaluate the effects of an intervention through comparison with a group that does not receive treatment. This comparison can be established via various methodologies, such as placebo controls—where participants receive an inert substance—or active controls, where they are given a standard treatment known to be effective. The selection of an appropriate control group must align with the research objectives and the specific nature of the intervention, ensuring that results are interpretable and meaningful.
Recent trends indicate a growing preference for employing multiple control groups in research studies, which enhances the reliability of results by minimizing potential biases associated with reliance on a single control group. For instance, research has shown that utilizing both placebo and active controls can provide a more comprehensive understanding of treatment effectiveness. Moreover, the integration of real-world data (RWD) is becoming increasingly prevalent, facilitating more accurate baseline comparisons and improved patient recruitment strategies.
Incorporating effective control group methodologies is paramount for preserving the integrity of research trials. Researchers are urged to implement randomization and blinding techniques to reduce bias and ensure comparability between treatment and control groups. As the landscape of medical research evolves, mastering the nuances of control group selection and execution will remain vital for generating trustworthy and valid results.

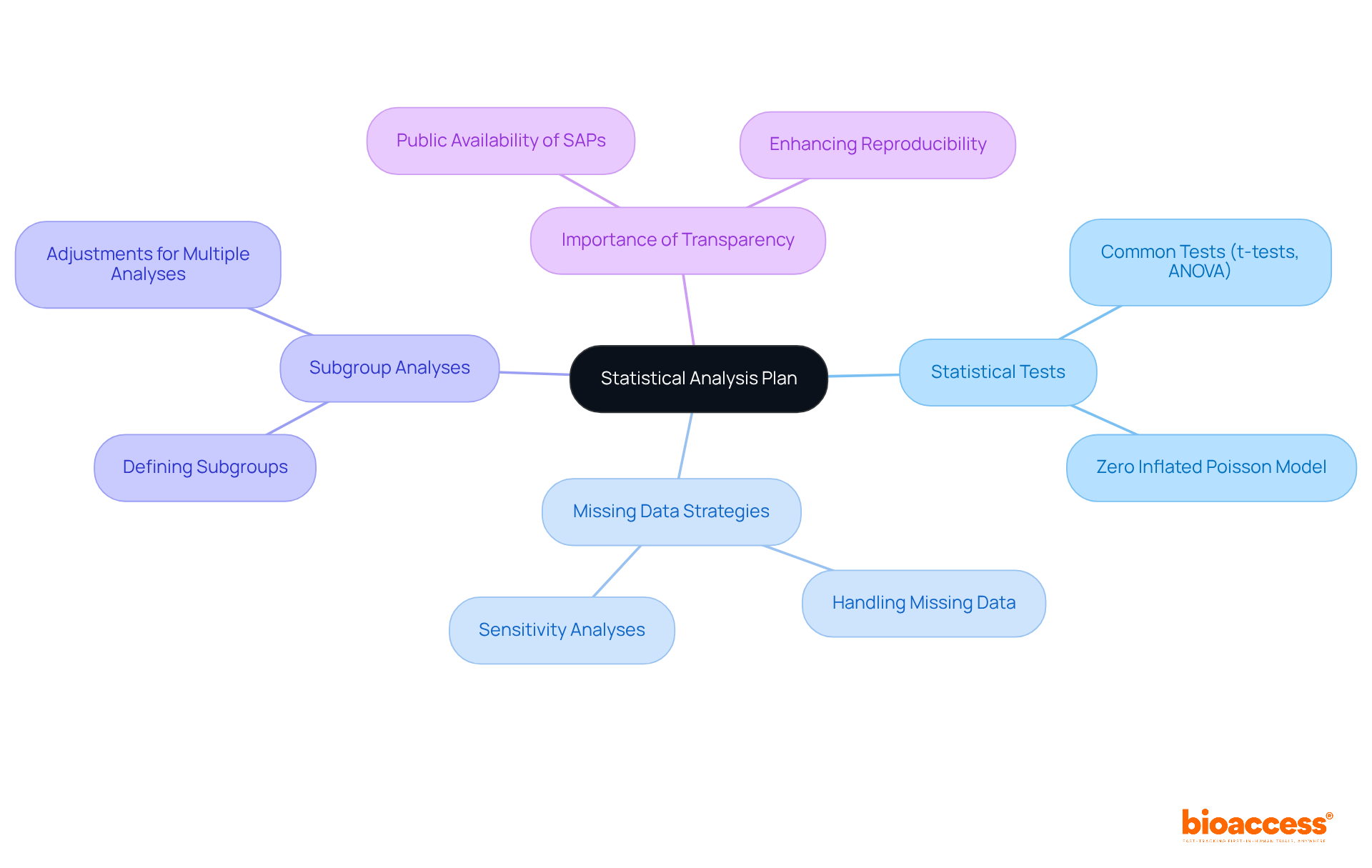
A statistical analysis plan (SAP) serves as a critical blueprint for the methods and procedures employed in the clinical study design to analyze data collected during a clinical trial. It meticulously outlines the statistical tests to be utilized, strategies for addressing missing data, and the framework for conducting subgroup analyses. By establishing a comprehensive clinical study design prior to the commencement of data collection, researchers can ensure a systematic approach that aligns directly with the study's objectives. This proactive strategy not only bolsters the integrity of the research but also promotes reproducibility, a fundamental aspect of clinical study design that is underscored by experts in the field.
Carrol Gamble, PhD, notably emphasizes that a clear and comprehensive clinical study design is essential for enhancing reproducibility in research studies. Alarmingly, fewer than 1% of researchers publish their SAPs in peer-reviewed journals, underscoring the critical need for transparency and the necessity for thorough SAPs in medical studies. The prevalence of common statistical tests, such as t-tests and ANOVA, in medical research further highlights the importance of a well-structured clinical study design to effectively guide these methodologies.
Furthermore, conducting multiple analyses without appropriate adjustments can inflate the alpha spend, thereby increasing the likelihood of Type I error. This reality accentuates the urgent need for a structured clinical study design analysis plan. By prioritizing the development of a robust SAP, researchers can significantly enhance the quality and reliability of their studies.
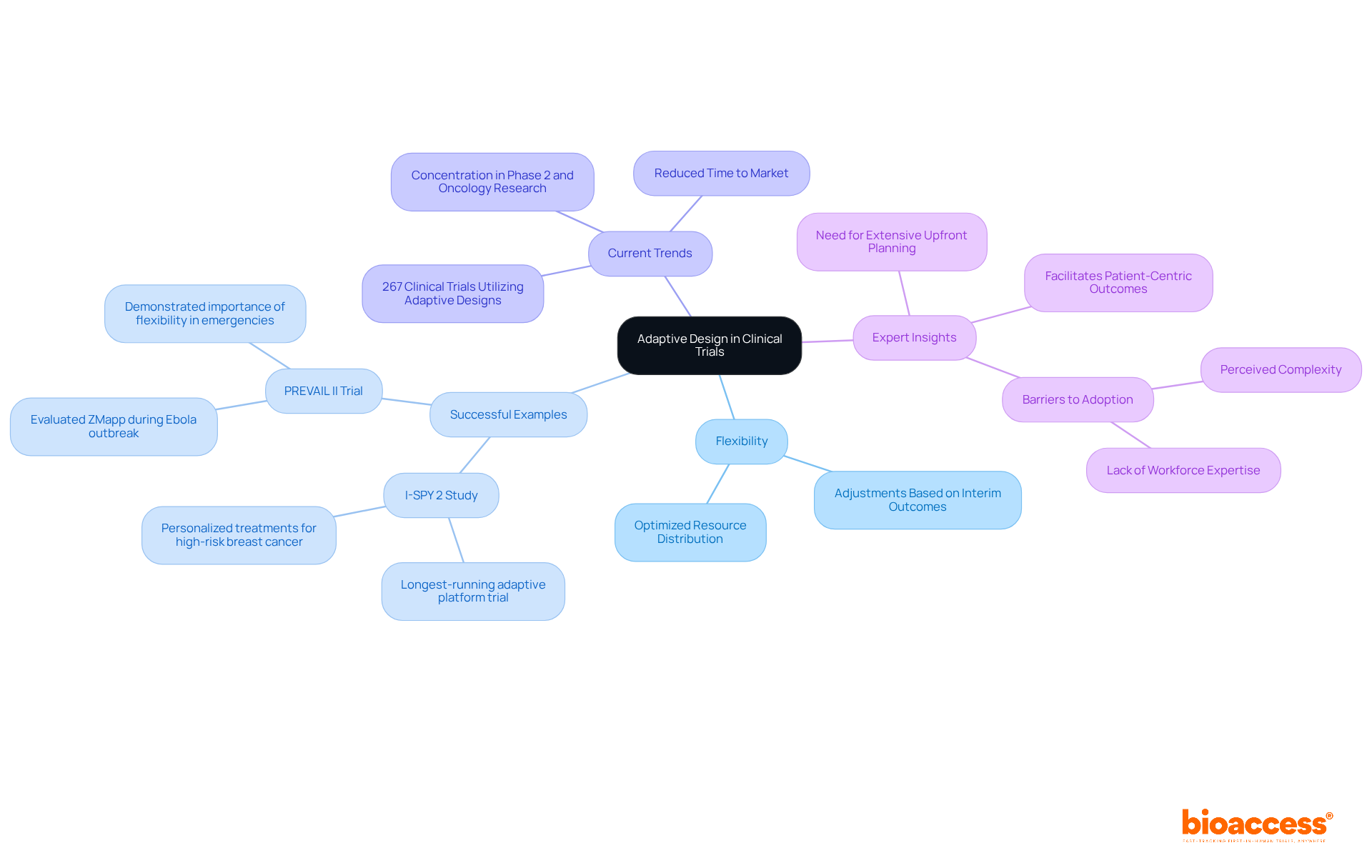
Adaptive design approaches empower researchers to implement predetermined modifications during studies based on interim outcomes. This inherent flexibility allows for adjustments in sample size, treatment regimens, and even endpoints, facilitating a more adaptable management strategy. By leveraging adaptive designs in clinical study design, researchers can optimize resource distribution and significantly enhance the likelihood of achieving meaningful results, ultimately leading to more effective clinical studies.
Successful examples of adaptive studies underscore their potential impact. The I-SPY 2 Study, launched in 2010, stands as the longest-running adaptive platform study, focusing on personalized treatments for high-risk breast cancer. It has effectively identified therapies tailored to various tumor subtypes, exemplifying the efficacy of adaptive methodologies in personalizing cancer treatment. Similarly, the PREVAIL II Trial, conducted during the Ebola outbreak, employed an adaptive design to swiftly evaluate the efficacy of ZMapp, highlighting the critical importance of flexibility in urgent public health scenarios.
Current trends reveal a growing acceptance of adaptive methodologies in clinical study design within the Medtech and Biopharma sectors. A 2023 study identified 267 clinical study designs utilizing adaptive designs, with a significant concentration in Phase 2 and oncology research. This shift signifies a sector-wide acknowledgment of the advantages adaptive studies offer, including reduced time to market and improved patient safety through continuous observation and modifications based on real-time data.
Experts note that while adaptive trials may necessitate more extensive upfront planning, their capacity to facilitate seamless transitions between trial phases and enhance patient-centric outcomes renders them invaluable. As the sector evolves, the integration of flexible approaches is expected to foster sustainable growth and innovation in healthcare studies.
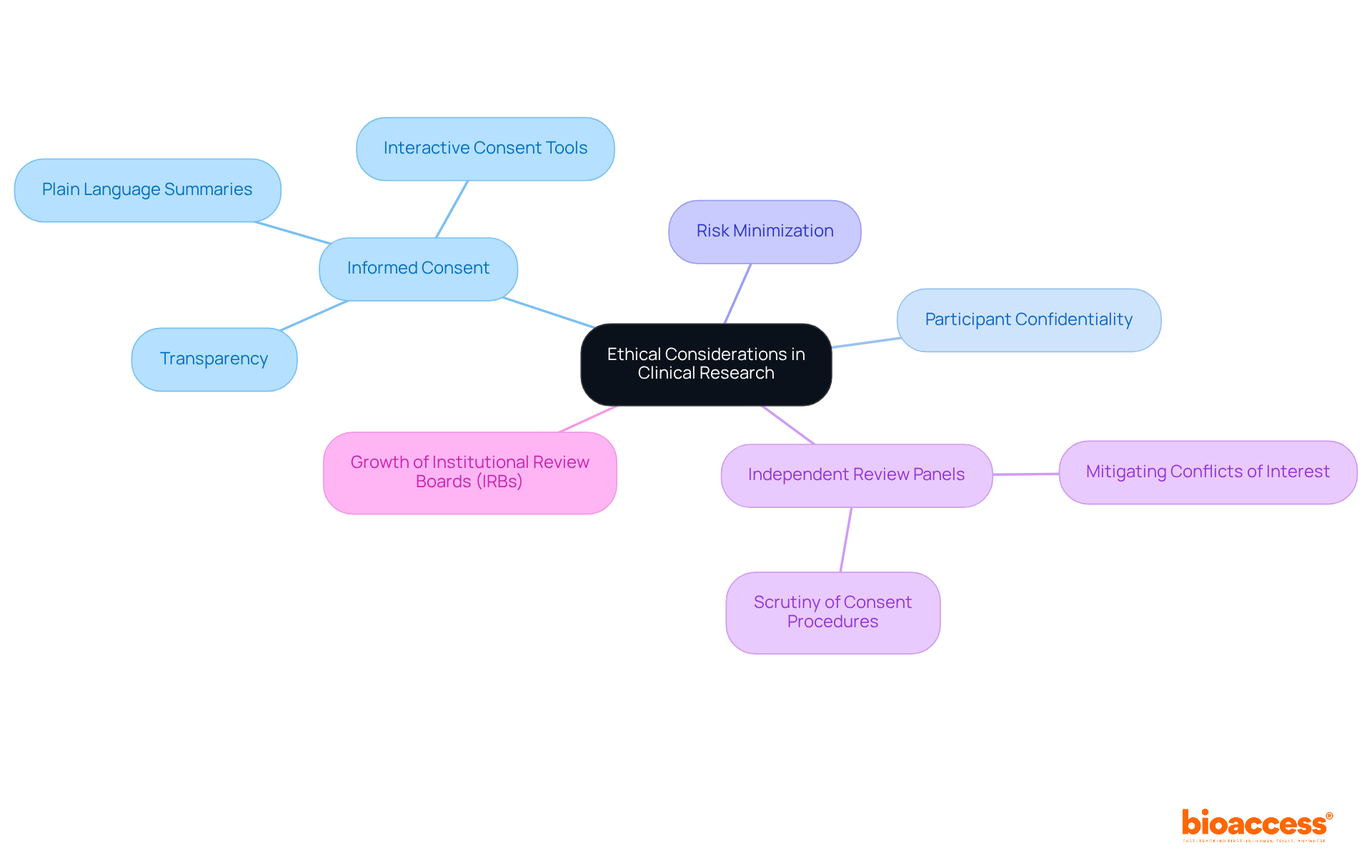
Ethical considerations in clinical research are paramount, encompassing responsibilities such as obtaining informed consent, safeguarding participant confidentiality, and minimizing risks. Informed consent is not merely a formality; it is a fundamental ethical requirement that ensures participants are fully aware of the study's purpose, methods, risks, and potential benefits. Recent trends highlight a shift towards more transparent and comprehensive informed consent processes, which are essential for fostering trust between researchers and participants.
For instance, the Belmont Report emphasizes the necessity of informed consent, mandating that participants understand the uncertainties surrounding treatment efficacy. This is especially significant in early-phase studies, where the risk-benefit ratio is often unclear. Moreover, independent review panels are increasingly scrutinizing informed consent procedures to ensure ethical acceptability and mitigate conflicts of interest. The significant growth in the number of Institutional Review Boards (IRBs), alongside the increase in clinical trials, further underscores the importance of this scrutiny.
Examples of effective informed consent processes include the use of plain language summaries and interactive consent tools that enhance participant understanding. Such practices not only adhere to ethical standards but also enable participants to make informed choices about their involvement in studies. Notably, only 63% of eligibility criteria are reported in journal articles, highlighting the need for transparency in informed consent processes.
Statements from specialists highlight the significance of informed consent:
"Has everything been done to minimize the risks and inconvenience to participants, to maximize the potential benefits, and to determine that the potential benefits to individuals and society are proportionate to, or outweigh, the risks?"
This underscores the ethical obligation to prioritize participant safety throughout the study process. The ethical evaluation of studies involving human participants has advanced considerably over the last two decades, emphasizing the importance of justifying eligibility standards in trials. By following these ethical guidelines, researchers can improve the integrity of their research and ensure the well-being of all participants.
Regulatory compliance stands as a cornerstone in medical research, necessitating unwavering adherence to laws and guidelines that vary across regions. This intricate process involves securing essential approvals from regulatory bodies and meticulously following research protocols. For instance, the FDA's guidelines, particularly ICH E6 and ICH E8, delineate comprehensive steps for integrating risk management into clinical study design, enabling researchers to identify adherence risks early in the clinical study design phase. Navigating these complex regulatory landscapes not only ensures that investigations are conducted ethically but also safeguards participant welfare and upholds the integrity of the work.
Recent trends indicate that pharmaceutical firms are increasingly outsourcing R&D tasks to contract organizations (CROs) to leverage their expertise in navigating these legal requirements. This shift is propelled by the imperative for compliance with evolving regulations and the aspiration to enhance operational efficiencies. Moreover, organizations that implement robust Good Clinical Practice (GCP) training programs experience markedly higher adherence rates, underscoring the significance of continuous staff education in maintaining compliance.
As the landscape of medical studies grows more intricate, with only 20% of research meeting deadlines due to inefficiencies, the integration of advanced technologies such as AI and data analytics becomes paramount. These tools not only streamline data management but also enhance adherence tracking, facilitating prompt corrective actions when necessary.
Informed consent remains a pivotal area, as over 50% of research participants find it challenging to fully comprehend key components, which can lead to ethical violations. Addressing these concerns is vital for safeguarding participant rights and ensuring the ethical practice of research studies. By prioritizing compliance and ethical standards, researchers can adeptly navigate the legal complexities of clinical study design, ultimately contributing to the success of their trials.
The success of clinical study design is fundamentally rooted in a meticulous understanding of its key elements, which collectively enhance the reliability and efficiency of research outcomes. By addressing critical components such as:
Researchers can significantly improve the validity of their findings. Furthermore, ethical considerations and regulatory compliance are pivotal in safeguarding participants and ensuring the integrity of the research process.
This article has highlighted various strategies, from leveraging global agility in clinical study design to utilizing adaptive methodologies that allow for flexibility in trial execution. The importance of engaging local healthcare providers for effective recruitment, the necessity of well-structured statistical analysis plans, and the critical role of control groups in establishing baselines have been underscored as essential practices. These insights not only aid in navigating the complexities of clinical research but also serve to foster innovation within the Medtech and Biopharma sectors.
As the landscape of clinical trials continues to evolve, embracing these best practices becomes increasingly vital. Researchers are urged to prioritize ethical standards, maintain regulatory compliance, and leverage advanced methodologies to enhance the quality and impact of their studies. By doing so, the clinical research community can drive meaningful advancements in healthcare, ultimately benefiting patients and society at large.
What is bioaccess® and what regions does it operate in?
bioaccess® is a company that strategically positions itself across Latin America, the Balkans, and Australia, providing flexibility in research design and accelerating clinical study timelines.
How does bioaccess® enhance clinical study design?
bioaccess® enhances clinical study design by leveraging rapid regulatory processes in Latin America, diverse patient populations in the Balkans, and robust ethical approval systems in Australia, significantly reducing the time to initiate and complete research projects.
What is the significance of the Latin American clinical trials market?
The Latin American clinical trials market generated USD 1,682.7 million in 2023, highlighting its increasing importance in the global clinical research landscape.
Why is defining the target population important in clinical studies?
Defining the target population is crucial for aligning participant traits with research objectives, enhancing the relevance of findings, and ensuring that results can be generalized to a broader patient demographic.
What strategies can improve participant recruitment in clinical studies?
Effective recruitment strategies include engaging local healthcare providers, utilizing community partnerships, employing culturally sensitive outreach, and leveraging digital tools and social media platforms to broaden outreach efforts.
Why is calculating the right sample size important in medical research?
A suitable sample size is vital as it affects the research's power, which is the likelihood of accurately identifying a genuine effect. An appropriate sample size enhances the reliability of the findings and supports the research hypotheses.
What is the recommended minimum sample size for clinical trials?
A minimum sample size of 100 participants per group is frequently advised to ensure sufficient power, especially in clinical trials where outcome occurrences can vary significantly.
What is statistical power and why is it important?
Statistical power is typically set at 80%, indicating the chance of detecting an effect if it exists. It is essential for ensuring that research can reliably support its hypotheses and avoid underpowered analyses.
What tools can assist researchers in calculating sample sizes?
Statistical software tools such as G-Power, OpenEpi, PASS, and R can help researchers perform complex calculations to estimate sample sizes based on different statistical analyses.
How does appropriate sample size calculation relate to ethical standards in research?
Appropriate sample size calculation is viewed as a prerequisite for research project approval by ethical committees, as it minimizes unnecessary risks to participants and maintains the integrity of the research.