

This article highlights the critical FDA medical device labeling requirements that manufacturers must adhere to for ensuring patient safety and regulatory compliance. It delineates essential components such as the device name, intended use, manufacturer information, and the Unique Device Identifier (UDI). Accurate labeling is not merely a regulatory obligation; it is vital for preventing misbranding issues and facilitating effective market access.
Navigating the complex landscape of FDA medical device labeling requirements is crucial for manufacturers seeking compliance and enhancing patient safety. As the demand for innovative medical solutions escalates, understanding the essential elements of labeling can significantly influence market access and regulatory adherence. However, with common pitfalls and evolving regulations, how can manufacturers effectively mitigate risks and optimize their labeling processes to avoid costly mistakes? This article delves into ten key FDA medical device labeling requirements, offering insights and best practices designed to empower stakeholders within the Medtech industry.
bioaccess® excels in assisting Medtech, Biopharma, and Radiopharma innovators in navigating the complexities of FDA medical device labeling requirements. This expertise is crucial in a landscape where rapid access to innovative medical solutions can profoundly influence patient outcomes.
With extensive knowledge of regulatory processes, bioaccess® streamlines adherence timelines, enabling clients to expedite their market entry while fully complying with essential guidelines. Recent initiatives reflect the FDA's commitment to enhancing communication and safety within the industry.
By subscribing to CDRH email lists, stakeholders can stay informed about critical updates, including recalls and safety communications, ensuring they remain compliant and responsive to regulatory changes. Through these efforts, bioaccess® not only promotes adherence but also empowers clients to effectively navigate the evolving regulatory environment.
To ensure compliance and safety, the FDA medical device labeling requirements must encompass several essential elements. These core components include:
Ensuring clarity in descriptions is crucial, as around 76% of healthcare tools lack essential information, resulting in misbranding problems. Additionally, research suggests that approximately 30% of medical equipment includes deceptive assertions, highlighting the significance of precise and transparent marking practices. By adhering to the FDA medical device labeling requirements, manufacturers can mitigate risks associated with regulatory non-compliance and improve their market position.
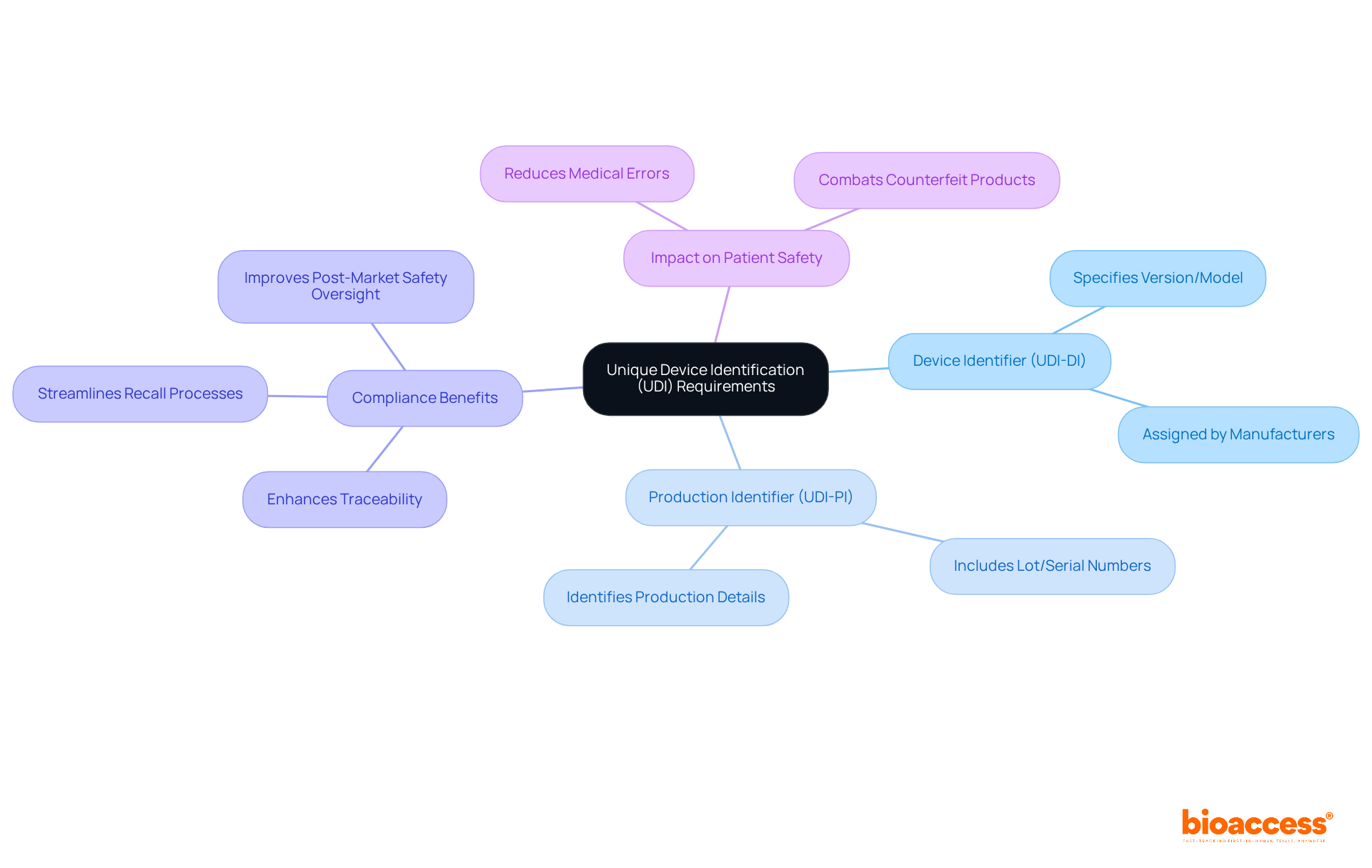
The Unique Product Identification (UDI) system is integral to the FDA medical device labeling requirements, which mandate that all medical products display a UDI on their labels and packaging. This system comprises two essential components:
Compliance with UDI requirements significantly enhances traceability, streamlining recall processes and bolstering patient safety.
Manufacturers must ensure the accurate submission of product information to the UDI database prior to market placement. The implementation of the UDI system is expected to improve post-market safety oversight and reduce medical errors, as it facilitates better tracking of products throughout their lifecycle. For instance, the new UDI system will be applied to all medical instruments and in-vitro diagnostic tools in the EU market, excluding custom-made items, thereby enhancing the traceability of these products. This initiative is projected to lead to more robust monitoring by regulatory authorities and improved waste disposal and stock-management strategies among healthcare institutions and economic operators.
Current expert opinions underscore the importance of UDI adherence for medical equipment, particularly as the healthcare landscape evolves towards increased digitalization. The UDI system not only helps producers comply with FDA medical device labeling requirements but also fosters a safer environment for patients by combating counterfeit products and minimizing the risk of medical errors. As the UDI system continues to be integrated into medical equipment labeling practices, its role in ensuring compliance and enhancing patient safety will only grow in significance.
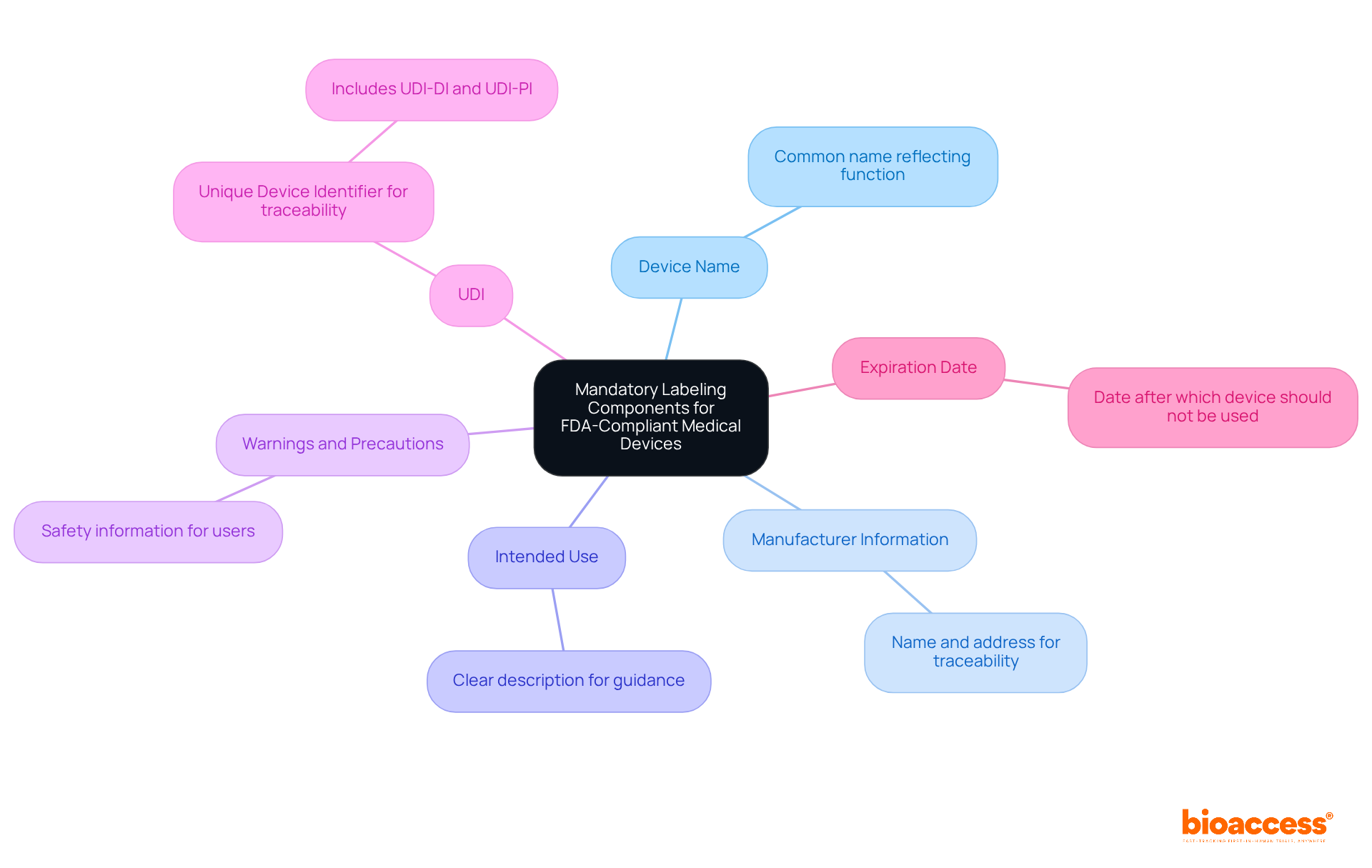
To ensure FDA compliance, medical device labels must include the following mandatory components:
Frequent marking errors in FDA submissions often arise from insufficient warnings or vague intended use descriptions, which can result in regulatory scrutiny and delays in market access. For instance, labels that fail to maintain legibility under various conditions can compromise safety and violate 21 CFR 820.120(a). Hence, compliance with the FDA medical device labeling requirements is not merely a regulatory duty but also a vital element in ensuring patient safety and efficient instrument use.
Producers frequently face significant challenges in complying with the FDA medical device labeling requirements, which can lead to substantial delays. Among the primary pitfalls are:
Non-compliance with formatting can result in rejection during audits, so adhering to FDA medical device labeling requirements is vital. Non-compliance in this domain is a common cause of FDA warning letters and can result in costly delays.
To mitigate these pitfalls, manufacturers should implement rigorous reviews and regular audits of their marking processes. Establishing a compliant documentation structure early in product design and risk management is essential, including maintaining records in the Device History Record (DHR) to confirm adherence. Additionally, staying informed about regulatory updates through FDA guidance subscriptions or participation in industry forums, alongside conducting usability testing for patient-facing products, can further bolster compliance and safety.

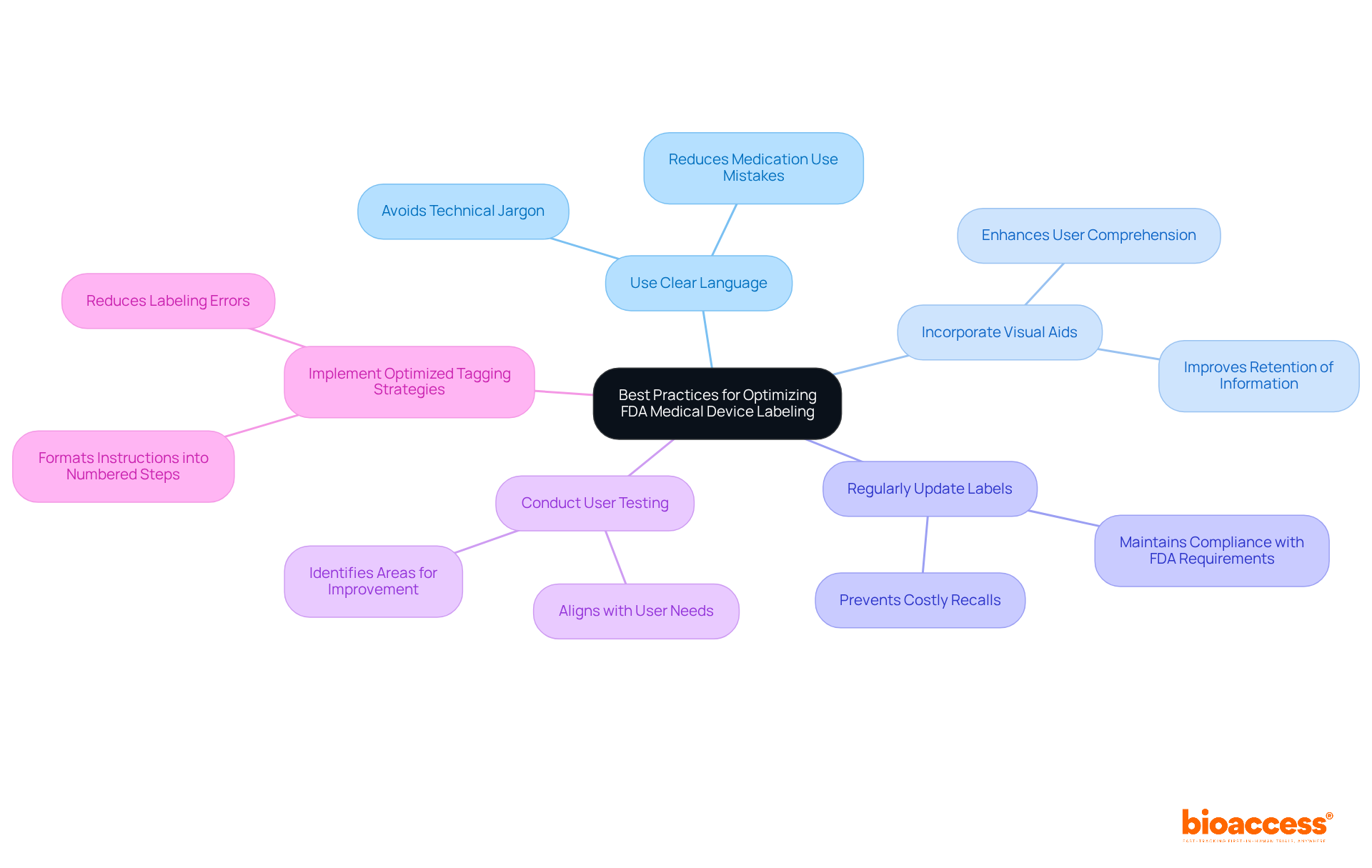
To optimize compliance with FDA medical device labeling requirements, manufacturers must embrace best practices that ensure clarity.
Use Clear Language: Simplifying language and avoiding technical jargon is essential for ensuring that instructions are easily understood by users. This approach can significantly reduce the likelihood of medication use mistakes, which are linked to inadequate identification in over 50% of cases. Moreover, implementing structured quality management practices has demonstrated potential savings of $175 million and the prevention of 1,500 lives lost.
Incorporate Visual Aids: Utilizing diagrams or symbols can greatly enhance user comprehension. Research shows that visual aids improve understanding and retention of critical information, making it easier for users to follow instructions accurately.
Regularly Update Labels: Labels must reflect the most current information and regulatory requirements. Regular audits and updates are crucial for maintaining compliance with FDA medical device labeling requirements and preventing costly recalls, which occur approximately once a month for clinically important drug recalls in the U.S. Furthermore, to adhere to FDA medical device labeling requirements, labels must remain legible and affixed under all storage, distribution, and use conditions as specified in 21 CFR 820.120(a).
Conduct User Testing: Engaging actual users in testing labels can uncover areas for improvement. This practice not only enhances label effectiveness but also aligns with the FDA medical device labeling requirements to ensure that markings meet user needs.
Implement Optimized Tagging Strategies: Successful tagging strategies in Medtech include formatting patient instructions into clearly numbered steps, significantly improving user comprehension and adherence to safety protocols. Avoiding frequent tagging errors, such as label mix-ups and missing required information, is essential for ensuring adherence and safety. By focusing on these strategies, manufacturers can enhance the clarity and effectiveness of their medical device labels, ultimately ensuring better patient safety and adherence.
Expert Insight: As Jesseca Lyons, a Senior Medical Device Guru at Greenlight Guru, emphasizes, defining risk categorization and indications for use is crucial in developing effective strategies for product information.
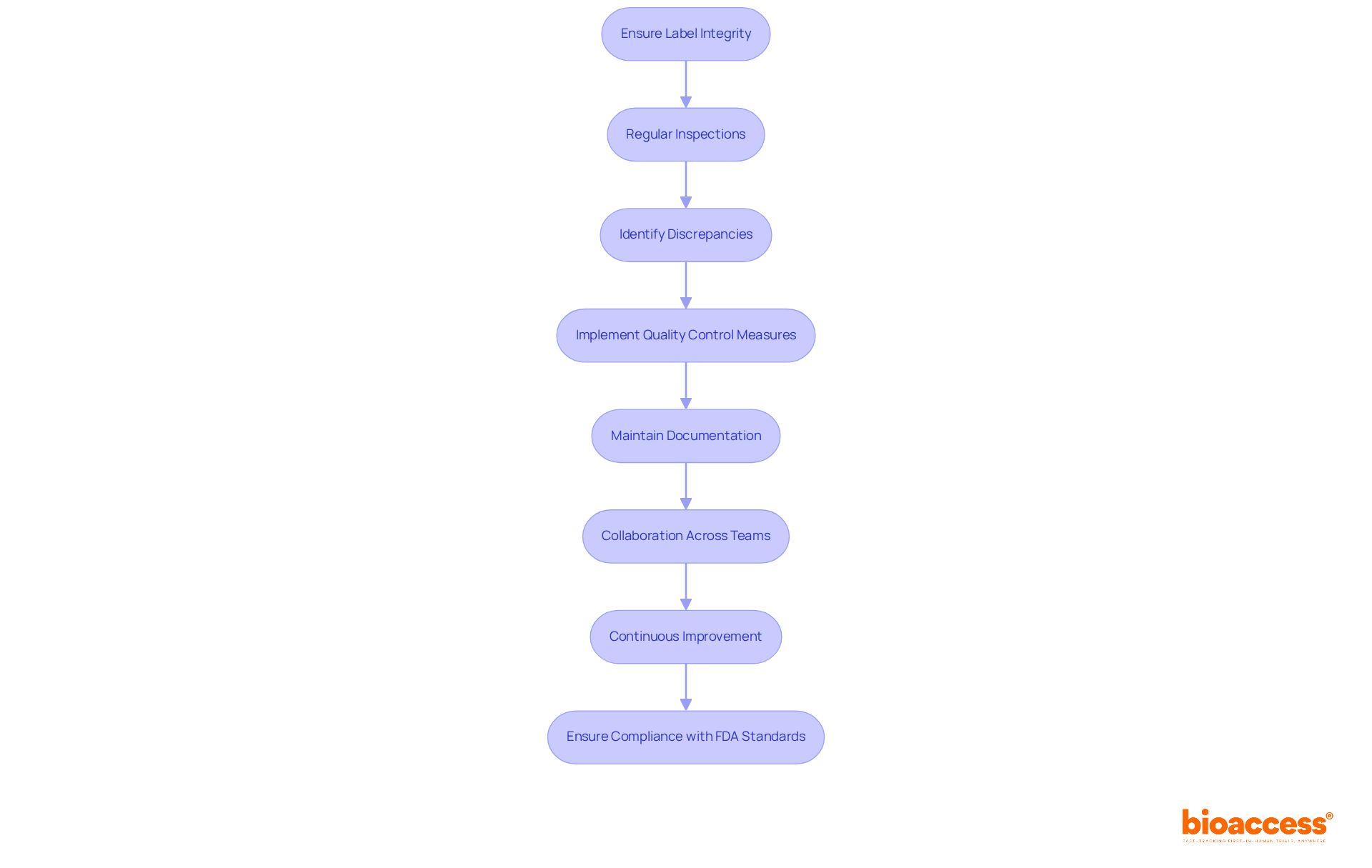
Ensuring label integrity involves several critical processes essential for compliance with the FDA medical device labeling requirements. Regular inspections are vital to confirm that labels are affixed correctly and remain legible throughout the product lifecycle. These inspections help identify any discrepancies early, thereby reducing the risk of non-compliance.
Quality control measures are imperative; manufacturers should implement robust protocols to identify errors in product information before products reach the market. This proactive strategy not only safeguards adherence to standards but also enhances product reliability. For instance, a case study emphasized that a structured checklist in the tagging process saved $175 million and 1,500 lives, underscoring the significant impact of effective quality control.
Documentation plays a crucial role in this process. Keeping thorough records of marking processes and inspections is essential for proving adherence during audits. This documentation should encompass specifics of inspections, corrective measures implemented, and any modifications made to marking practices. Complying with the FDA medical device labeling requirements in Section 820.120 guarantees that product integrity and inspection processes are included in good manufacturing practices.
Collaboration across teams is equally essential. Involving both engineering and non-engineering teams in the tagging process can lead to improved design controls, which greatly affect the final marking of medical devices. Jesseca Lyons highlights that this collaboration is vital for ensuring that all elements of the marking process are handled efficiently.
Finally, continuous improvement is necessary for upholding standards. Regularly updating quality management systems (QMS) and staying informed about regulatory changes are essential. Utilizing advanced QMS software can simplify tagging activities and enhance transparency, ensuring that all tagging elements are current and compliant with FDA standards. Furthermore, the UDI regulation mandates that manufacturers incorporate a unique identifier on tags and packaging, a crucial element of FDA requirements.
By prioritizing these processes, manufacturers can ensure that their labeling complies with FDA medical device labeling requirements, ultimately facilitating smoother market entry and enhancing patient safety.
Routine label inspections are essential for ensuring compliance with the FDA medical device labeling requirements in the medical device industry. These audits must include several critical components:
Review of Label Content: It is imperative to verify that all mandatory information is accurate and present, as inaccuracies can lead to significant compliance issues. A study indicated that 58% of errors stemmed from inaccurate categorization, highlighting the necessity for meticulous content checks. Assessing labeling processes is essential to ensure they comply with FDA medical device labeling requirements. This includes examining the methods used to record and monitor changes in tags, as improper practices can jeopardize trial integrity.
Feedback Mechanism: Establishing a robust system for collecting user feedback is vital to identify potential tagging issues. This proactive approach can mitigate risks associated with mislabeling, which was reported by 43.90% of respondents in a recent survey as a common challenge.
Incorporating these practices not only enhances compliance but also significantly contributes to patient safety and the overall success of clinical trials. Effective label audit processes in Medtech have shown that regular monitoring and staff training can markedly reduce errors, ensuring that products meet both regulatory requirements and user expectations.

The FDA medical device labeling requirements allow the use of symbols in medical equipment labeling to efficiently convey critical information. To maximize the effectiveness of symbols, manufacturers should adhere to the following guidelines:
Effective signage practices are crucial; studies indicate that clear instructions and warnings can significantly reduce the risk of misuse, which can lead to serious injuries or even fatalities. For instance, improper use of medical instruments can have dire consequences, underscoring the need for clear labeling. By applying these strategies, manufacturers can foster a positive user experience while ensuring compliance with FDA medical device labeling requirements. As Marco Theobold, an expert in medical equipment regulations, emphasizes, "Manufacturers should always refer directly to the text of the applicable laws to ensure that they are following the correct procedures and staying compliant." Regularly assessing packaging procedures against the FDA medical device labeling requirements is also advised to maintain continuous compliance.

Non-compliance with FDA medical device labeling requirements can lead to significant repercussions, including:
Product Recalls: Devices may be recalled if labeling is deemed misleading or inaccurate. In recent years, the FDA reported that around 20% of all medical product recalls were due to marking mistakes, highlighting the crucial significance of precise marking.
Fines and Penalties: Manufacturers can incur substantial fines for violations of FDA regulations. The FDA has the authority to impose fines that can reach millions of dollars, depending on the severity of the violation and the potential risk to patient safety. For instance, a producer encountered a $5 million penalty for repeated marking violations that jeopardized patient safety.
Legal Action: Non-compliance can expose manufacturers to lawsuits or other legal actions. Legal experts emphasize that the risks associated with non-compliance with FDA medical device labeling requirements can lead to costly litigation, which not only drains financial resources but also diverts attention from core business operations. Dr. Kesselheim observes, "The financial consequences of failing to adhere can be devastating, often resulting in settlements that surpass the initial expenses of following regulations."
Reputation Damage: Failing to meet labeling requirements can severely damage a company's reputation, eroding trust with consumers and healthcare providers. A tarnished reputation can have long-lasting effects on market position and customer loyalty, making compliance not just a regulatory necessity but a strategic imperative for manufacturers.

Navigating the FDA medical device labeling requirements is essential for manufacturers aiming to ensure compliance and promote patient safety. Adhering to these guidelines not only facilitates market access but also enhances the credibility and reliability of medical devices. The clarity and accuracy of labeling are pivotal, as they directly impact how devices are perceived and used in healthcare settings.
Key insights discussed in the article highlight the necessity of including critical elements such as:
Furthermore, understanding and implementing best practices, such as conducting regular audits and utilizing symbols for clarity, can significantly mitigate the risk of non-compliance. The consequences of failing to adhere to these requirements can be severe, ranging from product recalls and hefty fines to reputational damage.
Ultimately, the importance of FDA medical device labeling compliance cannot be overstated. By prioritizing accurate and clear labeling practices, manufacturers can not only fulfill regulatory obligations but also contribute to improved patient outcomes and safety. Embracing these standards is not just a legal requirement; it is a commitment to quality and responsibility in the medical device industry.
What is bioaccess® and how does it assist with FDA medical device labeling requirements?
bioaccess® specializes in helping Medtech, Biopharma, and Radiopharma innovators navigate FDA medical device labeling requirements, streamlining adherence timelines to expedite market entry while ensuring compliance with essential guidelines.
Why is compliance with FDA medical device labeling important?
Compliance is crucial as it influences patient outcomes by ensuring that medical devices are clearly labeled, safe to use, and effectively communicated, which helps prevent misinterpretation and misuse.
What are the core elements that must be included in FDA medical device labels?
Core elements include the device name, intended use, manufacturer information, instructions for use, warnings and precautions, and the Unique Device Identifier (UDI).
What is the Unique Device Identifier (UDI) system?
The UDI system requires all medical products to display a UDI on their labels and packaging, consisting of a Device Identifier (UDI-DI) for version or model specification and a Production Identifier (UDI-PI) for production details like lot or serial numbers.
How does the UDI system enhance patient safety and traceability?
The UDI system improves traceability, streamlines recall processes, and bolsters patient safety by facilitating better tracking of products throughout their lifecycle, thus reducing medical errors and combating counterfeit products.
What are the consequences of not adhering to FDA medical device labeling requirements?
Non-compliance can lead to misbranding issues, regulatory penalties, and increased risks associated with patient safety, ultimately affecting a manufacturer's market position.
How can stakeholders stay informed about FDA updates and regulatory changes?
Stakeholders can subscribe to CDRH email lists to receive critical updates, including recalls and safety communications, ensuring they remain compliant and responsive to regulatory changes.