


The article titled "10 Key Insights into Catheter Manufacturing for Clinical Research Leaders" presents essential factors influencing catheter manufacturing within the clinical research landscape. It underscores the significance of understanding market dynamics, regulatory compliance, technological advancements, and the crucial collaboration between manufacturers and healthcare providers. These elements are vital for driving innovation and enhancing patient outcomes in catheter development, making this overview highly relevant for clinical research leaders.
The landscape of catheter manufacturing is rapidly evolving, driven by technological advancements and an increasing demand for innovative medical solutions. As clinical research leaders navigate this complex environment, it is crucial to understand key insights into manufacturing processes to maintain a competitive edge.
What challenges lie ahead?
How can companies effectively harness new technologies and market dynamics to enhance patient care and streamline production?
These questions are vital for those aiming to succeed in this dynamic field.
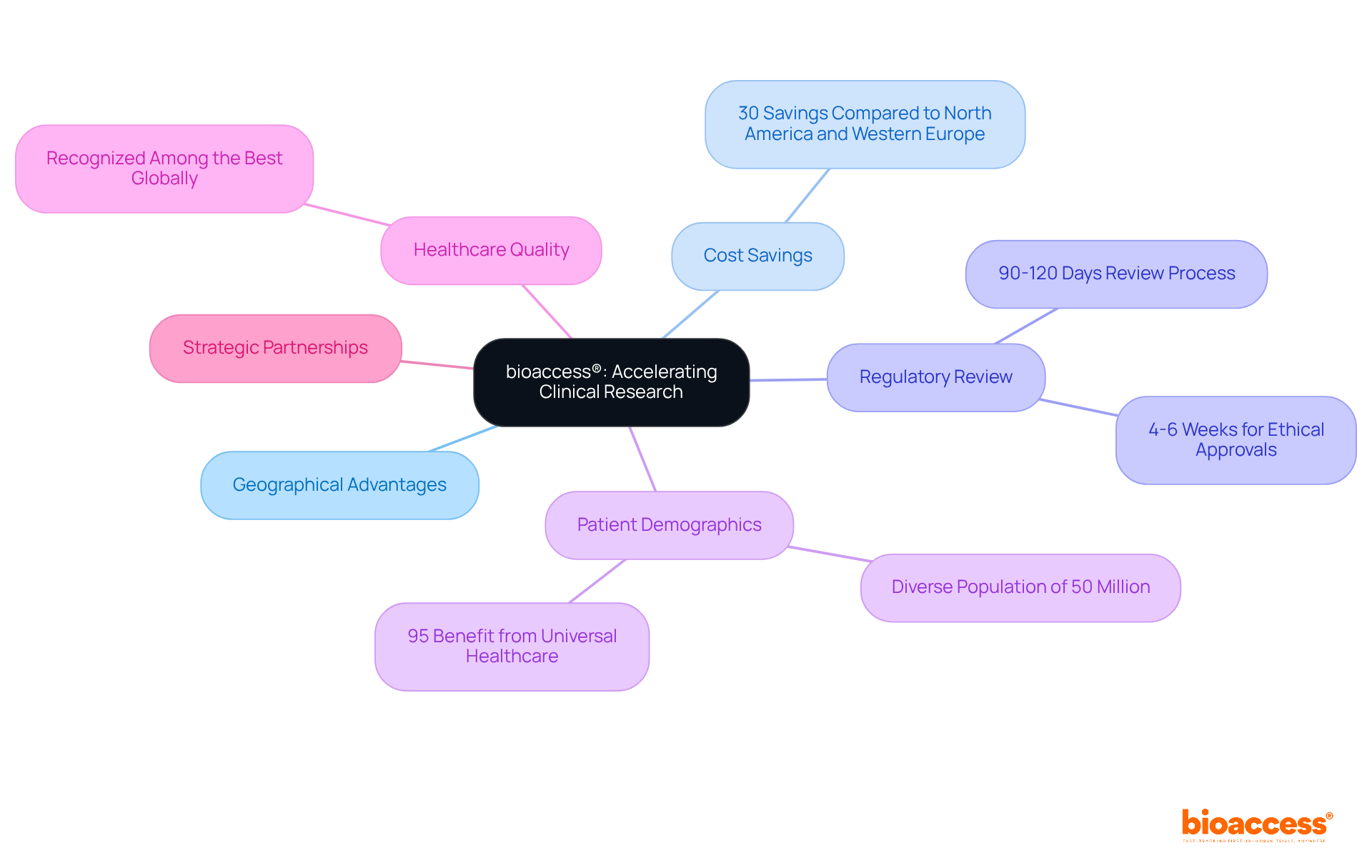
bioaccess® is dedicated to facilitating rapid clinical studies for medical device innovations, leveraging its unique geographical advantages in Colombia. With cost savings exceeding 30% compared to North America and Western Europe, and a regulatory review process that typically spans just 90-120 days, bioaccess® can secure ethical approvals in as little as 4-6 weeks. This remarkable agility, combined with access to a diverse patient population from Colombia's 50 million residents—95% of whom benefit from universal healthcare—empowers catheter manufacturing companies to expedite their research and development efforts.
Moreover, Colombia's high-quality healthcare system, recognized among the best globally, guarantees that innovative products are brought to market more swiftly and efficiently. The R&D tax incentives available in Colombia further enhance the financial appeal for companies, establishing it as a prime destination for clinical trials. Strategic partnerships play a pivotal role in realizing these objectives, underscoring bioaccess®'s position as a catalyst for innovation within the Medtech sector.
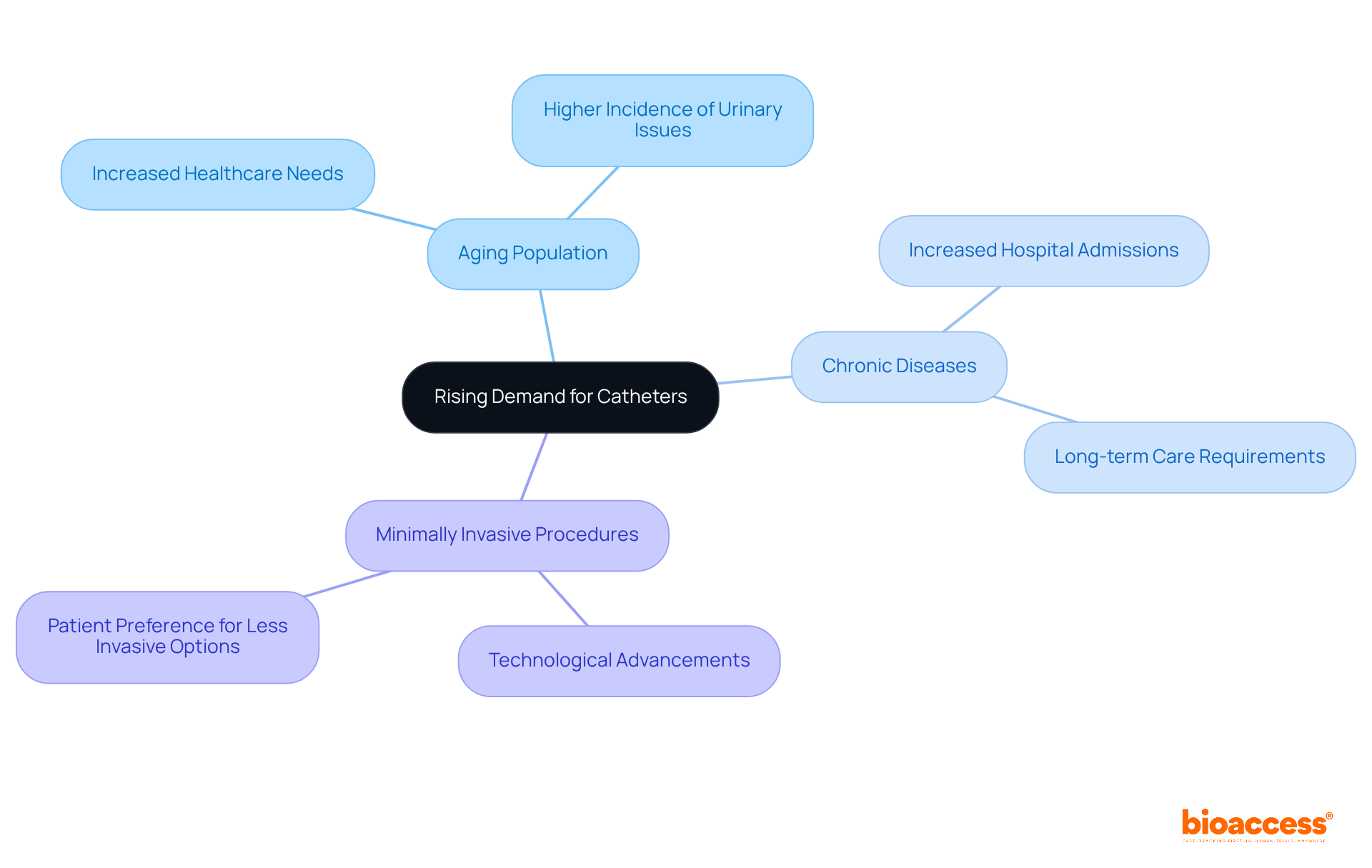
The demand for urinary tubes is escalating, driven by an aging population, a rising prevalence of chronic diseases, and advancements in minimally invasive procedures. Clinical study leaders must remain vigilant about these market dynamics to strategically position their products. Furthermore, comprehending regional variations in demand enables manufacturers to tailor their strategies effectively to meet specific market needs.
It is highly recommended that firms conduct comprehensive market analyses to identify opportunities and challenges in catheter manufacturing, ensuring they maintain competitiveness in a rapidly evolving landscape.
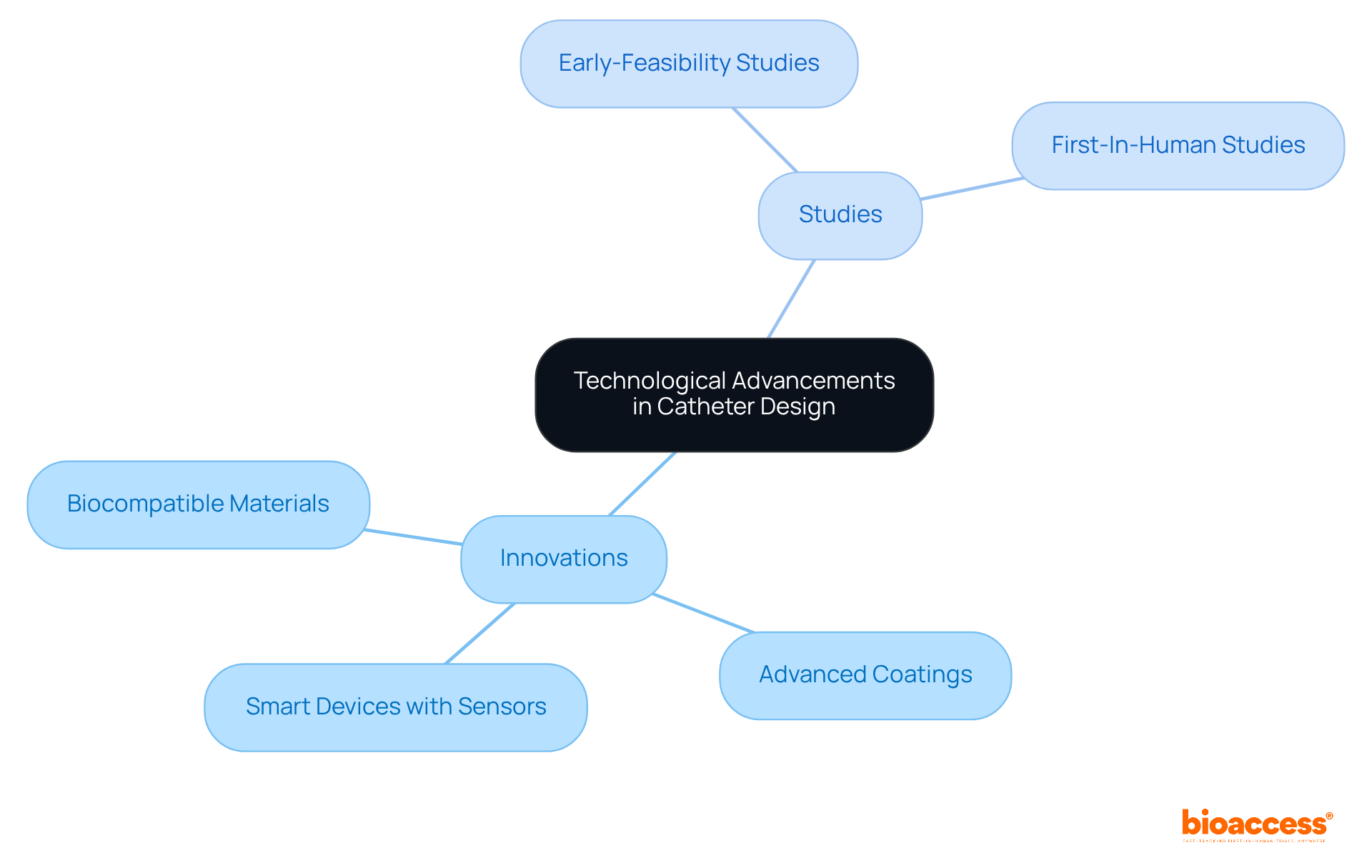
Recent technological advancements in tube design have led to the emergence of biocompatible materials, advanced coatings, and smart devices equipped with sensors. These innovations not only enhance patient outcomes but also significantly improve the overall effectiveness of medical tubes. Clinical study leaders must prioritize investing in research and development to explore these technologies, as they can profoundly impact the effectiveness and safety of catheter manufacturing.
Moreover, collaborating with skilled trial management services such as bioaccess® can facilitate the implementation of:
The importance of working together with technology suppliers and clinical study organizations cannot be overstated; such collaborations are crucial for remaining at the forefront of these advancements.
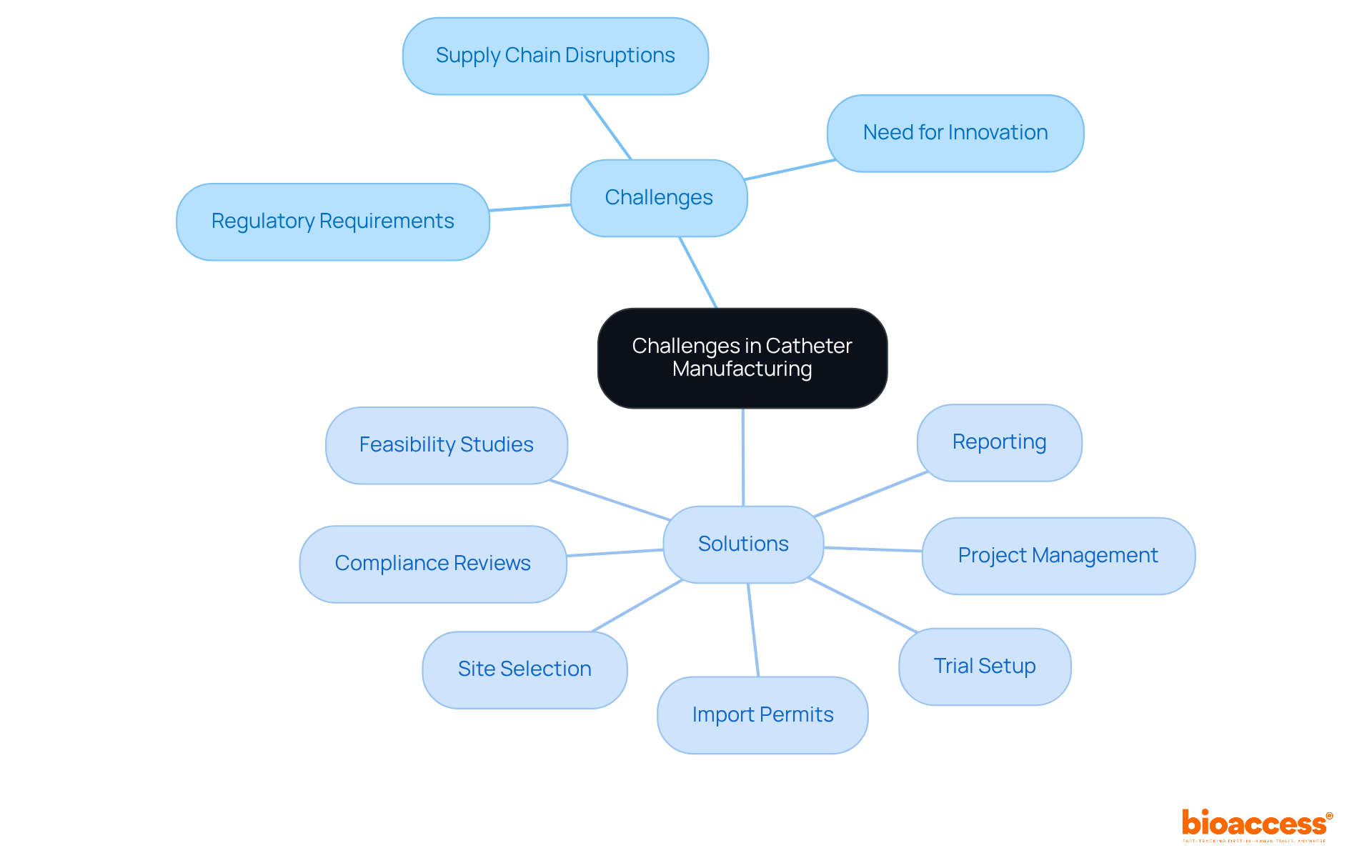
Manufacturers in catheter manufacturing encounter significant challenges, including stringent regulatory requirements, supply chain disruptions, and the imperative for continuous innovation. Addressing these complexities necessitates a proactive approach to risk management and strategic planning. Clinical research leaders must collaborate closely with their teams to identify potential obstacles early in the development process and implement effective solutions to mitigate risks. Engaging with comprehensive clinical trial management services, such as those provided by bioaccess, can greatly facilitate this effort.
These services encompass essential components like:
All crucial for ensuring adherence to regulatory standards. It is advisable for organizations to foster a culture of adaptability and resilience, enabling them to respond effectively to these challenges. By doing so, they can not only navigate the complexities of the Medtech landscape but also enhance their operational efficiency and compliance.
In catheter manufacturing, regulatory compliance is paramount, as it directly influences patient safety and the effectiveness of the device. Clinical research leaders must ensure that their offerings align with the stringent standards established by regulatory bodies. This obligation encompasses conducting comprehensive preclinical and clinical trials to gather critical data on safety and effectiveness. Experts underscore the necessity of remaining vigilant regarding regulatory changes and proactively engaging with regulatory agencies early in the development process, thereby facilitating smoother approval pathways.

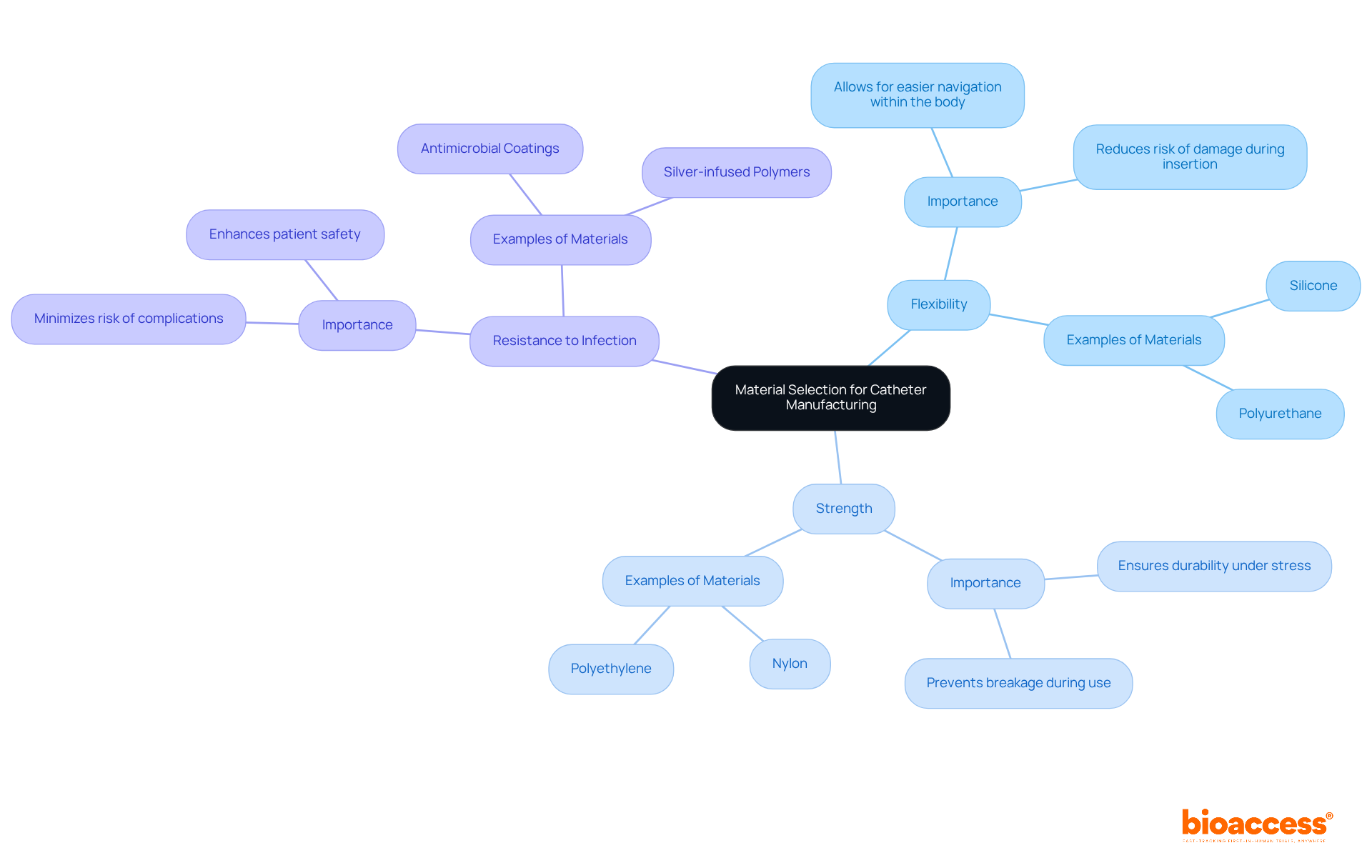
The selection of materials is a pivotal factor in the manufacturing of medical devices, significantly affecting their performance, biocompatibility, and durability. Manufacturers must evaluate critical aspects such as:
when determining appropriate materials. It is recommended that thorough testing of these materials be conducted to ensure compliance with safety and efficacy standards. Collaborating with material scientists can provide valuable insights into innovative materials that may enhance device performance.
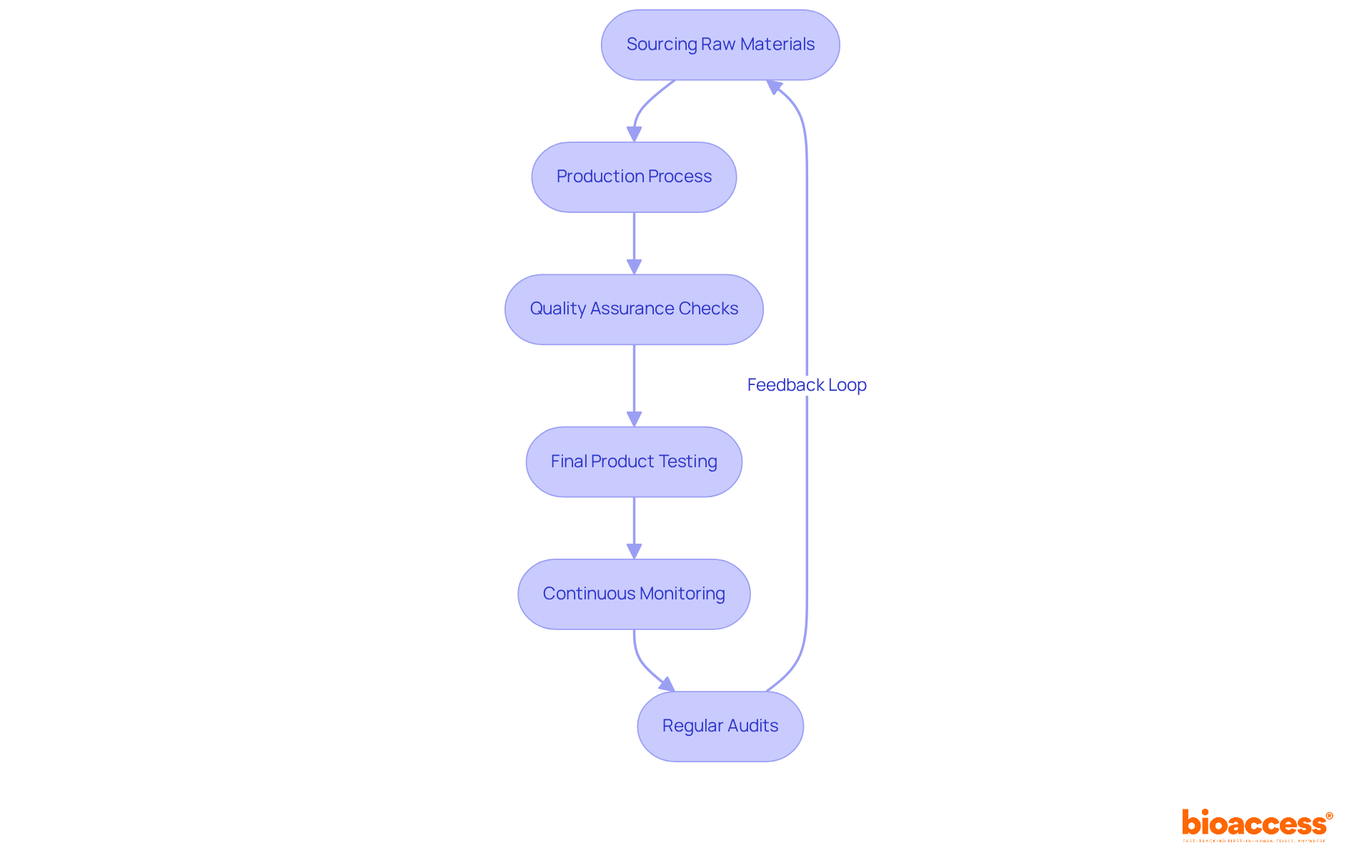
Quality control stands as a pivotal element in tube manufacturing, ensuring that products adhere to established standards and specifications. Manufacturers are urged to implement rigorous quality assurance processes throughout the entire production cycle, from sourcing raw materials to conducting final product testing. The significance of continuous monitoring and improvement of quality control measures cannot be overstated, as it plays a vital role in preventing defects and safeguarding patient safety. Furthermore, regular audits and compliance checks are essential in maintaining high standards within the tube manufacturing sector.
Smart Technologies: The Future of Catheter Manufacturing
The integration of smart technologies into catheter manufacturing is poised to revolutionize the industry. Innovations such as IoT-enabled catheters, which can monitor patient data in real-time, are becoming increasingly prevalent. These technologies not only enhance patient care but also offer valuable information for manufacturers to refine their offerings.
Moreover, as these advancements arise, it is essential for research leaders in healthcare to consider how they can utilize bioaccess's expertise in managing trials, including:
to validate these innovations. Collaborating with tech companies can facilitate the exploration of advancements in catheter manufacturing, ensuring that manufacturers remain competitive in the market while also adhering to regulatory standards.

Cooperation between catheter manufacturing companies and healthcare professionals is essential for developing solutions that fulfill medical needs. By collaborating with healthcare experts, catheter manufacturing companies gain valuable insights into the challenges faced in medical environments, allowing them to tailor their products effectively.
To bolster this collaboration, bioaccess provides comprehensive clinical trial management services, which encompass:
Establishing consistent communication pathways and joint initiatives fosters innovation and ensures that medical devices are designed with end-user requirements in mind. This positions bioaccess as a trusted CRO and consulting partner for U.S. medical device firms operating in Colombia.
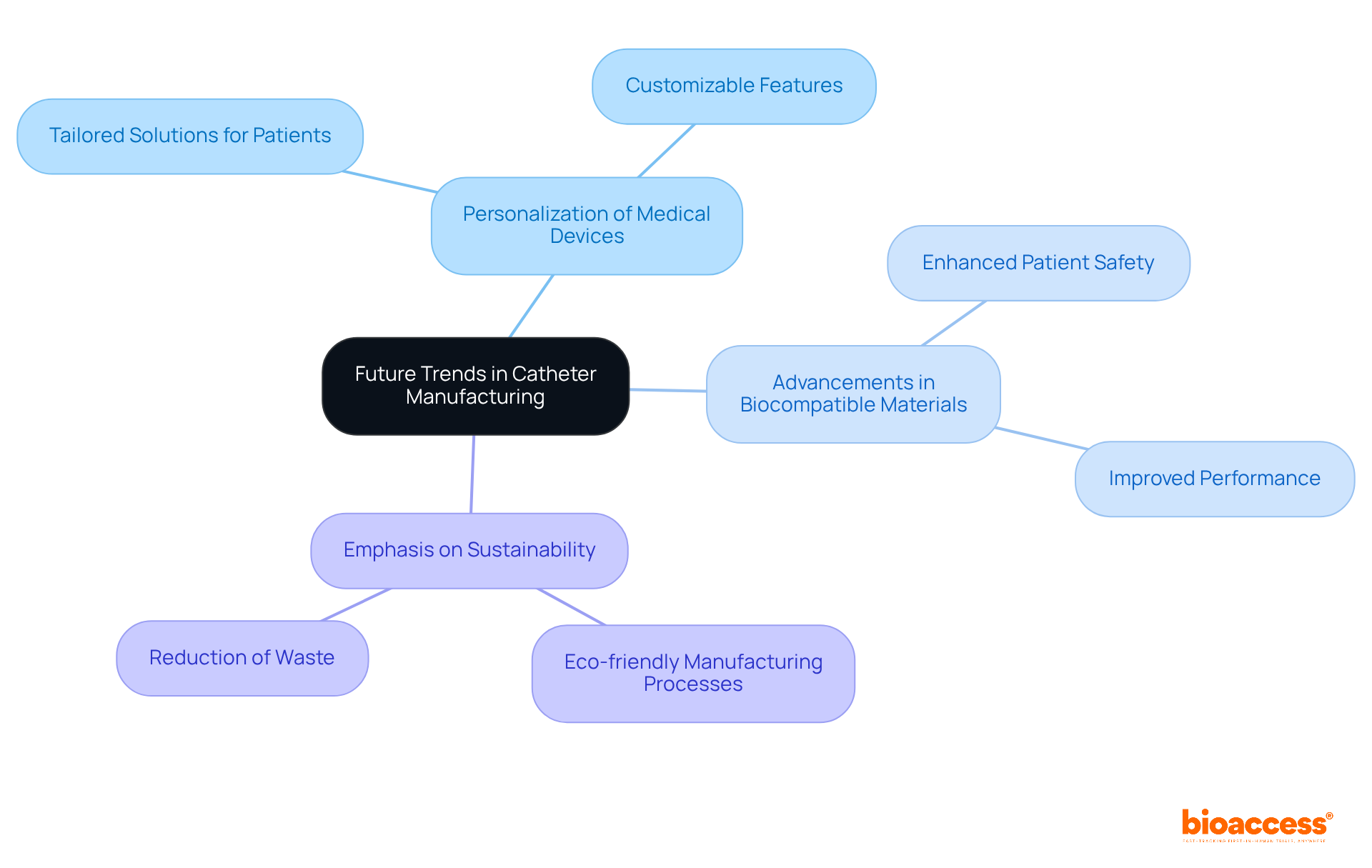
Several pivotal trends, including the increased personalization of medical devices, advancements in biocompatible materials, and the growing emphasis on sustainability within manufacturing processes, are poised to shape the future of catheter manufacturing. At bioaccess™, we are committed to advancing medical technologies through innovation and quality, ensuring that our offerings meet the highest standards in the industry. We invite healthcare leaders to join our dedicated team and share their expertise to help shape the future of medical care.
Staying informed about these trends is crucial for adapting strategies and ensuring that products remain relevant in an evolving market. Continuous innovation and responsiveness to emerging trends are essential for maintaining a competitive edge—principles that bioaccess™ embodies as a leader in medtech clinical research in Latin America. Together, we can navigate the challenges of the Medtech landscape and drive forward-thinking solutions that enhance patient outcomes.
The landscape of catheter manufacturing is evolving rapidly, driven by technological advancements, regulatory demands, and market dynamics. Understanding these changes is crucial for clinical research leaders aiming to maintain a competitive edge. By leveraging the unique advantages offered by bioaccess®, such as expedited clinical trials and access to a diverse patient population, manufacturers can enhance their research and development processes, ultimately leading to improved patient outcomes.
Key insights from the article underscore the importance of:
These factors are essential in ensuring the success of catheter manufacturing. The rising demand for catheters, coupled with the integration of smart technologies and a focus on regulatory compliance, highlights the necessity for manufacturers to continually adapt their strategies. By conducting thorough market analyses and fostering partnerships with healthcare providers, companies can effectively navigate the complexities of the Medtech landscape.
As the future of catheter manufacturing unfolds, embracing these trends will be vital for ensuring relevance and competitiveness. The call to action is clear:
This will drive advancements in medical technology. Together, stakeholders can navigate the challenges ahead and contribute to a healthcare environment that prioritizes safety, efficacy, and improved patient care.
What is bioaccess® and its role in clinical research for catheter innovations?
bioaccess® is an organization dedicated to facilitating rapid clinical studies for medical device innovations, particularly in catheter manufacturing. It leverages geographical advantages in Colombia to expedite research and development efforts.
What are the cost benefits of conducting clinical studies with bioaccess® in Colombia?
Companies can achieve cost savings exceeding 30% compared to North America and Western Europe when conducting clinical studies with bioaccess®.
How quickly can bioaccess® secure ethical approvals for clinical studies?
bioaccess® can secure ethical approvals in as little as 4-6 weeks, with the overall regulatory review process typically spanning 90-120 days.
What advantages does Colombia offer for clinical trials in terms of patient population?
Colombia has a diverse patient population of 50 million residents, with 95% benefiting from universal healthcare, which empowers catheter manufacturing companies to expedite their research.
How does Colombia's healthcare system contribute to the efficiency of bringing innovative products to market?
Colombia's high-quality healthcare system is recognized globally, ensuring that innovative products are brought to market swiftly and efficiently.
What financial incentives are available for companies conducting research and development in Colombia?
R&D tax incentives are available in Colombia, enhancing the financial appeal for companies and establishing the country as a prime destination for clinical trials.
What factors are driving the rising demand for urinary tubes in catheter manufacturing?
The rising demand for urinary tubes is driven by an aging population, increasing prevalence of chronic diseases, and advancements in minimally invasive procedures.
Why is it important for clinical study leaders to understand market dynamics in catheter manufacturing?
Understanding market dynamics allows clinical study leaders to strategically position their products and tailor their strategies to meet specific regional market needs.
What recent technological advancements are influencing catheter design and manufacturing?
Recent advancements include the development of biocompatible materials, advanced coatings, and smart devices equipped with sensors, which enhance patient outcomes and improve the effectiveness of medical tubes.
What types of studies should clinical study leaders prioritize to explore new technologies in catheter manufacturing?
Clinical study leaders should prioritize investing in research and development for early-feasibility studies, first-in-human studies, and other essential trials necessary for introducing innovations to the market.
How can collaboration benefit companies in the catheter manufacturing sector?
Collaborating with technology suppliers and clinical study organizations, such as bioaccess®, is crucial for remaining at the forefront of technological advancements and ensuring successful trial management.