


The article titled "10 Key Insights into Medical Device Regulatory Affairs for Success" serves as a vital resource for those navigating the complex landscape of medical device regulatory affairs. It underscores the necessity of comprehending local regulations and highlights effective communication with regulatory bodies as pivotal strategies. Furthermore, it discusses the critical role of clinical trials in expediting approvals and ensuring compliance. These insights are indispensable for achieving success in the competitive realm of medical devices.
The medical device industry is undergoing a significant transformation, particularly in regions such as Latin America, where regulatory frameworks are becoming increasingly intricate. For Medtech companies, grasping the nuances of these regulations is essential for successful market entry and sustained growth. This article explores ten crucial insights that can empower organizations to navigate the complex landscape of medical device regulatory affairs. It highlights strategies to overcome challenges and seize emerging opportunities.
How can companies leverage these insights not only to comply with regulations but also to accelerate their innovation and enhance their market presence?
bioaccess® leverages the dynamic environment of Latin America to secure medical device certifications within an impressive timeframe of 4-6 weeks. This swift process is essential for Medtech innovators aiming to expedite their market entry.
By harnessing local expertise and a comprehensive understanding of medical device regulatory affairs, bioaccess® enables companies to navigate the certification landscape effectively, significantly reducing time-to-market compared to traditional markets. This advantage is particularly crucial for startups and smaller companies that may lack the resources to manage lengthy validation procedures independently.
As the demand for healthcare products continues to rise, particularly with the projected growth of the LATAM medical equipment sector, the ability to obtain rapid approvals emerges as a vital asset for success in this competitive arena.
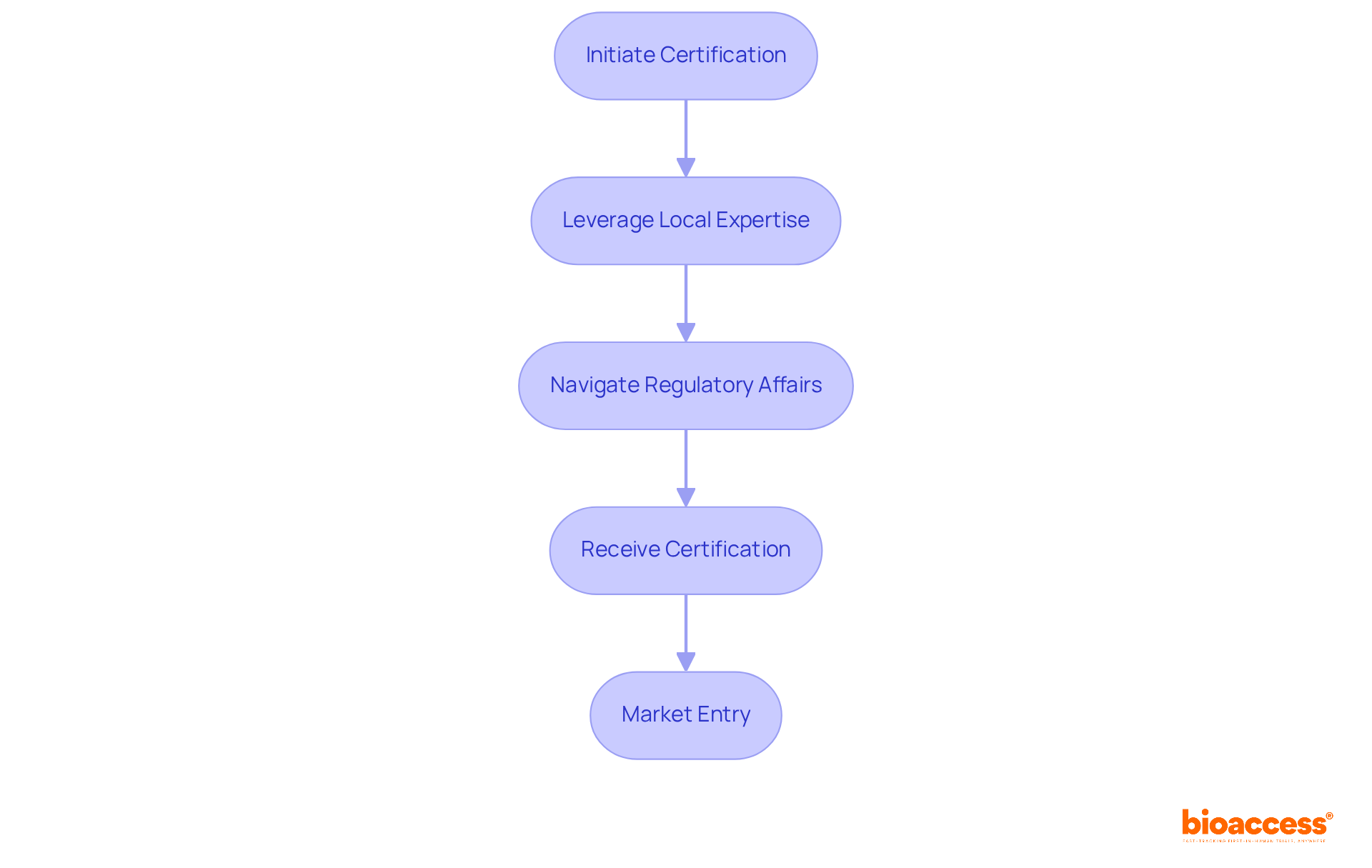
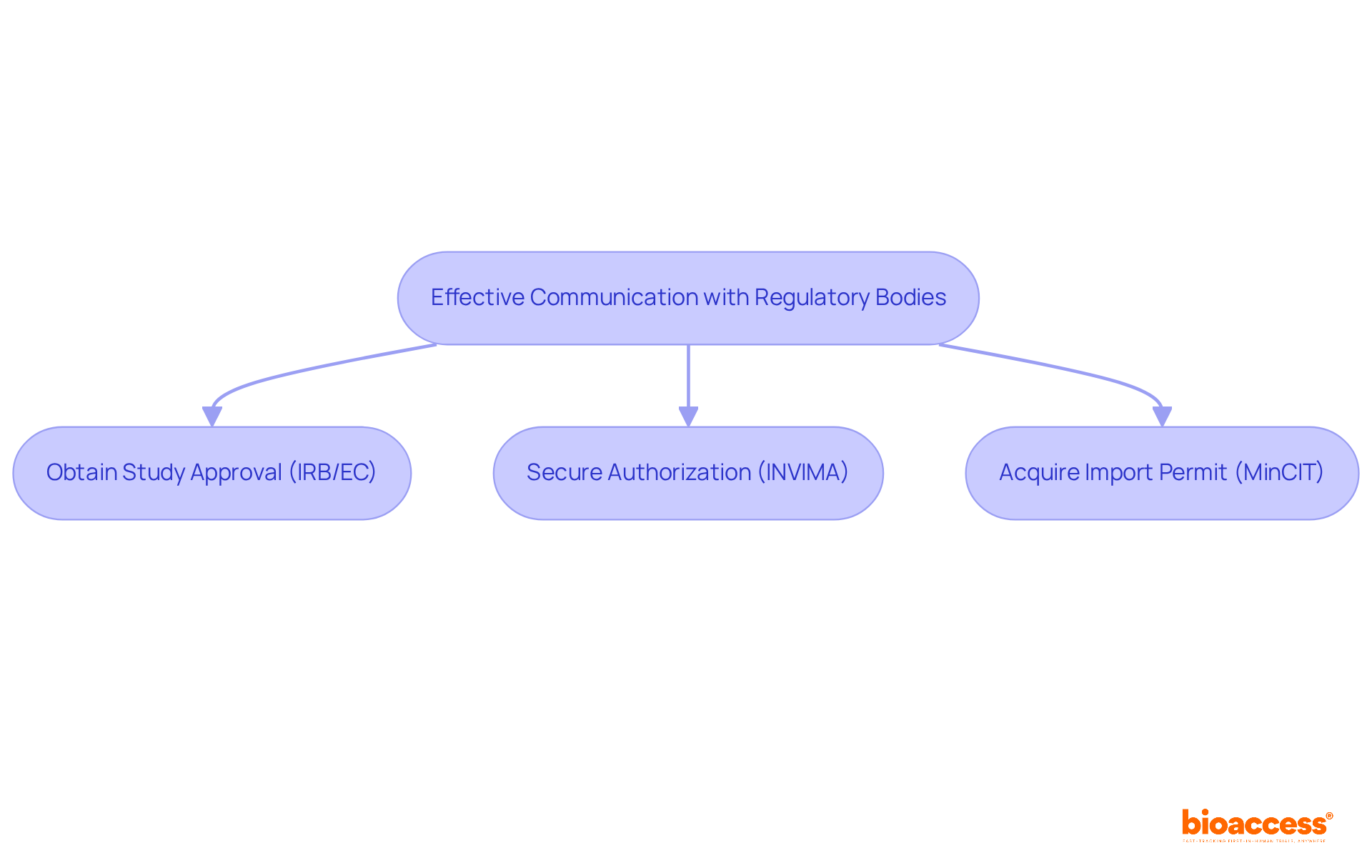
In Latin America, each nation has its own regulatory body and specific criteria for medical equipment authorization, making it imperative for firms to comprehend these regulations to avoid setbacks. For instance, in Colombia, the process begins with obtaining study consent from the site's institutional review board (IRB) or ethics committee (EC), followed by approval from INVIMA, the country's regulatory agency, and securing an import permit from the Ministry of Industry and Commerce (MinCIT) for investigational devices.
Similarly, Brazil's ANVISA and Argentina's ANMAT have distinct processes that can significantly influence timelines and documentation requirements. Understanding these nuances is crucial for Medtech firms aiming to develop effective compliance strategies in medical device regulatory affairs that align with local expectations and expedite their validation processes.
Furthermore, bioaccess® offers professional services that support Medtech, Biopharma, and Radiopharma startups in navigating these challenges, ensuring faster compliance and activation of studies, ultimately paving a more streamlined path to market.

Ethical permissions are essential for conducting clinical trials involving human subjects, ensuring adherence to ethical standards that protect participants' rights and well-being. This critical process involves obtaining informed consent and designing studies that minimize risks.
In Latin America, bioaccess® has forged strong relationships with ethics committees, significantly accelerating ethical review processes. Specifically, bioaccess facilitates the acquisition of study authorization from the site's institutional review board (IRB) or ethics committee (EC), regulatory endorsement from Colombia's INVIMA, and import permits from the Ministry of Industry and Commerce (MinCIT). This strategic collaboration not only shortens authorization timelines but also enhances the credibility of the research conducted.
Recent statistics reveal that the median time for ethics approval in the region is approximately 48 days, with streamlined governance processes contributing to expedited site activations. Organizations that prioritize robust connections with ethics committees have demonstrated increased efficiency in navigating compliance environments related to medical device regulatory affairs, ultimately enhancing their ability to swiftly introduce innovative medical products to the market.
As the clinical study market is projected to reach $70 billion USD by 2028, the significance of effective ethical review processes cannot be overstated.

Clinical studies are essential for gathering evidence that supports the safety and effectiveness of medical devices, which is a key aspect of medical device regulatory affairs necessary for regulatory approval. In Latin America, bioaccess® distinguishes itself in early-phase clinical studies, particularly First-In-Human (FIH) and Early-Feasibility Studies (EFS). These studies are vital for evaluating new technologies and refining product designs early in development. For example, Tioga Cardiovascular's Luna™ Transcatheter Mitral Valve Replacement (TMVR) System is currently undergoing initial human testing, highlighting the innovative advancements being pursued in the region.
Current trends reveal an increasing focus on FIH and EFS studies, with numerous Medtech companies incorporating these assessments into their development strategies. This proactive approach not only identifies potential challenges prior to extensive testing but also enhances the likelihood of favorable outcomes. Indeed, studies indicate that involving non-industrial partners in FIH studies can boost success rates by 11.3 percentage points, emphasizing the significance of collaboration.
Experts in the field underscore the critical nature of these early-phase studies. As Dr. Jorge Hernando Ulloa stated, 'The recent presentation at the Charing Cross International Symposium showcased one-year data from the first-in-human VenoValve® study, underscoring advancements in vascular medicine and the importance of meticulous study management.' Additionally, Dr. John B. Simpson's research on OCT-guided atherectomy further illustrates the innovative landscape of clinical studies in the region. Furthermore, Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, recounted his positive experience with bioaccess® during its initial human testing in Colombia, demonstrating how bioaccess® adeptly navigates the complex compliance environment that often hinders startups.
By effectively conducting FIH and EFS studies, bioaccess® empowers clients to produce the robust clinical data necessary for medical device regulatory affairs, while also reducing costs and timelines. The Latin America clinical trials market is anticipated to reach USD 2,654.0 million by 2030, with a compound annual growth rate (CAGR) of 6.7% from 2024 to 2030, reinforcing the strategic importance of conducting studies in this region. This strategic focus positions bioaccess® as a leader in fostering medical innovation in Latin America.

Establishing clear channels of communication with overseeing organizations is paramount for successful medical equipment endorsements. Companies must proactively engage with regulators in the realm of medical device regulatory affairs to clarify requirements and address any concerns early in the process. bioaccess® underscores the importance of clarity and responsiveness in its interactions with oversight bodies, which fosters trust and streamlines the validation process. This includes:
Furthermore, bioaccess® connects innovative Medtech, Biopharma, and Radiopharma startups with top-ranked clinical research sites, facilitating a more efficient pathway to clinical trial initiation and success. Regular updates and feedback loops are essential in medical device regulatory affairs to significantly mitigate misunderstandings and delays.
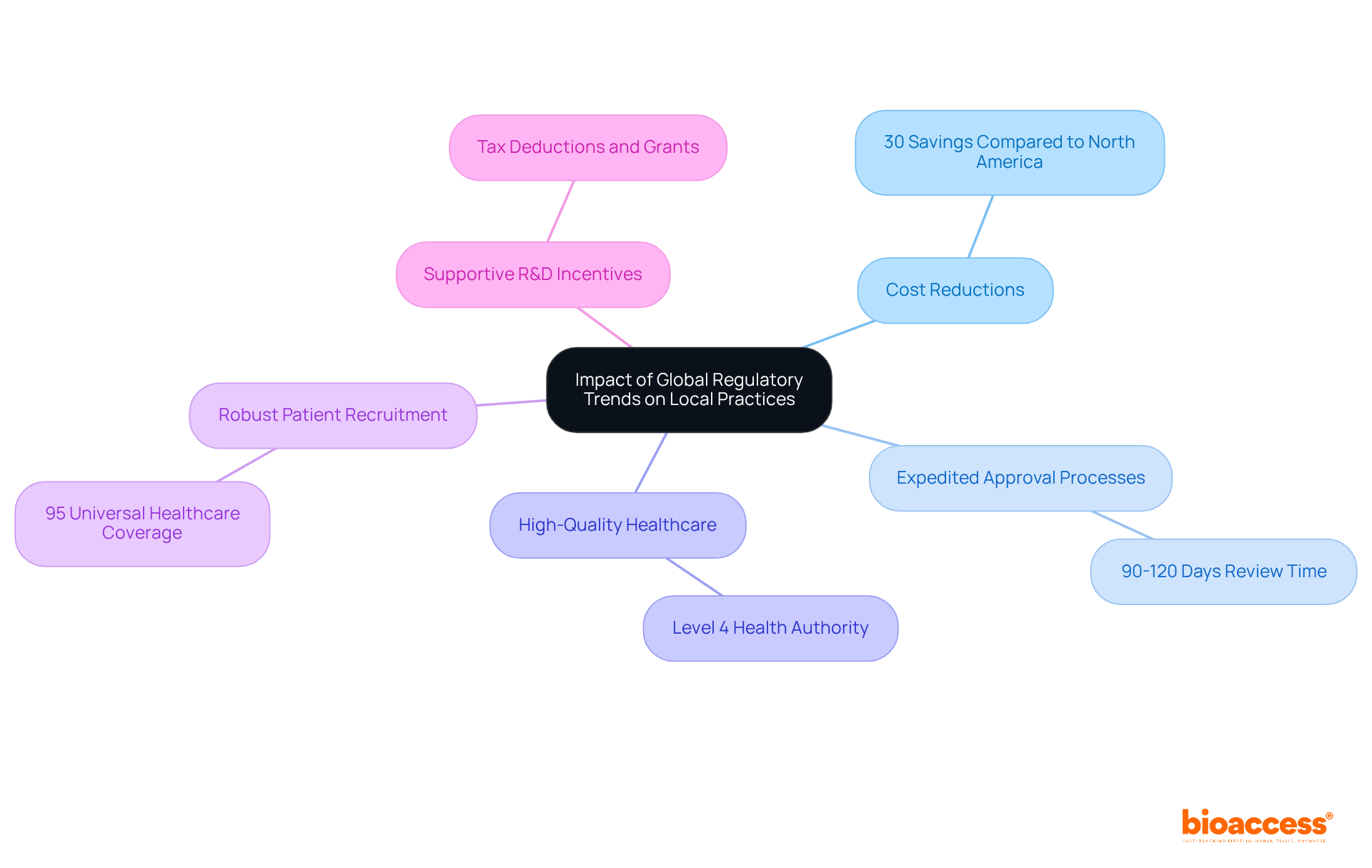
The healthcare equipment sector is undergoing significant transformation, driven by global compliance trends that increasingly influence local practices. In Latin America, and particularly in Colombia, the environment is exceptionally advantageous for medical device companies, bolstered by several competitive benefits.
Colombia offers cost reductions exceeding 30% compared to trials in North America or Western Europe, alongside expedited approval processes. The average review times for total IRB/EC and INVIMA (Colombia's National Food and Drug Surveillance Institute) are only 90-120 days. This efficiency is further supported by a high-quality healthcare system, recognized by the World Health Organization as a Level 4 health authority and ranked among the best in Latin America.
Moreover, Colombia's population of over 50 million provides a robust patient recruitment base, with approximately 95% of individuals covered by universal healthcare. The country also promotes research and development through substantial tax deductions and grants, positioning itself as an attractive destination for Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH).
As the compliance landscape continues to evolve, it is imperative for Medtech companies to remain informed about these trends to achieve success in both local and global markets.
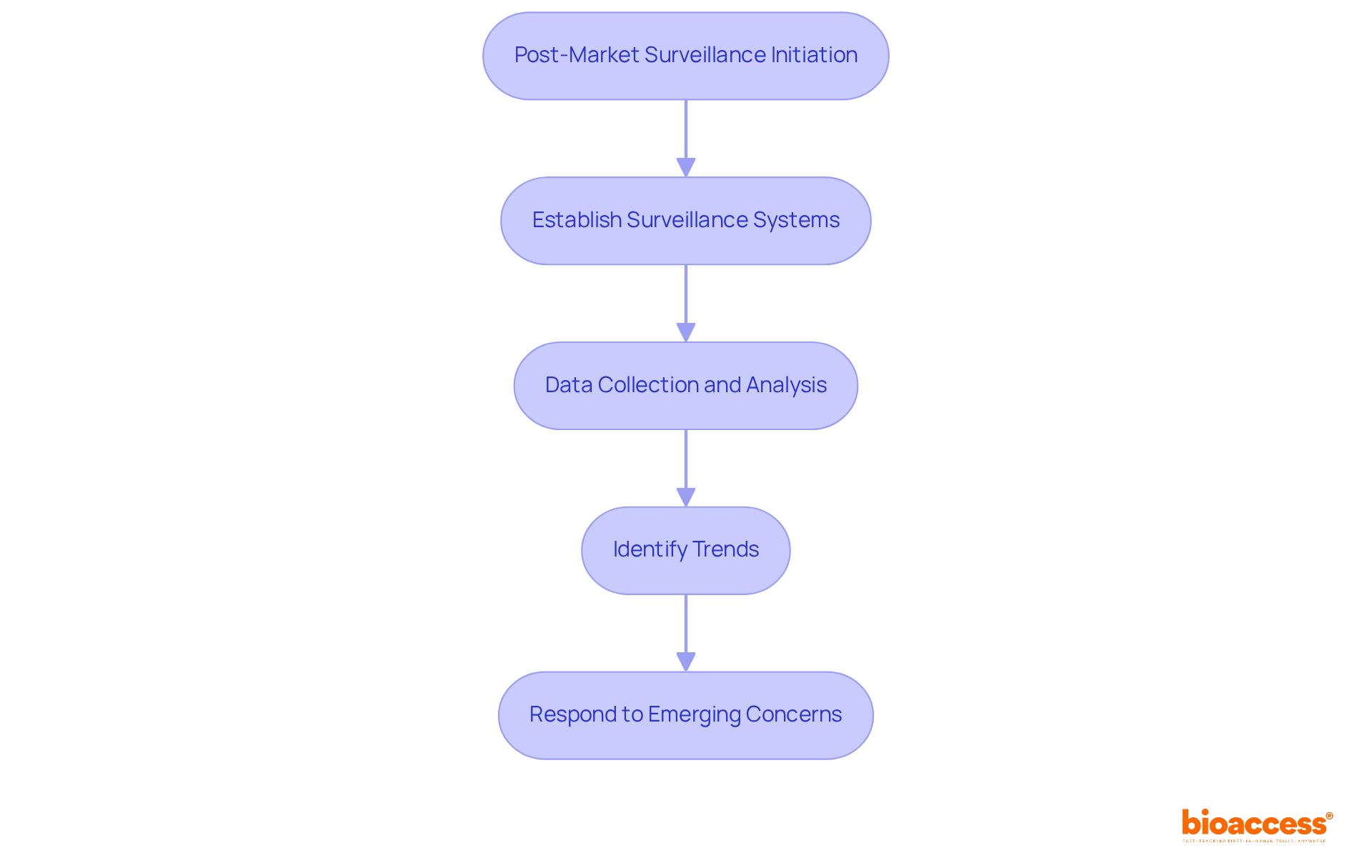
Post-market surveillance is vital for the continuous monitoring of medical devices in medical device regulatory affairs once they enter the market. This process is crucial in medical device regulatory affairs for identifying potential safety concerns and ensuring compliance with legal standards.
In Latin America, regulatory authorities require manufacturers to establish comprehensive post-market surveillance systems as part of medical device regulatory affairs. These systems must encompass mechanisms for ongoing data collection and analysis, enabling the identification of trends and effective responses to emerging concerns.
Companies that adopt robust monitoring practices not only enhance patient safety but also bolster their credibility with regulators. For example, organizations that utilize advanced data analytics and AI technologies have notably improved their response times and compliance rates.
bioaccess® plays a critical role in assisting clients in developing these systems, ensuring they can monitor performance and swiftly address any issues, thus maintaining a favorable benefit-risk ratio.
Continuous training and education are essential for maintaining adherence to the standards of medical device regulatory affairs. Companies must equip their teams with up-to-date knowledge of medical device regulatory affairs and best practices to effectively navigate the complexities of the industry. As we look to 2025, the landscape of medical device regulatory affairs is anticipated to change, making ongoing education even more vital.
bioaccess® offers customized training programs aimed at equipping clients with knowledge on critical subjects including:
Our comprehensive services include:
All aimed at advancing medical device trials efficiently. By investing in comprehensive education on medical device regulatory affairs, organizations can significantly reduce the risk of non-compliance, enhance product quality, and cultivate a culture of excellence in regulations. Statistics show that firms with strong training initiatives see a 30-50% rise in employee retention, highlighting the significance of continuous education in sustaining a skilled workforce capable of meeting compliance standards.
Insights from compliance instructors emphasize best practices, stressing the necessity for regular updates and interactive learning techniques to stay aligned with the changing legal landscape. This proactive approach not only ensures compliance but also positions companies for success in the competitive Medtech landscape.

The landscape of medical device regulatory affairs is undergoing significant transformation, driven by key trends such as the increasing reliance on real-world evidence (RWE) and the integration of artificial intelligence (AI) into regulatory processes. RWE is becoming essential for demonstrating the safety and effectiveness of medical devices, with payers increasingly valuing it for assessing long-term durability and rare adverse events. Despite its potential, many manufacturers have yet to submit RWE as the primary evidence for premarket approvals, often relying on pivotal clinical studies supplemented by RWE. This gap emphasizes the need for clearer oversight guidance and successful case examples to encourage broader adoption.
AI is also transforming the governance framework, providing innovative solutions for data analysis and decision-making. As companies utilize AI to enhance their compliance strategies, they can improve efficiency and accuracy in navigating complex requirements. Industry analysts highlight that the combination of AI and RWE can result in more resilient submissions, ultimately promoting greater transparency and trust in clinical trial data.
To remain competitive in the field of medical device regulatory affairs, companies must proactively adapt to these evolving trends. bioaccess® is dedicated to providing clients with the insights and strategies necessary to successfully navigate the future regulatory environment, ensuring they are well-equipped to meet the challenges and opportunities presented by these advancements.

The intricate landscape of medical device regulatory affairs in Latin America presents both challenges and opportunities for Medtech companies. Understanding the nuances of regional regulations and leveraging local expertise are essential for achieving rapid market entry and sustained success. By focusing on strategic compliance and effective communication with regulatory bodies, organizations can navigate this complex environment and enhance their innovation capabilities.
Throughout this article, key insights have been discussed, including:
The advantages of working with knowledgeable partners like bioaccess® highlight how companies can significantly reduce time-to-market and improve compliance outcomes. Moreover, the emphasis on post-market surveillance and adapting to global regulatory trends reinforces the necessity of a proactive approach in this evolving field.
As the medical device sector continues to grow, it is crucial for companies to remain agile and informed about emerging trends and regulatory changes. Engaging with local regulatory bodies, investing in comprehensive training, and adopting innovative practices will not only facilitate compliance but also position organizations for long-term success in a competitive market. Embracing these insights will empower Medtech companies to navigate the regulatory landscape effectively, ensuring that they can bring innovative medical solutions to those who need them most.
What is bioaccess® and what does it offer for medical device regulatory approvals in Latin America?
bioaccess® is a service that accelerates the certification of medical devices in Latin America, achieving approvals within 4-6 weeks. This rapid process is particularly beneficial for Medtech innovators seeking to expedite their market entry.
How does bioaccess® help companies navigate the certification landscape?
bioaccess® utilizes local expertise and a comprehensive understanding of regulatory affairs to help companies effectively navigate the certification processes, significantly reducing time-to-market compared to traditional methods.
Why is rapid approval important for startups and smaller companies in the Medtech sector?
Rapid approval is crucial for startups and smaller companies as they often lack the resources to manage lengthy validation procedures independently, making quick market entry essential for their success.
What challenges do firms face regarding regulatory frameworks in Latin America?
Each country in Latin America has its own regulatory body and specific criteria for medical equipment authorization, which can lead to setbacks if firms do not fully understand these regulations.
Can you provide an example of the regulatory process in a specific Latin American country?
In Colombia, the process involves obtaining study consent from an institutional review board (IRB) or ethics committee, followed by approval from INVIMA, and an import permit from the Ministry of Industry and Commerce (MinCIT) for investigational devices.
How does bioaccess® assist with ethical approvals in medical device regulations?
bioaccess® has established strong relationships with ethics committees, which helps to expedite the ethical review processes necessary for conducting clinical trials involving human subjects.
What is the significance of ethical approvals in clinical trials?
Ethical approvals are crucial for ensuring that clinical trials adhere to standards that protect participants' rights and well-being, involving informed consent and risk minimization.
What are the current statistics regarding ethics approval timelines in Latin America?
The median time for ethics approval in the region is approximately 48 days, with streamlined governance processes contributing to faster site activations.
Why is the clinical study market important and what is its projected growth?
The clinical study market is projected to reach $70 billion USD by 2028, highlighting the importance of effective ethical review processes in facilitating the introduction of innovative medical products to the market.