


The article titled "10 Key Insights for Effective Syringe Manufacturing" presents essential strategies and practices that significantly enhance the syringe manufacturing process. It clearly states the relevance of these insights to clinical research, emphasizing that:
These are critical for improving efficiency, safety, and overall quality in syringe production. This approach directly addresses the evolving demands of the healthcare sector, highlighting the importance of adapting to new challenges.
In the context of the Medtech landscape, these insights underscore the role of bioaccess in tackling key challenges faced by manufacturers. By leveraging data and established methodologies, companies can streamline their operations and better meet the needs of healthcare providers. The incorporation of statistics and case studies throughout the article serves to engage readers, prompting them to reflect on their own challenges in clinical research and manufacturing.
In conclusion, the article reinforces the importance of collaboration among stakeholders in the syringe manufacturing process. The next steps involve embracing these strategies to foster innovation and improve outcomes in the healthcare industry, ultimately driving progress and enhancing patient safety.
The intricate world of syringe manufacturing is undergoing a transformative shift, driven by the need for enhanced safety, efficiency, and innovation. As healthcare demands evolve, manufacturers face the challenge of balancing cost-effectiveness with stringent quality and regulatory standards. This article delves into ten key insights that illuminate best practices for effective syringe production and explores the impact of clinical research, advanced materials, and user-centric design on the future of this critical industry. How can manufacturers navigate these complexities to ensure their products meet the highest standards while remaining accessible and affordable?
bioaccess® leverages its extensive clinical research experience to optimize syringe manufacturing processes. By integrating clinical insights into development, the organization tailors injections to meet the precise needs of healthcare providers and patients. This strategic approach not only expedites the production timeline of syringe manufacturing but also significantly elevates the quality of the syringes, ensuring their suitability for clinical applications. As industry leaders emphasize, embedding clinical research into product development is vital for fostering innovation and ensuring reliability in syringe manufacturing.
To achieve cost efficiency in syringe manufacturing, companies must embrace lean principles that emphasize waste reduction and productivity enhancement. Lean methodologies, such as value stream mapping and continuous improvement, have proven effective in significantly lowering production costs while elevating quality standards. Case studies reveal that organizations implementing lean strategies can enhance their Overall Equipment Effectiveness (OEE), with many production lines operating at a mere 60% productivity, highlighting substantial opportunities for improvement.
Furthermore, incorporating automation into production lines amplifies these advantages by reducing labor costs and boosting output. Automation technologies, including robotic assembly and automated inspection systems, not only streamline processes but also decrease human error, resulting in higher-quality products. As Anders Wilhelmsson states, "Lean Manufacturing is an important focus. This methodology can help streamline and enhance production processes to provide greater benefits for customers, while saving time and money by removing waste."
Additionally, integrating Lean 4.0 technologies can improve efficiency and minimize waste in healthcare, presenting a superior alternative to conventional waste management methods. By adopting these strategies, syringe manufacturing producers can effectively navigate the complexities of the MedTech supply chain while maintaining competitive pricing and high standards.
Achieving accuracy in syringe manufacturing is paramount, necessitating stringent quality control measures and advanced production technologies. The implementation of computer numerical control (CNC) machines is pivotal; these machines facilitate precise cutting and shaping of syringe components, ensuring adherence to the tight tolerances essential for patient safety. Frequent calibration of equipment, combined with strict adherence to production protocols, guarantees that each syringe meets the necessary specifications. This meticulous approach not only enhances the reliability of syringes but also significantly reduces the risks associated with inaccuracies in medical devices.
For instance, CNC Swiss machines play a crucial role in creating complex components with minimal variation, which is essential for upholding high standards in medical production. Experts in the field emphasize that the precision offered by CNC machining is not merely beneficial but a necessity in the medical industry, where even minor discrepancies can have serious implications for patient care. By utilizing advanced production technologies, syringe manufacturing can ensure that the products are both safe and effective, ultimately contributing to improved healthcare outcomes.
Maintaining sterility in the process of syringe manufacturing is paramount, necessitating the implementation of stringent cleaning and sterilization protocols. Manufacturers must utilize validated sterilization methods, such as ethylene oxide or gamma radiation, to guarantee that all injection devices remain free from contaminants. Furthermore, sustaining a controlled environment during production and packaging is crucial to avert any potential breaches in sterility. This rigorous approach not only safeguards patient health but also upholds the integrity of clinical research.
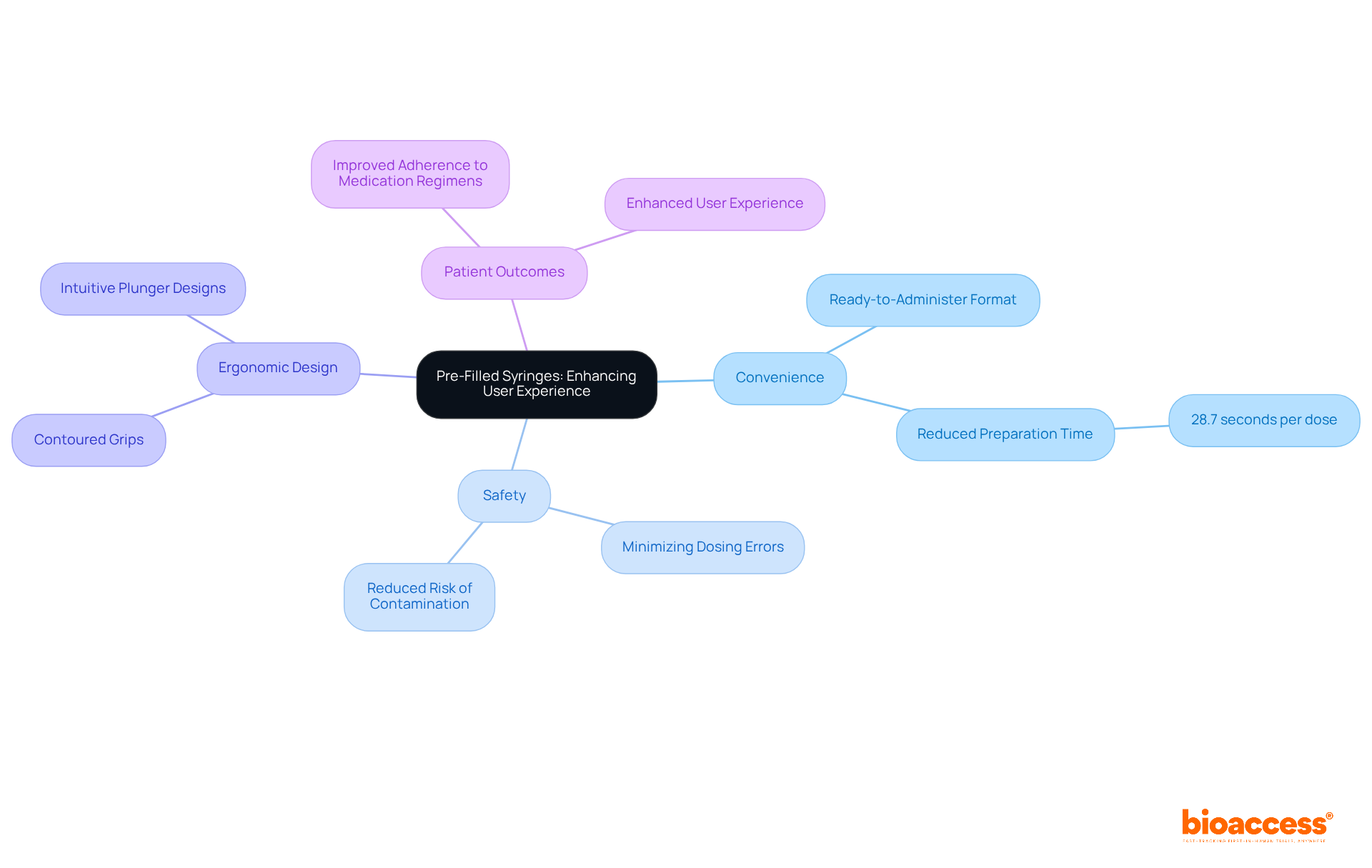
Pre-filled injectors represent a significant advancement in drug administration, offering a convenient solution for healthcare professionals and patients alike. By eliminating the need for manual filling, these devices not only minimize the risk of dosing errors and contamination but also enhance overall safety.
Manufacturers are increasingly focusing on ergonomic designs that facilitate ease of handling and injection, catering to patients across all age groups. Case studies have demonstrated that ergonomic features, such as contoured grips and intuitive plunger designs, greatly enhance user experience, making injections less intimidating and more efficient.
Usability experts assert that thoughtful design can lead to improved adherence to medication regimens, ultimately enhancing patient outcomes. As the demand for pre-filled injectors rises, prioritizing these ergonomic enhancements will be essential in addressing the evolving needs of the healthcare landscape.
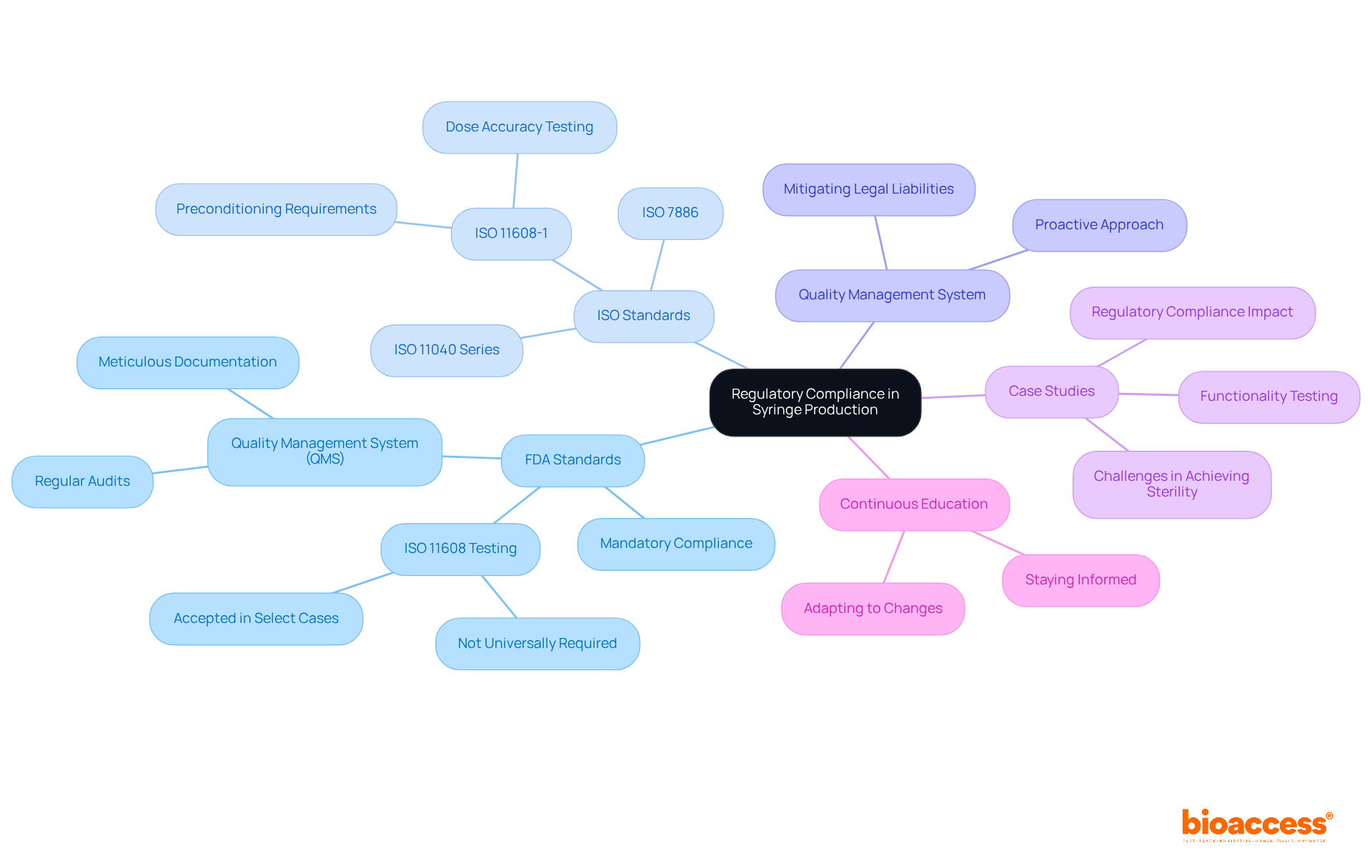
Navigating the complex regulatory landscape is essential for syringe manufacturing companies aiming for market entry. In syringe manufacturing, compliance with standards established by the FDA and ISO is not just recommended; it is mandatory. Experts such as Ana Criado, Director of Regulatory Affairs and a professor in biomedical engineering, stress that following these standards can greatly improve credibility and safety.
Although the FDA has not systematically required ISO 11608 testing for prefilled containers (PFS) or prefilled containers with needle safety devices (PFS/NSD) in NDA or BLA approvals over the last five years, syringe manufacturing companies should implement a robust quality management system (QMS) that includes regular audits and meticulous documentation to demonstrate compliance with all applicable regulations. This proactive approach not only ensures product safety and efficacy in syringe manufacturing but also mitigates legal liabilities and enhances speed to market.
For instance, case studies highlight that strict compliance with guidelines focusing on sterility and material compatibility is crucial for preventing contamination and ensuring accurate dosing. Furthermore, the FDA has explicitly acknowledged ISO 11608-1 testing as a valid method for demonstrating device performance in select cases, underscoring the importance of these standards in maintaining high-quality standards for syringe manufacturing processes.
As the industry evolves, staying informed about regulatory changes and integrating advanced technologies into production will be vital for maintaining competitiveness and ensuring patient safety. Katherine Ruiz, another expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, also reinforces the need for continuous education and adaptation to these evolving standards.
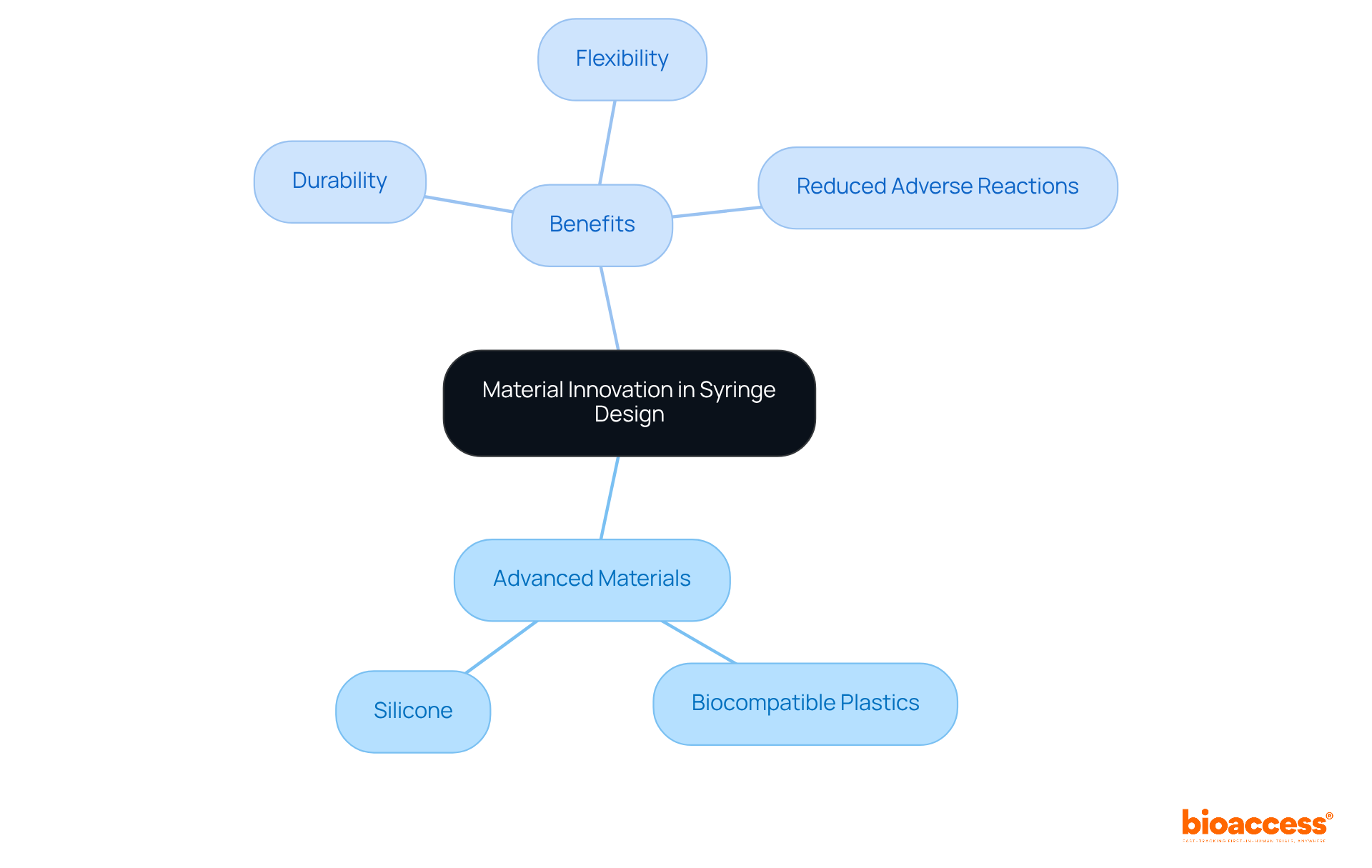
The incorporation of advanced materials, including biocompatible plastics and silicone, plays a pivotal role in significantly enhancing the performance of syringes. These innovative materials not only bolster the durability and flexibility of syringes but also mitigate the risk of adverse reactions in patients.
It is imperative for manufacturers in syringe manufacturing to invest in research and development to explore new material options that could yield competitive advantages in the market. This strategic focus on material innovation is essential for addressing the evolving challenges within the Medtech landscape.
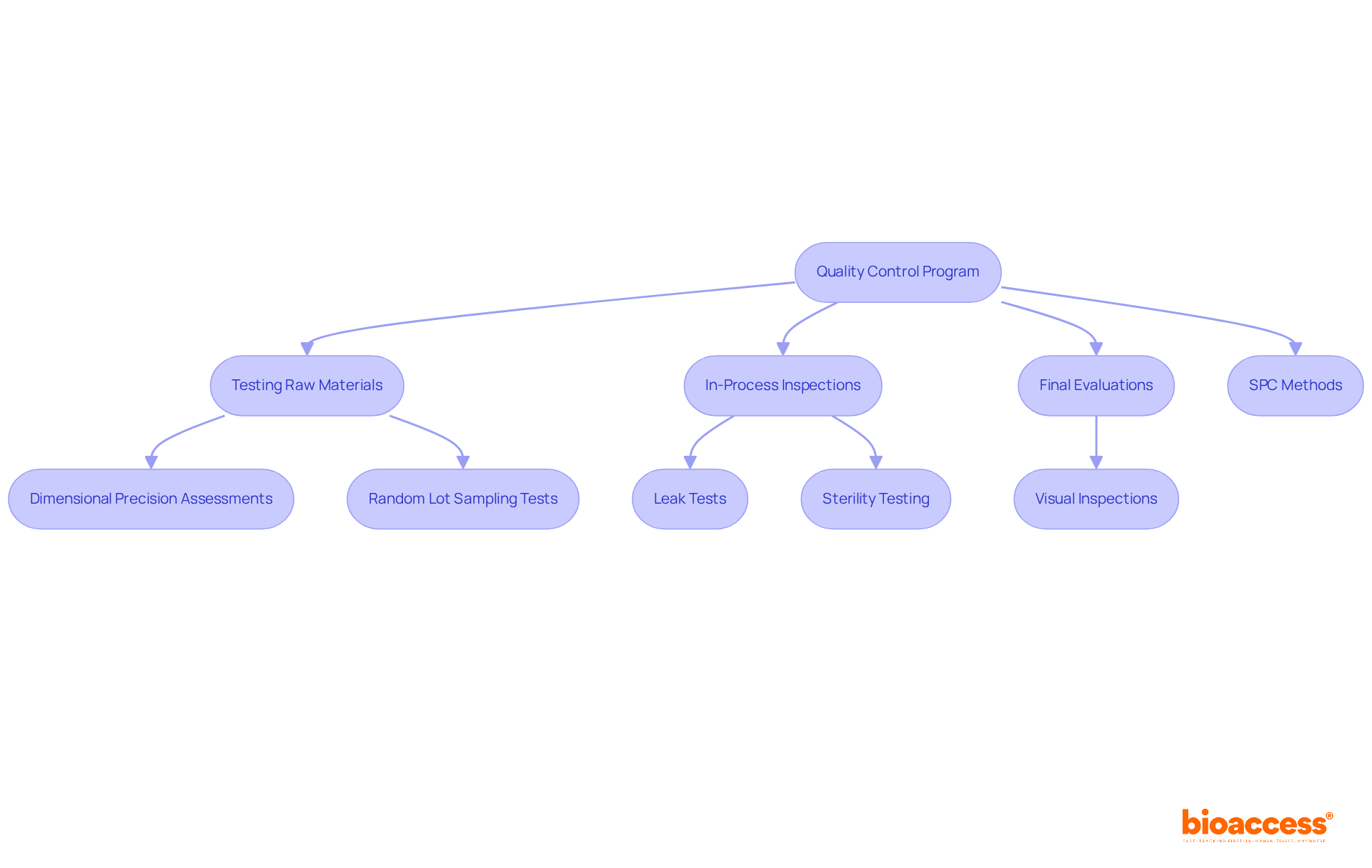
Implementing a comprehensive control program is essential for syringe manufacturing. This program encompasses regular testing of raw materials, in-process inspections, and final evaluations of outcomes. Employing statistical process control (SPC) methods assists in detecting variations in the manufacturing process, enabling prompt corrective measures to uphold item standards and safety.
Key elements of this program include:
The implementation of SPC not only enhances accountability through traceability systems but also fosters a culture of continuous improvement, essential for adapting to evolving industry standards in syringe manufacturing. Frequent assessments of standards and training for inspectors are vital practices that uphold high control benchmarks. As emphasized by assurance leaders, maintaining high safety and efficacy standards in medical devices is a collective responsibility that necessitates collaboration and commitment across all levels of production. Moreover, guaranteeing packaging integrity inspections is essential for preserving sterility and item standards during transit.
The process of syringe manufacturing encompasses several crucial steps:
Each of these stages demands meticulous planning and execution to guarantee that the final product adheres to all quality standards. Manufacturers must adopt a systematic approach to process management, employing tools such as value stream mapping to identify and eliminate inefficiencies effectively. This structured methodology not only enhances productivity but also reinforces the commitment to excellence in the Medtech landscape.
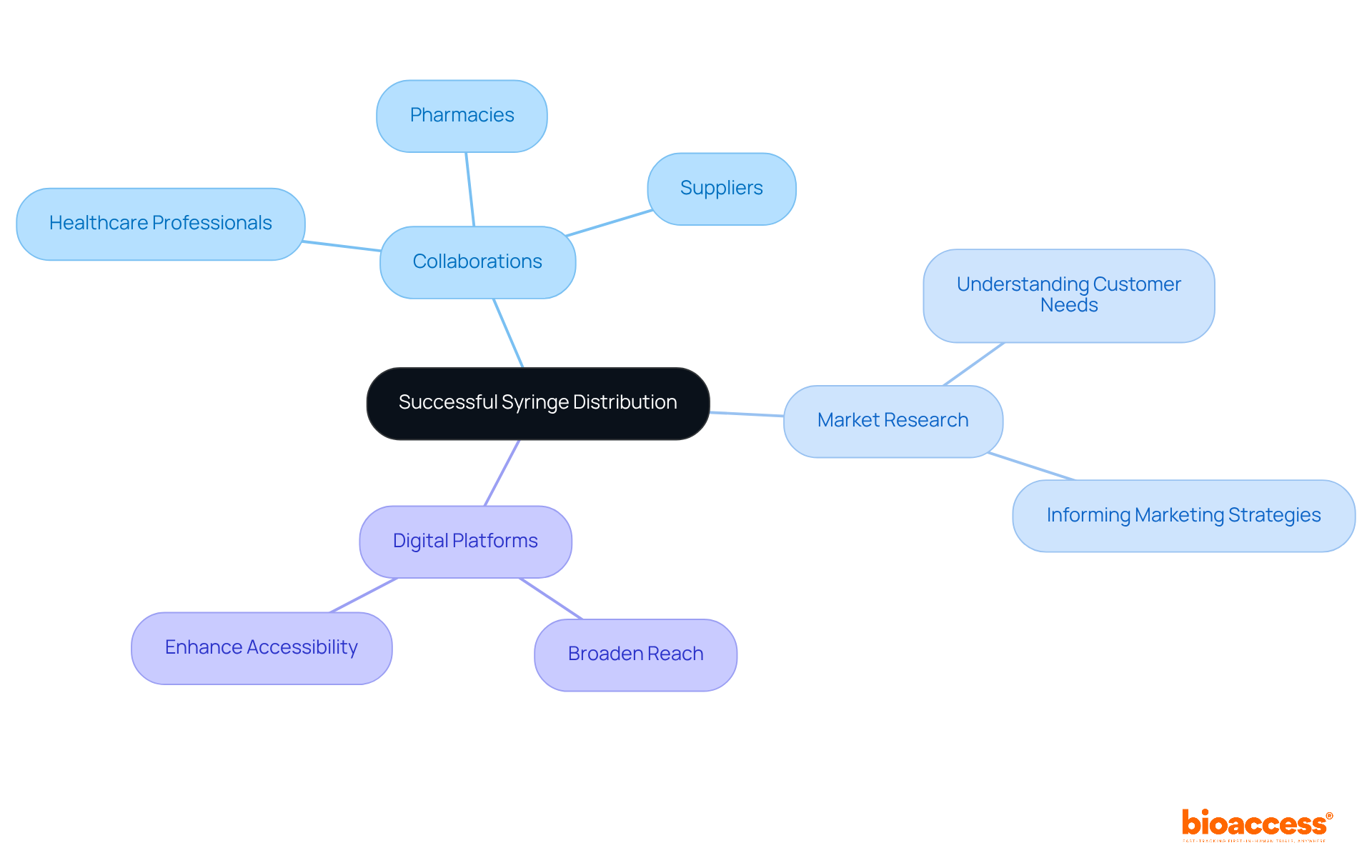
To ensure effective market entry for injection devices, producers must develop a robust distribution plan that emphasizes collaborations with healthcare professionals, pharmacies, and suppliers. Engaging in thorough market research is essential to grasp the needs and preferences of target customers, significantly informing marketing strategies and boosting product visibility. Furthermore, harnessing digital platforms for marketing and sales not only broadens reach but also enhances accessibility, aligning with the growing trend of digital transformation in healthcare.
For instance, companies like Unilife Corporation have effectively utilized strategic collaborations to enhance their market presence, demonstrating the value of partnerships in navigating complex distribution channels. As noted by industry experts, a well-executed digital marketing strategy can amplify the impact of traditional distribution methods, ensuring that syringe manufacturing efficiently and effectively reaches the intended users.
The insights shared on effective syringe manufacturing underscore the critical need for integrating clinical research, cost efficiency, precision, and regulatory compliance to elevate the production process. By concentrating on these pivotal areas, manufacturers can enhance product quality and ensure that syringes adapt to the evolving demands of healthcare providers and patients alike. This strategic approach lays the groundwork for innovation and reliability within the medical device industry.
Throughout the article, several strategies have been delineated, including:
Each of these components is essential in guaranteeing that syringes are safe, effective, and user-friendly. Furthermore, the significance of material innovation and robust distribution strategies accentuates the necessity for manufacturers to remain competitive in a dynamic market.
Ultimately, the future of syringe manufacturing is contingent upon a steadfast commitment to excellence through continuous improvement and adaptation. By embracing these insights and best practices, stakeholders can adeptly navigate the complexities of the MedTech landscape while prioritizing patient safety and satisfaction. Engaging in proactive measures today will pave the way for advancements in syringe production, ensuring that the industry is well-equipped to meet the challenges of tomorrow's healthcare environment.
How does bioaccess® enhance syringe manufacturing?
bioaccess® leverages its clinical research expertise to optimize syringe manufacturing by integrating clinical insights into the development process, tailoring injections to meet the specific needs of healthcare providers and patients. This approach expedites production timelines and elevates syringe quality for clinical applications.
What strategies can be employed to achieve cost efficiency in syringe manufacturing?
Companies can achieve cost efficiency by embracing lean principles that focus on waste reduction and productivity enhancement. Lean methodologies, such as value stream mapping and continuous improvement, are effective in lowering production costs while improving quality. Additionally, incorporating automation technologies, like robotic assembly and automated inspection systems, can reduce labor costs and increase output.
What role does automation play in syringe manufacturing?
Automation plays a significant role in syringe manufacturing by streamlining processes, reducing labor costs, and minimizing human error. This leads to higher-quality products and improved productivity on production lines.
Why is accuracy important in syringe production?
Accuracy is crucial in syringe production to ensure patient safety and product reliability. Stringent quality control measures and advanced production technologies, such as computer numerical control (CNC) machines, are necessary to maintain tight tolerances and minimize variations in syringe components.
How do CNC machines contribute to syringe manufacturing?
CNC machines facilitate precise cutting and shaping of syringe components, ensuring adherence to strict specifications. This precision is essential in the medical industry, as even minor discrepancies can have serious implications for patient care.
What are Lean 4.0 technologies and how do they benefit syringe manufacturing?
Lean 4.0 technologies enhance efficiency and minimize waste in healthcare by integrating advanced digital solutions into traditional lean manufacturing practices. This approach offers a superior alternative to conventional waste management methods, helping syringe manufacturers navigate the complexities of the MedTech supply chain while maintaining competitive pricing and high quality standards.