


The landscape of women's health research is evolving rapidly, shaped by historical underrepresentation and persistent disparities in clinical trials. Recent insights from the Phase 2 Women's Health Report underscore the urgent need for inclusive practices and innovative methodologies.
What concrete steps can we take to ensure that women's unique health challenges are met with tailored solutions? How can stakeholders collaborate effectively to bridge the gaps in understanding drug efficacy and safety for women?
This article delves into ten key insights that highlight the critical importance of addressing these issues and the potential for transformative change in women's health research.
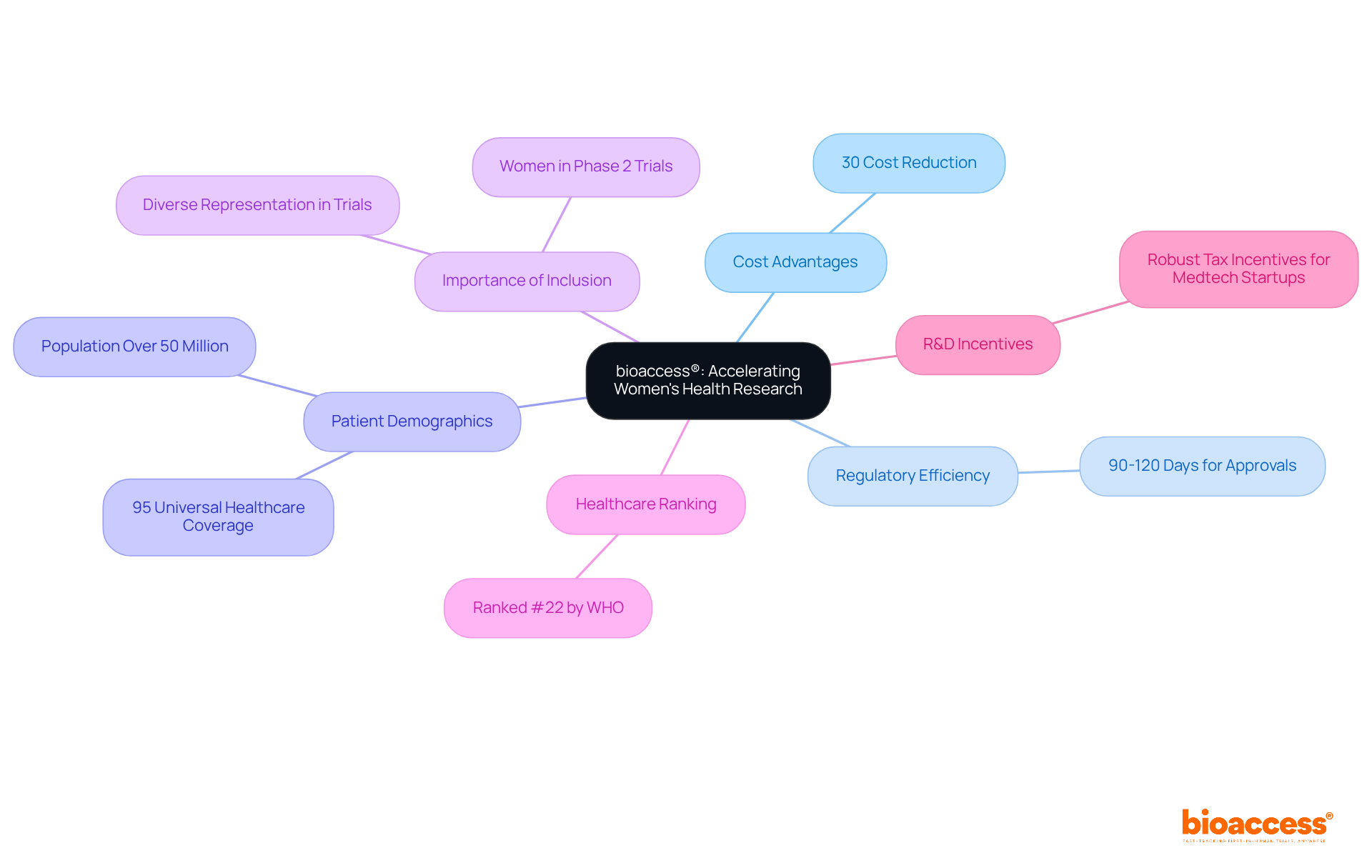
bioaccess® leverages its extensive expertise in early-stage research to drive advancements in phase 2 women's health. By capitalizing on Colombia's competitive advantages, such as significant cost reductions exceeding 30% compared to North America and Western Europe, bioaccess® accelerates the trial process. The country's swift regulatory procedures allow for ethical approvals in just 90-120 days, a crucial factor in addressing the unique medical challenges faced by women. This expedited timeline facilitates the introduction of new therapies and medical devices to the market much faster than traditional methods allow.
With a population exceeding 50 million and 95% coverage under universal healthcare, Colombia presents a diverse patient demographic that enhances recruitment efforts. The rapid approval process not only improves access to essential health solutions but also underscores the pressing need for diverse representation in phase 2 women's health research studies. Dr. Beth Garner, Chief Scientific Officer of Ferring Pharmaceuticals US, emphasizes that including women in phase 2 women's health trials is vital to ensure the effectiveness of the products developed for the populations they serve.
Moreover, Colombia's healthcare system ranks #22 by the World Health Organization, and hospitals must undergo a rigorous ICH/GCP certification process before conducting studies with pharmaceutical drugs. Additionally, Colombia's robust R&D tax incentives support Medtech startups in their innovation pursuits, positioning bioaccess® as a leading CRO for expedited research services in Latin America.
Historically, females have faced significant underrepresentation in research studies, often excluded due to concerns about hormonal fluctuations and potential pregnancy risks. This exclusion has resulted in critical gaps in understanding how various treatments impact females, leading to a lack of tailored therapies. For example, prior to 1993, females were seldom included in clinical studies, a trend that has had enduring consequences on drug efficacy and safety for female patients.
The thalidomide tragedy of the 1960s, which resulted in over 10,000 children suffering from severe birth defects, prompted the FDA to implement stringent guidelines that inadvertently further marginalized females in research. Consequently, many healthcare practices for females are based on studies that are over 17 years old, perpetuating outdated treatment protocols. The National Institutes of Health has indicated that it can take up to 17 years for new evidence to be integrated into standard medical practice, underscoring the long-lasting effects of historical exclusion.
Moreover, in 2020, females represented less than 40% of participants in heart disease and stroke studies, highlighting the ongoing issue of underrepresentation in critical areas of research. Addressing this historical oversight is essential for enhancing health outcomes for females today, especially in phase 2 women's health, as evidenced by the persistent advocacy for greater inclusion of females in research studies. The FDA's January 2022 guidance encourages the participation of individuals, particularly those of reproductive capability, in early-phase trials focused on phase 2 women's health, aiming to rectify past injustices and improve the safety and efficacy of treatments for females.
Danielle Mitchell, CEO of Black Women in Clinical Research, stresses the importance of including diverse populations in clinical trials, asserting that this inclusion is vital for both social justice and scientific advancement.

Despite advancements in clinical research, significant gaps persist in understanding how drugs and medical devices function in females. Research shows that females often experience different side effects and drug interactions compared to males. Alarmingly, many studies still fail to adequately represent female participants. For example, a recent analysis revealed that less than 30% of participants in early-phase trials are female, resulting in a critical lack of data on how treatments specifically affect them. This gap highlights the urgent need for more inclusive research practices.
At bioaccess®, we recognize this challenge and are committed to addressing it. We offer extensive clinical trial management services tailored specifically for phase 2 women's health in Latin America. Our expertise spans:
By employing customized methodologies, we effectively bridge these gaps, providing essential data to enhance understanding of treatment efficacy and safety in phase 2 women's health for female patients.
Collaboration is key in overcoming these challenges. By working together, we can ensure that clinical research is more representative and effective for all patients. Let's take the next step towards inclusive research practices that prioritize female health.

The report on phase 2 women's health provides critical insights into the ongoing disparities impacting females, particularly in cardiovascular and reproductive wellness. Notably, females are 1.5 to 2 times more likely to experience adverse drug reactions (ADRs) compared to males, with these reactions leading to approximately 250,000 hospital admissions annually in Australia alone. This statistic underscores the significant role ADRs play, representing roughly 10% of all unexpected hospital admissions, which further highlights their broader influence on female well-being.
This disparity is exacerbated by the reality that females often receive less personalized care, resulting in poorer health outcomes. For instance:
These figures emphasize the urgent need for more targeted investigation in these areas. Case studies, such as Walgreens' commitment to including females in trial studies, illustrate the importance of integrating gender factors into research protocols. Walgreens has engaged with over 4 million patients for potential enrollment, with more than 60% being female, showcasing a significant effort to enhance female representation in medical studies.
These findings highlight the necessity of a gender-aware approach in medical research and healthcare delivery, particularly in phase 2 women's health, ensuring that the unique needs of females are adequately addressed. The call for collaboration and further investigation is clear, as addressing these disparities is essential for improving health outcomes for women.

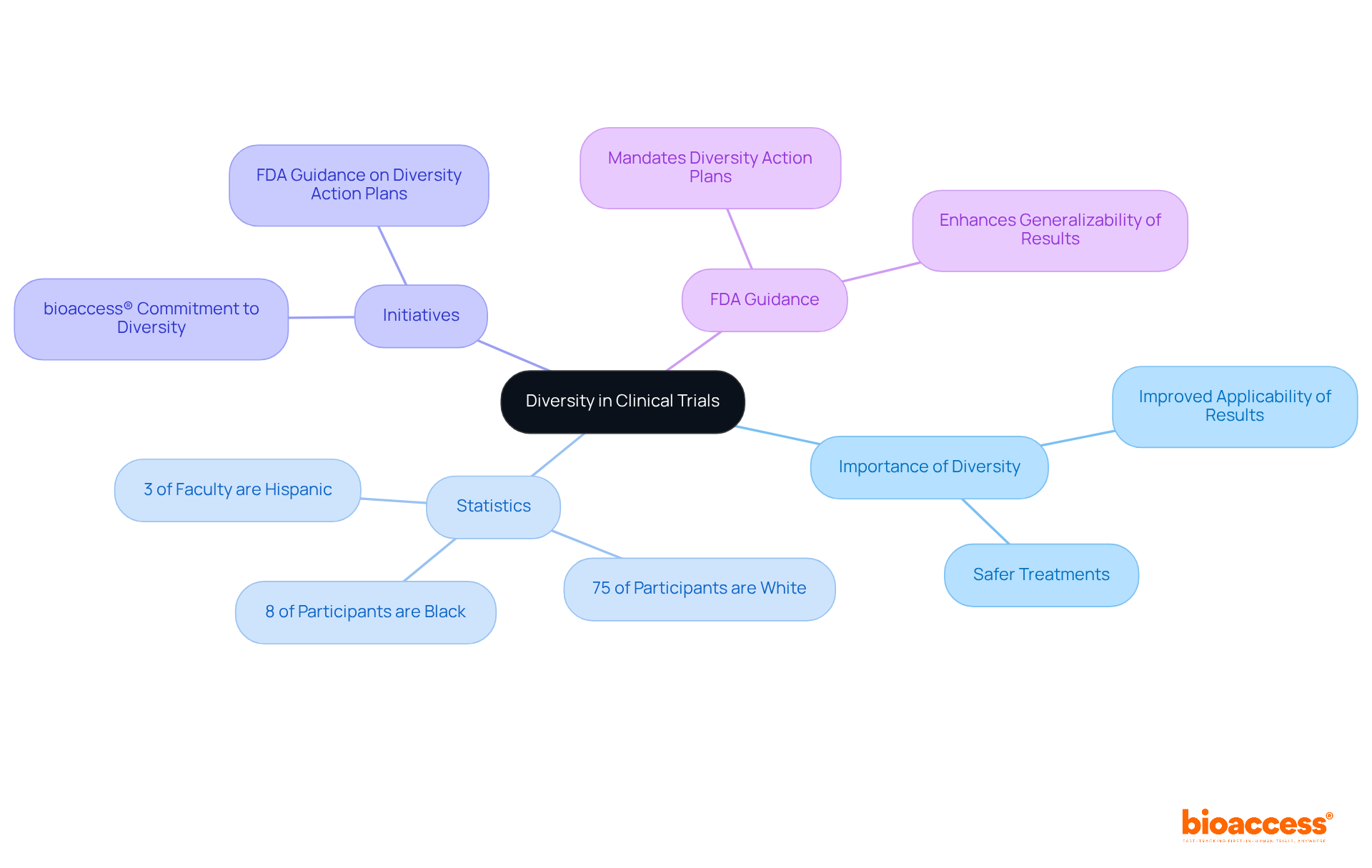
Enhancing variety in clinical trials is crucial for advancing findings related to phase 2 women's health. Diverse participant pools not only improve the applicability of results across various demographics but also lead to more effective and safer treatments. For instance, studies reveal that involving individuals from diverse ethnic backgrounds yields outcomes that more accurately reflect the general population, ultimately enhancing well-being results. In 2020, only 8% of participants in new drug trials were Black, underscoring the urgent need for more inclusive practices.
Initiatives like bioaccess®'s commitment to promoting diversity in research exemplify the essential role of inclusivity in advancing phase 2 women's health. Furthermore, the upcoming FDA guidance on Diversity Action Plans aims to bolster participant diversity in clinical trials, reinforcing the significance of these efforts. By prioritizing inclusivity, these initiatives strive to address disparities in wellness and ensure that medical interventions are relevant and effective for everyone.
Advancements in research techniques are vital for enhancing women's wellness studies. Adaptive trial designs, which allow for modifications based on interim results, significantly boost the efficiency and relevance of clinical trials. For instance, seamless designs in phase 2 women's health have been effectively utilized in studies focused on conditions like breast cancer and reproductive health, enabling real-time adjustments that improve patient outcomes.
Furthermore, incorporating patient-reported outcomes and qualitative methodologies deepens the understanding of female-related issues, ensuring that studies accurately reflect the real-world experiences of female patients. By embracing these innovative approaches, researchers can more effectively address the unique needs of women in clinical trials, ultimately leading to more impactful medical interventions.

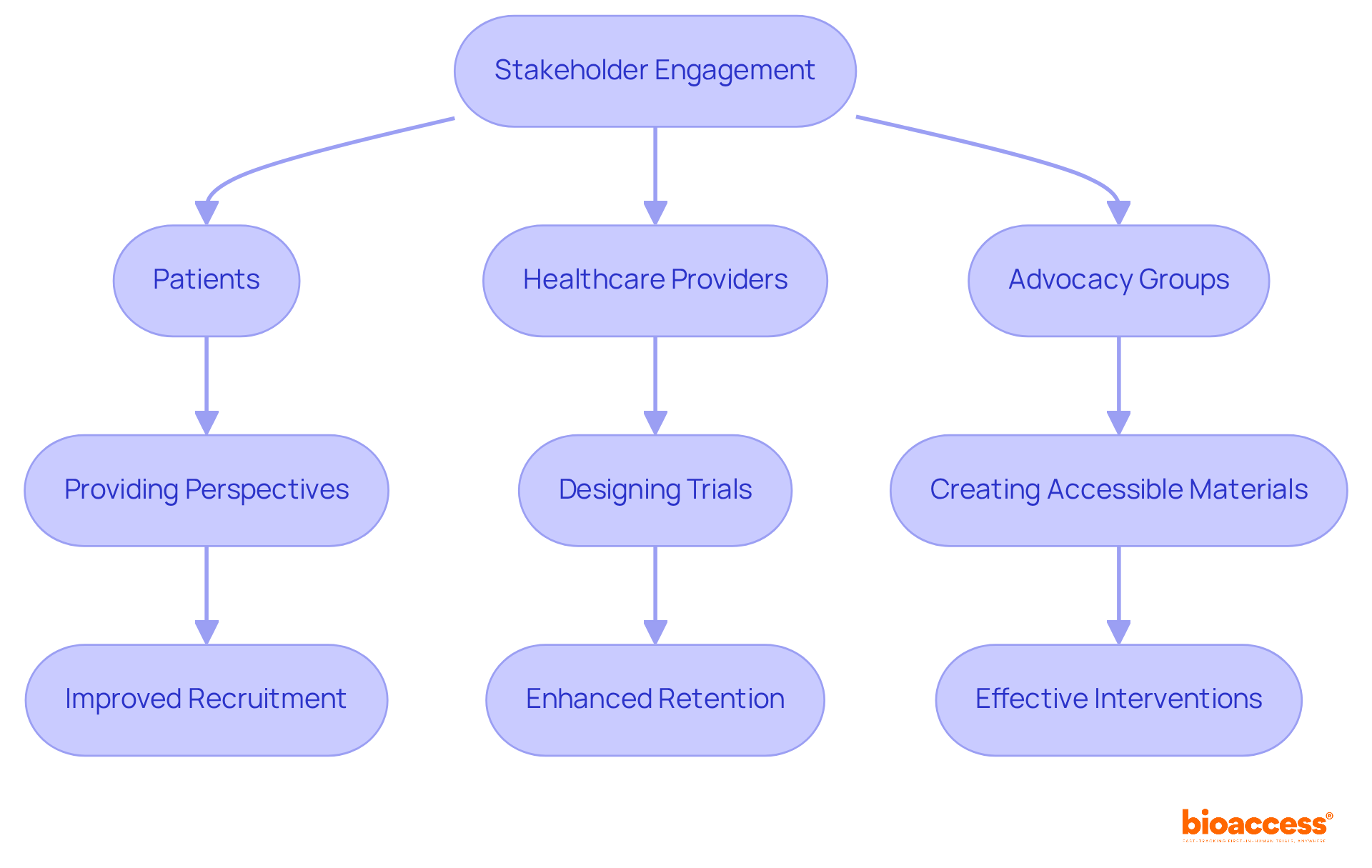
Effective stakeholder engagement is crucial for advancing health research in phase 2 women's health. By integrating the perspectives of patients, healthcare providers, and advocacy groups, organizations can ensure that studies are not only relevant but also tailored to meet the actual needs of women. Collaborative efforts have shown a significant impact on participant recruitment and retention in research trials. For instance, a systematic review revealed that only 5% of randomized clinical trials included patient or family representatives in their design, highlighting a substantial opportunity for improvement in stakeholder engagement.
Successful initiatives, such as those involving community representatives in trial design, demonstrate that including women's voices leads to more effective interventions and policies. In one notable case, community members contributed to creating accessible trial materials, which improved participant engagement and overall study design. Moreover, studies suggest that patient engagement can enhance the likelihood of enrollment by an odds ratio of 1.16 (95% CI 1.01 to 1.34), underscoring the significance of incorporating patient viewpoints throughout the investigation process. As Joanna C Crocker aptly stated, "Scientists don’t know what they’re missing - the influence of patient participation in studies."
By prioritizing stakeholder involvement, the future of phase 2 women's health studies can be shaped to more accurately represent and address the needs of the community it serves. This approach not only fosters trust but also paves the way for more impactful health research outcomes.
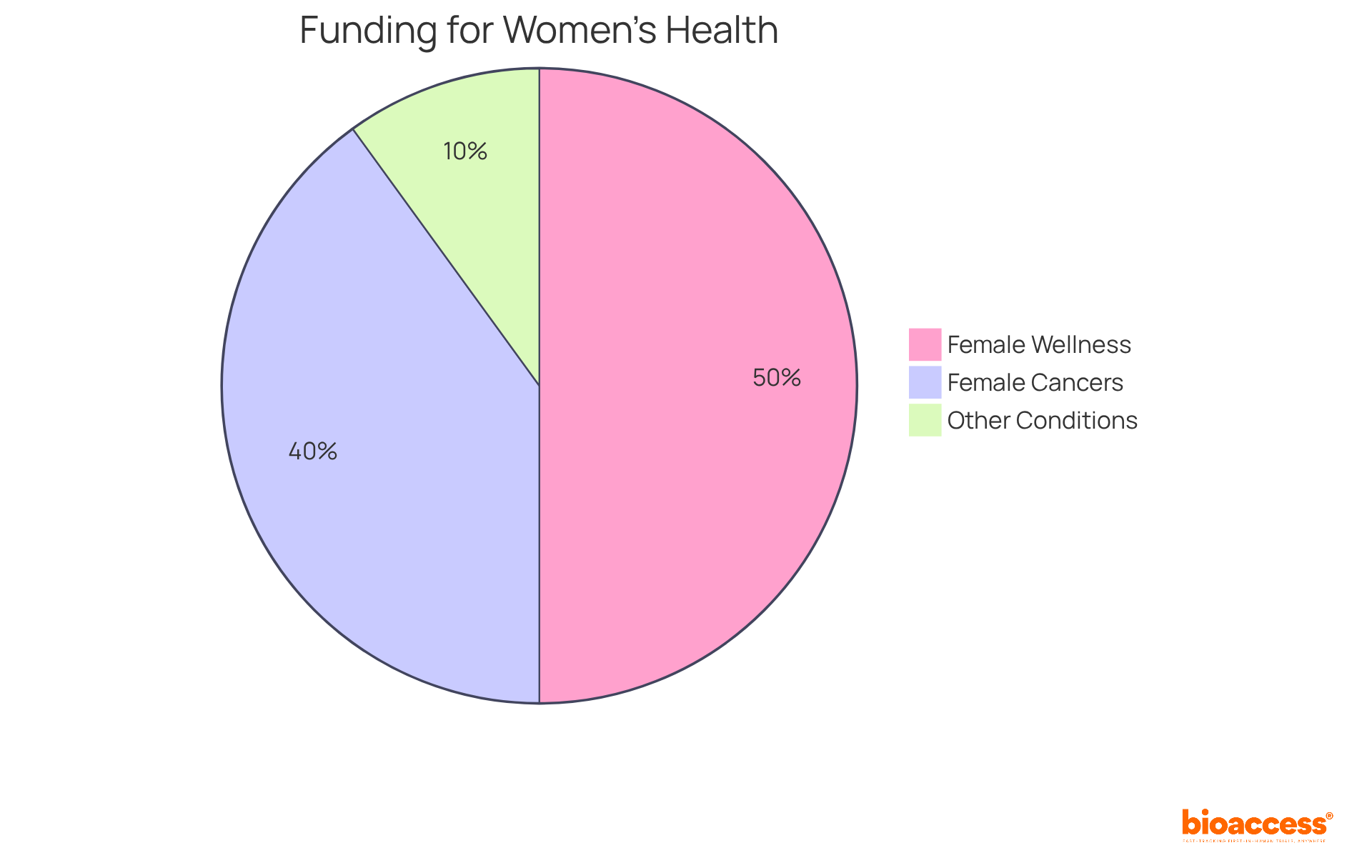
Recent findings on female wellness studies underscore the urgent need for policymakers to prioritize funding for initiatives aimed at women's well-being. Currently, a mere 5% of global R&D financing is allocated to female wellness - of which only 4% is directed towards female cancers and 1% for all other female-specific medical conditions. This stark reality highlights the necessity of translating study findings into actionable policies to address historical disparities.
Requiring female participation in clinical trials is a crucial step toward establishing a fairer healthcare system. This approach not only enhances the significance of research but also ensures that the unique wellness needs of female populations are adequately represented. By investing in female well-being, stakeholders can unlock significant economic advantages. Estimates suggest that closing investment gaps could boost the global economy by $1 trillion annually by 2040, as reported by the World Economic Forum.
Moreover, operating with a limited evidence base on female wellness is costing trillions, emphasizing the financial repercussions of inadequate funding. As we approach 2025, it is imperative that funding priorities reflect these insights, focusing on innovative solutions that empower individuals and enhance well-being across diverse populations.
Education and awareness are essential for enhancing outcomes for females in healthcare. Comprehensive training programs for healthcare professionals that focus on the unique conditions and treatment responses specific to females can significantly improve the quality of care provided. For example, emphasizing active listening skills in medical training validates patient concerns, which leads to timely and effective care. Dr. Naira Chobanyan underscores this importance, stating, "Validating a patient’s concerns is just as important as the treatment itself."
Furthermore, programs that educate females about their wellness entitlements and available resources empower them to advocate for their own health needs. With females now comprising 54.6% of students enrolled in M.D.-granting medical schools and 73.6% of residents in Pediatrics, their role in shaping healthcare practices is increasingly significant. By fostering informed medical choices through targeted education for both providers and patients, we can achieve improved wellness outcomes in phase 2 women's health and address the disparities that have historically affected care for women.
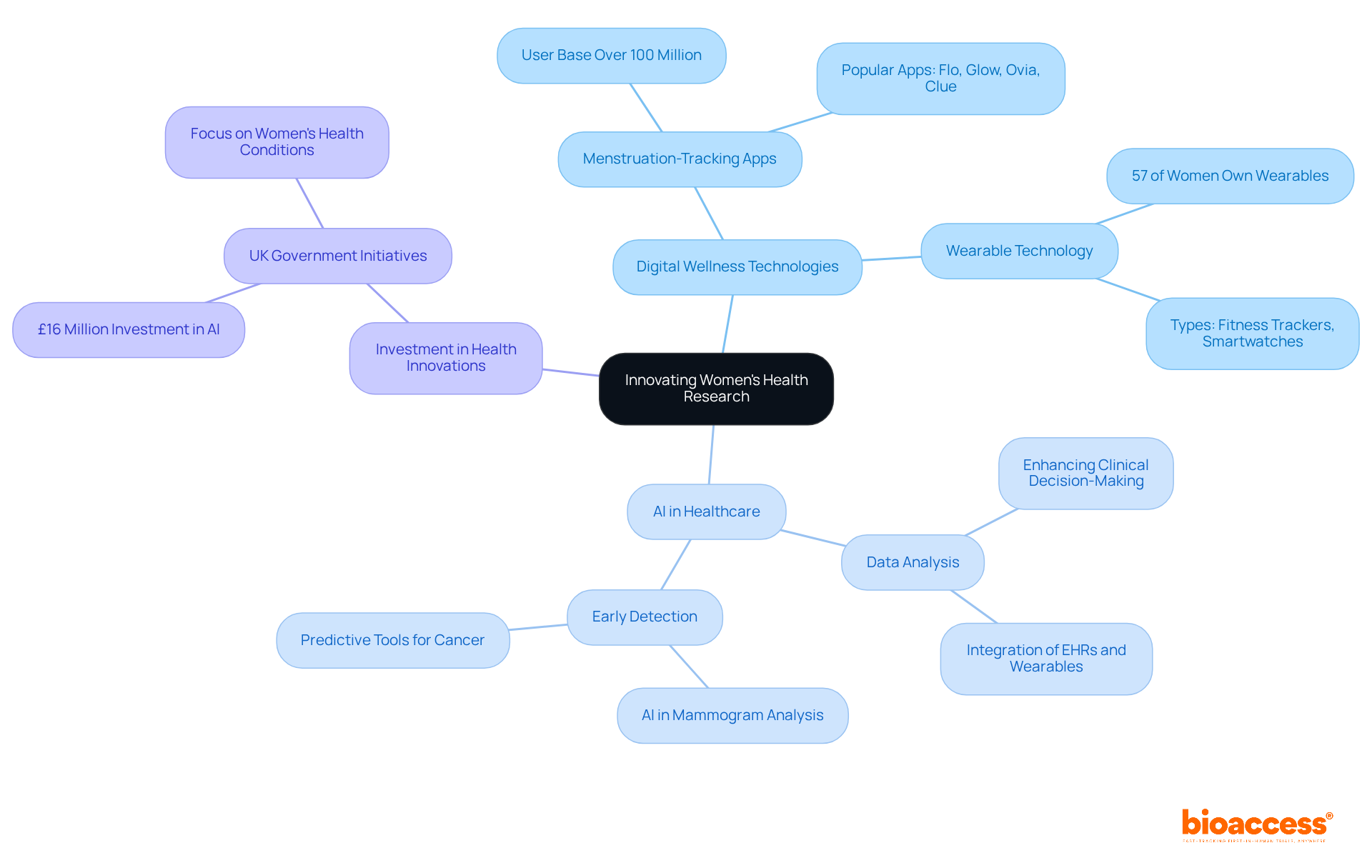
The future of female well-being research is increasingly intertwined with innovation and technology. Digital wellness technologies are revolutionizing the landscape by enabling remote monitoring and tailored treatment plans that cater to individual needs. For example, over 100 million individuals actively use menstruation-tracking applications, empowering them to manage their health proactively. Additionally, 57% of women globally own wearable technology, such as fitness trackers and smartwatches, highlighting a broader trend of engagement with digital wellness tools. Meanwhile, artificial intelligence is crucial in enhancing data analysis, facilitating the identification of trends that inform clinical decision-making. AI algorithms are employed to analyze mammograms and ultrasounds, significantly boosting early detection rates for diseases like breast and ovarian cancer.
Investments in AI technology, exemplified by the UK Government's £16 million initiative aimed at improving diagnosis and treatment for various medical conditions - including breast, colon, and prostate cancers, as well as neurological disorders like dementia and Parkinson's - underscore a commitment to integrating these advancements into healthcare systems. This strategic focus on phase 2 women's health not only addresses the unique wellness challenges faced by women but also promotes gender equality in medical access. As digital wellness continues to evolve, it is poised to drive significant improvements in phase 2 women's health outcomes, establishing personalized care as a standard practice in the coming years. The integration of digital health solutions and AI technologies is vital for enhancing phase 2 women's health by creating a more effective, responsive, and inclusive healthcare environment for women.
The insights derived from the Phase 2 Women's Health Report highlight the critical importance of inclusive and innovative approaches in clinical research focused on women's health. Addressing the historical underrepresentation of women in clinical trials and emphasizing the need for diverse participant demographics, the report advocates for a transformative shift in how women's health research is conducted. This ensures that therapies are effective and safe for all women.
Key findings reveal alarming disparities in drug efficacy and adverse reactions experienced by women, underscoring the necessity for tailored research methodologies that prioritize female health. Furthermore, the report emphasizes the role of stakeholder engagement and policy reform in enhancing research outcomes. It urges collaboration among patients, healthcare providers, and policymakers to create a more equitable healthcare landscape.
As the future of women's health research unfolds, harnessing technological advancements and fostering educational initiatives that empower both women and healthcare professionals is imperative. By committing to these changes, the healthcare community can significantly improve health outcomes for women, ensuring their unique needs are met and that they receive the quality care they deserve. Embracing this call to action is essential for paving the way toward a more inclusive and effective healthcare system for women everywhere.
What is bioaccess® and its role in women's health research?
bioaccess® is a clinical research organization that leverages its expertise in early-stage research to drive advancements in phase 2 women's health, aiming to accelerate the trial process and improve access to essential health solutions.
How does Colombia contribute to the efficiency of clinical trials conducted by bioaccess®?
Colombia offers significant cost reductions exceeding 30% compared to North America and Western Europe, along with swift regulatory procedures that allow for ethical approvals in just 90-120 days, facilitating faster introduction of new therapies and medical devices.
Why is diverse representation in phase 2 women's health research important?
Diverse representation is crucial to ensure the effectiveness of products developed for women, as emphasized by Dr. Beth Garner, Chief Scientific Officer of Ferring Pharmaceuticals US. Inclusion of women in trials helps address unique medical challenges and improves health outcomes.
What historical challenges have women faced in clinical trials?
Women have historically been underrepresented in clinical trials due to concerns about hormonal fluctuations and pregnancy risks, resulting in critical gaps in understanding treatment effects and leading to outdated healthcare practices.
What impact did the thalidomide tragedy have on women's participation in clinical research?
The thalidomide tragedy led to stringent FDA guidelines that further marginalized women in research, contributing to a long-lasting lack of tailored therapies and reliance on studies that may not accurately reflect female health needs.
What are the current statistics regarding women's participation in clinical trials?
As of 2020, females represented less than 40% of participants in heart disease and stroke studies, and less than 30% of participants in early-phase trials, highlighting ongoing issues of underrepresentation.
How does bioaccess® address the gaps in understanding drug efficacy and device performance for women?
bioaccess® offers clinical trial management services specifically tailored for phase 2 women's health, including Early-Feasibility Studies, First-In-Human Studies, and Pivotal Studies, to provide essential data on treatment efficacy and safety for female patients.
What is the significance of the FDA's January 2022 guidance regarding clinical trials?
The FDA's guidance encourages the participation of individuals, particularly those of reproductive capability, in early-phase trials focused on phase 2 women's health, aiming to rectify past injustices and improve treatment safety and efficacy for females.
Why is collaboration important in clinical research for women's health?
Collaboration is key to overcoming challenges in clinical research, ensuring that studies are more representative and effective for all patients, particularly for enhancing female health outcomes.