


The article presents an overview of the ten essential features of clinical trial software crucial for the success of Medtech startups. These features encompass:
Together, they significantly enhance operational efficiency and expedite the clinical research process. Ultimately, these capabilities empower startups to bring innovative medical devices to market more effectively.
The landscape of clinical trials is rapidly evolving, driven by the urgent need for efficiency and innovation in Medtech. As startups race to bring life-saving technologies to market, the right clinical trial software emerges as a game-changer, offering features that not only streamline processes but also enhance compliance and data integrity.
With a plethora of options available, what essential features can truly make a difference in achieving Medtech success? By exploring these key functionalities, we uncover how they empower organizations to navigate the complexities of clinical research, optimize resources, and improve patient engagement.
bioaccess® stands out by providing expedited medical studies services specifically tailored for Medtech startups. By harnessing the regulatory speed of Latin America, the diverse patient populations in the Balkans, and Australia's efficient pathways, bioaccess® achieves ethical approvals in an impressive 4-6 weeks. This swift turnaround is crucial for startups aiming to introduce innovative medical devices to the market promptly, enabling them to outpace competitors and address pressing healthcare demands.
With over 15 years of experience, bioaccess® possesses a profound understanding of the regulatory environment and operational challenges that Medtech firms encounter, establishing itself as a vital partner in the medical study process.
Industry leaders emphasize that rapid ethical approvals are not merely beneficial but essential for fostering innovation and ensuring timely access to life-saving technologies. As the landscape of medical investigations evolves, the focus on regulatory speed and efficiency continues to shape successful Medtech startups, underscoring the importance of strategic alliances with entities like bioaccess®.
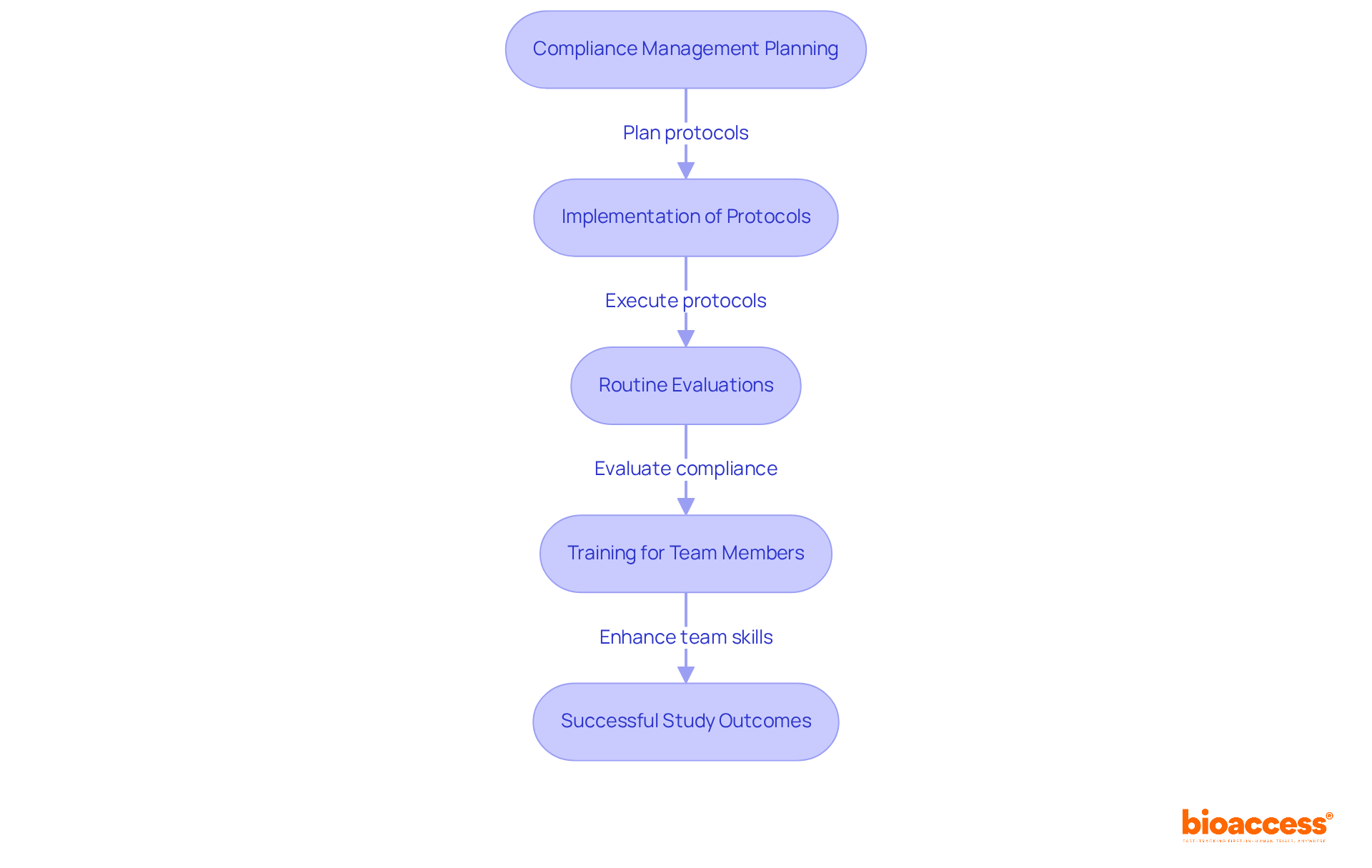
Effective compliance management is essential for ensuring that clinical studies meet the regulatory standards set by authorities such as the FDA and EMA. This necessity demands careful planning and implementation of protocols, alongside routine evaluations and comprehensive training for all team members involved in the process.
By adopting robust compliance management systems, Medtech companies can significantly mitigate the risks associated with non-compliance, which may lead to costly delays and reputational damage. Staying informed about evolving regulations is crucial for ensuring compliance and achieving successful study outcomes.
Implementing best practices in regulatory adherence not only safeguards participant safety but also enhances the integrity of the research, ultimately supporting the advancement of innovative medical solutions.
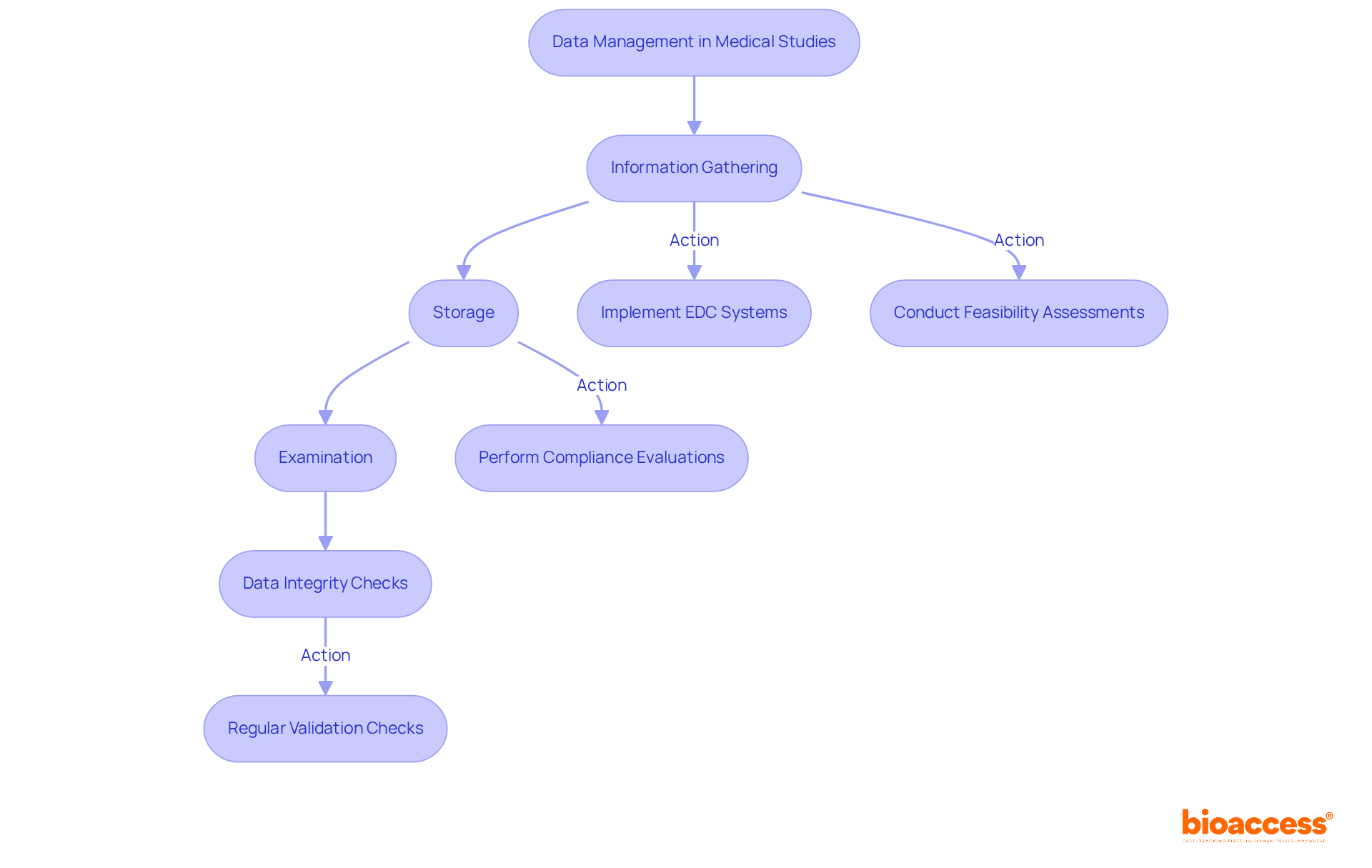
Information management in medical studies is essential for the organized gathering, storage, and examination of data, ensuring its integrity and precision. Implementing electronic data capture (EDC) systems significantly simplifies this process, reducing the risk of errors associated with manual entry.
Furthermore, bioaccess underscores the importance of comprehensive clinical trial software in managing services, which encompass:
Establishing clear data management protocols, including regular data validation checks and audits, is crucial for maintaining high data quality. This approach not only enhances the reliability of test outcomes but also supports regulatory compliance and facilitates informed decision-making throughout the testing process.
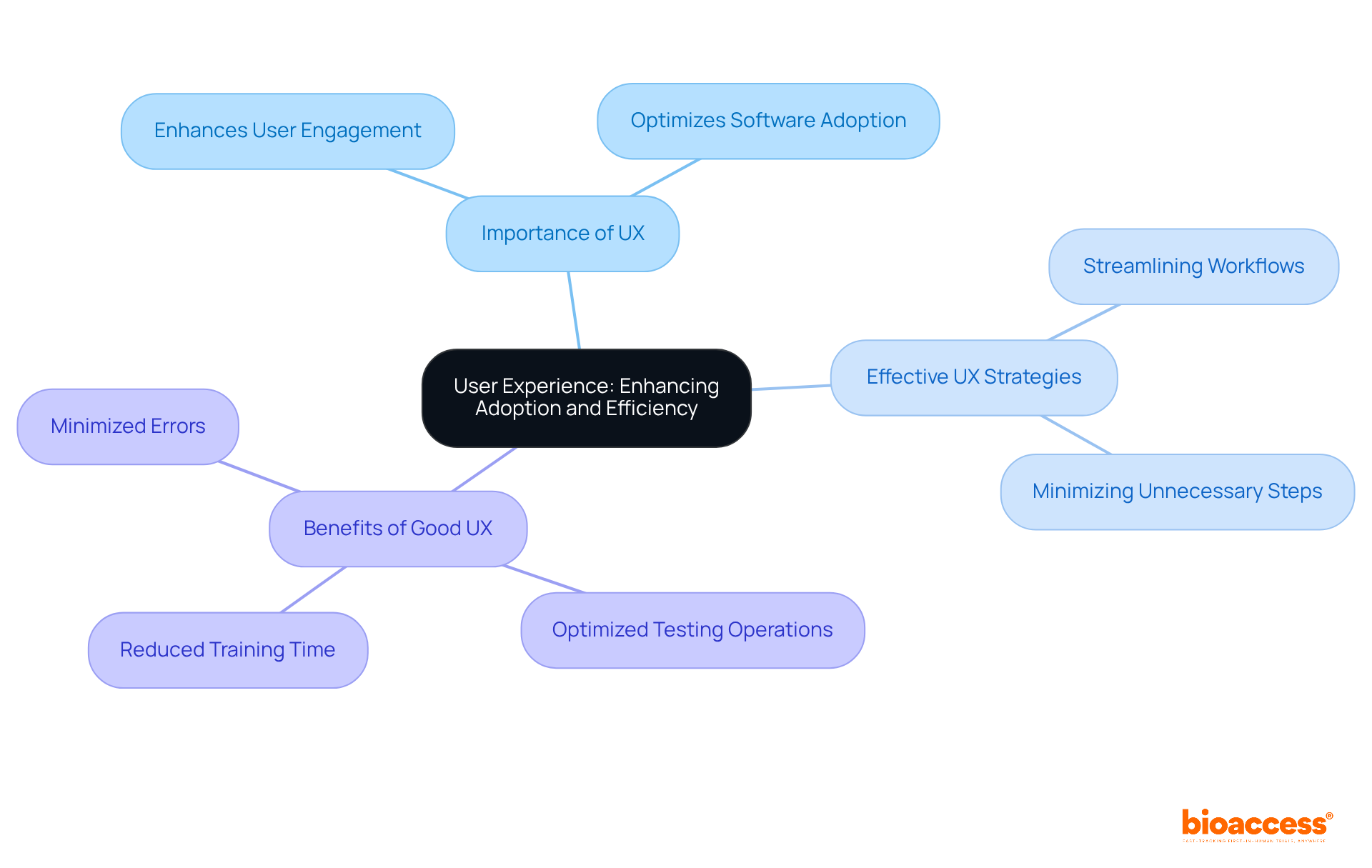
User experience (UX) is essential for the successful uptake of research software; intuitive interfaces and user-friendly designs significantly enhance user engagement. By prioritizing UX, Medtech companies can optimize testing operations, reduce training time, and minimize errors. Engaging end-users in the design process not only ensures that the software aligns with their needs and preferences but also fosters a sense of ownership and satisfaction.
Effective UX design strategies, such as:
can profoundly influence research efficiency. Ultimately, an emphasis on user experience aids in smoother adoption of research software and enhances the overall success of Medtech initiatives.
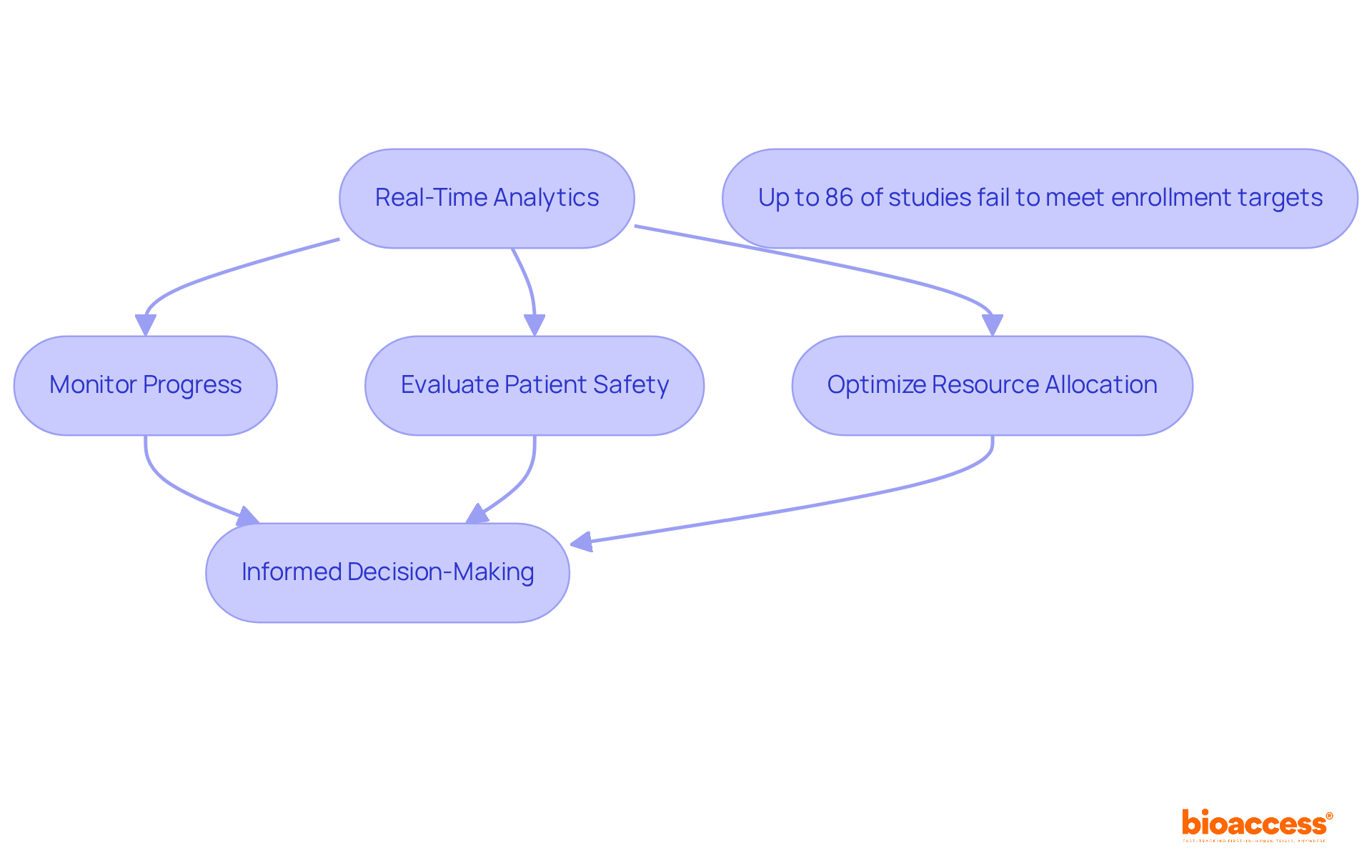
Real-time analytics empower clinical research teams to meticulously monitor progress and make informed decisions grounded in current data. By leveraging advanced analytics tools, researchers can discern trends, evaluate patient safety, and optimize resource allocation throughout the research process. The Clinical Research Management Expert underscores that the application of real-time information not only bolsters operational efficiency but also facilitates proactive risk management, enabling teams to tackle potential issues before they escalate. This approach is particularly vital, as research indicates that up to 86% of studies fail to meet their enrollment targets, underscoring the necessity for prompt and effective decision-making within the Medtech industry.
Integration features are essential for enhancing information flow across various clinical study systems, including electronic information capture (EIC), clinical trial software, and clinical study management systems (CSMS) along with statistical analysis software. Efficient interaction among these systems is crucial for reducing information silos, minimizing errors, and improving overall process efficiency.
Industry specialists advocate for platforms that provide robust integration capabilities, empowering teams to access and analyze data from multiple sources in real-time. This functionality not only streamlines workflows but also significantly enhances decision-making and operational agility, ultimately leading to more successful clinical trials through the use of clinical trial software.
Notably, studies indicate that individuals spend 60% to 80% of their time trying to locate information, underscoring the critical role of integration in alleviating this inefficiency. As Douglas Merrill aptly states, 'Big information isn’t about bits; it’s about talent,' highlighting the necessity for skilled professionals to effectively manage and interpret integrated information.
Furthermore, the challenges posed by disorganized information present substantial opportunities for analysis, reinforcing the need for strong integration capabilities in medical research. Additionally, 67% of analytics leaders identify organizational culture as a significant barrier to becoming analytics-focused, which can hinder efforts to improve information flow.
Thorough assistance and education are essential for empowering users to optimize the efficiency of research software. This includes:
Investing in user education significantly boosts software adoption, resulting in enhanced data quality and improved trial outcomes. Industry professionals underscore that fostering a culture of ongoing education within Medtech organizations equips teams to adeptly navigate the complexities of medical studies. Successful training programs not only concentrate on technical skills but also highlight the significance of comprehending user needs and the broader research process. By prioritizing user education, Medtech firms can drive innovation and ensure the success of their medical studies.
Scalability is a crucial aspect of clinical trial software, enabling it to meet the evolving demands of studies as they progress. Whether overseeing a small pilot study or a large multi-site experiment, clinical trial software effectively manages varying participant counts and data complexities. Industry specialists underscore the necessity for Medtech firms to select clinical trial software solutions that can seamlessly adapt to their testing requirements. This adaptability not only optimizes resource utilization but also enhances the overall efficiency of the clinical trial software used in the trial.
Notably, bioaccess® accelerates patient enrollment by 50% compared to Western sites, achieving significant cost savings of $25K per patient with FDA-ready data—no rework, no delays. As Khone, Lead Data Management and Clinical Data Science at Roche Diagnostics, states, 'Standardization helps our teams be more efficient,' which highlights the importance of clinical trial software as a scalable solution in streamlining processes.
Furthermore, the shift towards automation and interoperability in research management emphasizes the need for solutions that facilitate real-time data integration and optimize workflows, ultimately fostering successful outcomes across various study sizes. Additionally, considering that monitoring constitutes 25% to 30% of total healthcare study expenses, the financial benefits of adopting scalable clinical trial software solutions are substantial.
Cost management is pivotal for the success of clinical research operations, and utilizing clinical trial software directly influences the financial sustainability of research projects. By implementing effective budgeting strategies and leveraging clinical trial software, organizations can significantly optimize expenditures during tests. Regular budget reviews are essential; they enable organizations to identify inefficiencies and uncover potential cost savings.
For instance, the median expenditure for critical studies aiding drug approvals is estimated at US$48 million, while the median cost per medication approved with a single study is US$28 million. This data provides a broader context regarding study expenses. By concentrating on financial management, Medtech companies can enhance their operational efficiency, ensuring that research studies are not only feasible but also successful.
Furthermore, utilizing centralized budgeting tools grants real-time access during negotiations, facilitating smoother adjustments. Continuous budget monitoring is vital for the early detection of deviations and timely responses. As highlighted by industry specialists, Nitya Maddodi emphasizes that effective budget management is crucial for the success and sustainability of research facilities.
Customized clinical trial software solutions for budget management enable real-time financial analysis, ensuring resources are allocated efficiently and projects remain on course financially. Additionally, incorporating flexibility in budget strategies allows organizations to accommodate unforeseen events, further enhancing their financial resilience.
With bioaccess®'s expertise in comprehensive research study management services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting—organizations can navigate the complexities of studies while optimizing their financial strategies. This approach not only supports the success of individual experiments but also contributes to job creation, economic growth, and healthcare enhancement in local economies.
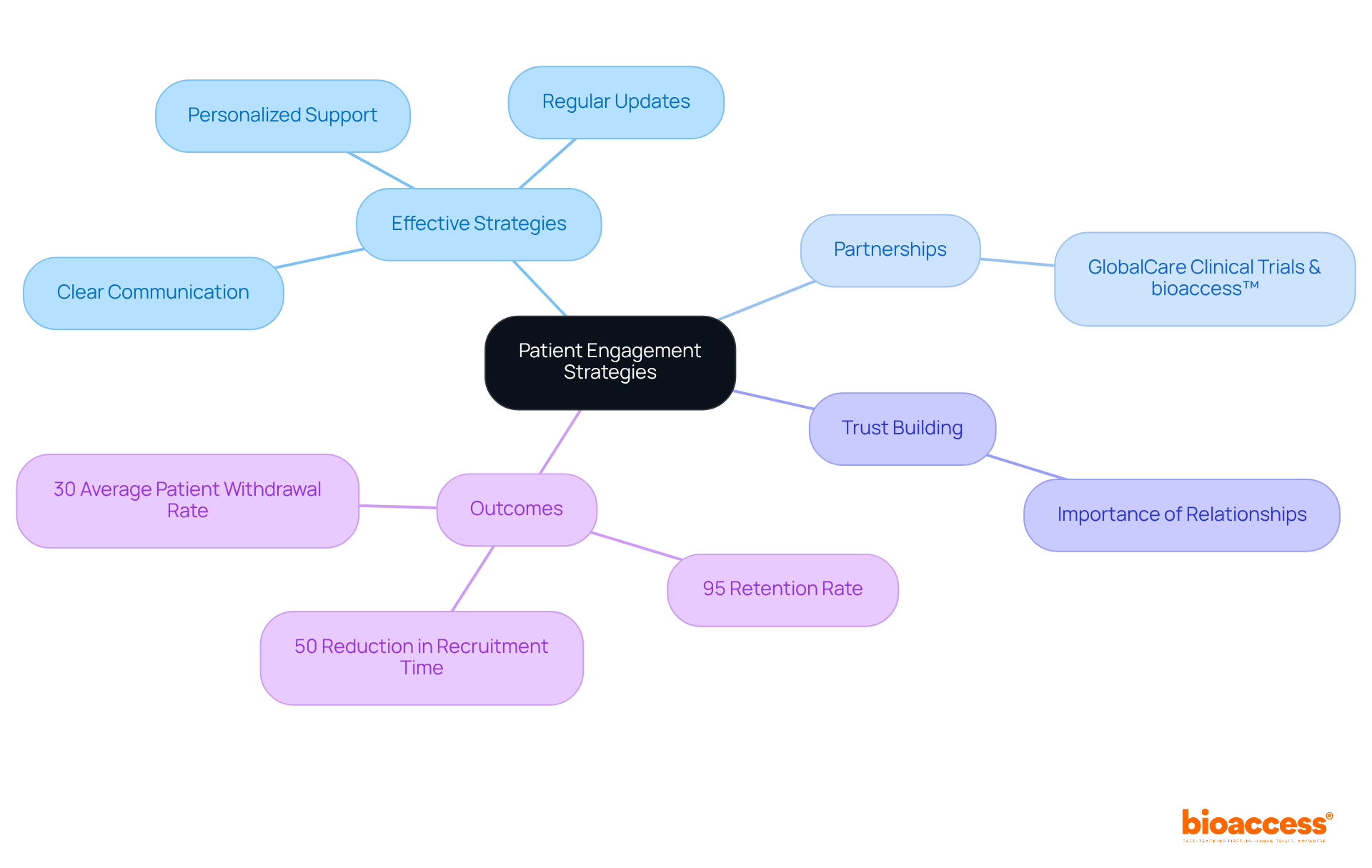
Improving patient involvement is essential for increasing retention rates and overall satisfaction in research studies. Effective strategies, such as clear communication, regular updates, and personalized support, significantly enrich the patient experience.
A recent partnership between GlobalCare Clinical Trials and bioaccess™ exemplifies the impact of these strategies, achieving over a 50% reduction in recruitment time and an impressive 95% retention rate in Colombia. This collaboration leverages bioaccess™'s significant presence in Colombia to assist global pharmaceutical clients with their patient needs at home.
Craig Lipset emphasizes that establishing trust is crucial before recruitment, underscoring the necessity of relationship-building with participants. Statistics reveal that, on average, 30% of patients withdraw from research trials, highlighting the importance of these engagement strategies.
By fostering strong connections with participants, Medtech companies can enhance retention and gather authentic feedback that surpasses insights from academic experts, guiding future initiatives. This patient-centered approach is vital for achieving meaningful outcomes and nurturing trust within the clinical research community.
The success of Medtech startups is fundamentally anchored in the effective utilization of clinical trial software, which acts as a cornerstone for navigating the intricate landscape of medical research. By underscoring essential features such as:
organizations can significantly streamline their operations and bolster their overall research outcomes. These elements not only facilitate a more efficient study process but also guarantee that innovative medical solutions are delivered to the market in a timely manner.
Throughout this discussion, various critical aspects have been examined, including:
This discourse also highlights the value of patient engagement strategies in enhancing retention and satisfaction, ultimately cultivating trust in the clinical research process.
In summary, leveraging advanced clinical trial software is indispensable for Medtech companies aiming for success in an increasingly competitive landscape. By prioritizing these key features and implementing best practices, organizations can not only optimize their research processes but also contribute to the advancement of healthcare solutions. As the industry continues to evolve, embracing these innovations will be crucial for meeting the demands of regulatory bodies and the patients they serve.
What services does bioaccess® provide for Medtech startups?
bioaccess® offers expedited medical studies services tailored specifically for Medtech startups, achieving ethical approvals in 4-6 weeks by leveraging regulatory speed in Latin America, diverse patient populations in the Balkans, and efficient pathways in Australia.
Why is the quick turnaround of ethical approvals important for Medtech startups?
A swift turnaround is crucial for startups to introduce innovative medical devices to the market promptly, allowing them to outpace competitors and address pressing healthcare demands.
How long has bioaccess® been in operation, and what is its expertise?
bioaccess® has over 15 years of experience and possesses a profound understanding of the regulatory environment and operational challenges faced by Medtech firms.
What role does compliance management play in clinical studies?
Effective compliance management is essential for ensuring that clinical studies meet regulatory standards set by authorities like the FDA and EMA, involving careful planning, implementation of protocols, and routine evaluations.
What are the risks of non-compliance in clinical studies?
Non-compliance can lead to costly delays and reputational damage for Medtech companies, making it crucial to adopt robust compliance management systems.
How can Medtech companies enhance data management in clinical studies?
By implementing electronic data capture (EDC) systems and comprehensive clinical trial software, Medtech companies can ensure organized data gathering, storage, and examination, reducing the risk of errors.
What key services are included in bioaccess®'s clinical trial management?
Key services include feasibility assessments, site selection, compliance evaluations, study setup, and project management.
Why is maintaining high data quality important in medical studies?
High data quality enhances the reliability of test outcomes, supports regulatory compliance, and facilitates informed decision-making throughout the testing process.