


This article delivers essential insights for clinical research directors on compliance with 21 CFR Part 807, which governs the registration and listing of medical devices. Understanding these regulatory requirements is crucial for success in clinical trials. Accurate documentation and the adoption of best practices are not just recommendations; they are vital strategies that streamline the approval process. By enhancing these aspects, clinical research directors can significantly improve the efficiency and success rates of their trials.
In the ever-evolving Medtech landscape, the role of bioaccess becomes increasingly important in addressing key challenges. As regulatory frameworks become more complex, staying informed and compliant is not merely beneficial; it is imperative. This article aims to equip clinical research directors with the knowledge needed to navigate these complexities effectively.
Collaboration is key in this endeavor. By working together and sharing insights, clinical research teams can tackle the challenges posed by regulatory compliance head-on. The next steps involve not only understanding these regulations but also implementing strategies that foster a culture of compliance and excellence in clinical research.
Navigating the complex landscape of regulatory compliance presents a critical challenge for clinical research directors, particularly with the evolving mandates of 21 CFR Part 807. This regulation governs not only the registration and listing of medical devices but also shapes the entire clinical trial process, significantly influencing timelines and success rates for innovative medical solutions. As directors strive to enhance their compliance strategies, the stakes are undeniably high: failure to adhere to these regulations can result in costly delays and potential penalties.
So, how can clinical research directors effectively leverage the insights from 21 CFR Part 807 to optimize their operations? By understanding and implementing these regulations, they can ensure timely market entry for groundbreaking medical products, ultimately driving success in their clinical research endeavors.
Bioaccess® leverages its deep understanding of 21 CFR Part 807 to help clinical research directors achieve compliance. By offering tailored solutions that meet compliance standards, bioaccess® accelerates the clinical trial process, significantly boosting the success rates of medical innovations. Their expertise in navigating the intricate regulatory landscape is vital for directors aiming to optimize workflows and secure timely approvals.
With ethical approvals obtained in just 4-6 weeks, bioaccess® enables organizations to initiate studies more swiftly, ultimately enhancing the overall effectiveness of clinical research efforts.
All medical equipment establishments must register with the FDA under 21 CFR Part 807. This process requires crucial information, including the establishment's name, address, and a comprehensive list of the items produced. Registration isn't a one-time task; it needs to be updated annually and whenever there are operational changes. Adhering to these requirements is essential, as non-compliance can lead to penalties and hinder the legal promotion of products.
Understanding the nuances of FDA registration is vital for maintaining compliance. Current statistics reveal that while many establishments meet these requirements, a significant number still struggle with timely updates and accurate submissions. For instance, the FDA conducted 1,984 inspections of medical apparatus establishments in 2023, underscoring the ongoing scrutiny in this sector.
Successful medical equipment companies emphasize the significance of a robust quality management system (QMS) while navigating the registration process under 21 CFR Part 807. Companies utilizing solutions like Greenlight Guru have reported smoother registration experiences, ensuring that all necessary documentation—such as product descriptions, labeling information, and regulatory certificates—is meticulously maintained.
In summary, the key requirements for medical equipment registration under 21 CFR Part 807 are straightforward but demand diligence. Organizations must prioritize adherence to protect their market presence and uphold the safety and effectiveness standards expected in the medical equipment sector.
To ensure compliance with 21 CFR Part 807, it is crucial to promptly update device listings whenever there are changes in specifications or manufacturing processes. Establishing a routine review process and utilizing the FDA's electronic registration system can significantly enhance accuracy. Educating all team members on the importance of maintaining precise listings fosters a culture of adherence and vigilance. This proactive approach not only mitigates the risk of non-compliance but also streamlines approval processes and improves patient outcomes.
Statistics reveal that 67% of submissions lead to an Additional Information (AI) request, often resulting in delays. This underscores the necessity of maintaining accurate listings. To bolster adherence efforts, directors should consider scheduling regular evaluations of equipment listings, ideally every three months, to ensure ongoing precision and compliance with legal obligations, including 21 CFR Part 807. Insights from experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, can guide organizations in navigating the complexities of regulatory frameworks and enhancing their compliance strategies.
Foreign equipment establishments must adhere to the stringent registration and listing protocols specified in 21 CFR Part 807. This includes the mandatory appointment of a U.S. agent, the submission of comprehensive registration information, and ensuring that all devices intended for import into the U.S. are properly listed. The U.S. agent plays a crucial role, facilitating communication with the FDA and managing compliance-related inquiries, particularly during time-sensitive situations like recalls or safety investigations. Non-compliance with these regulations can lead to significant delays, import refusals, and potential legal repercussions. This highlights the necessity for clinical research directors to maintain a robust understanding of these procedures.
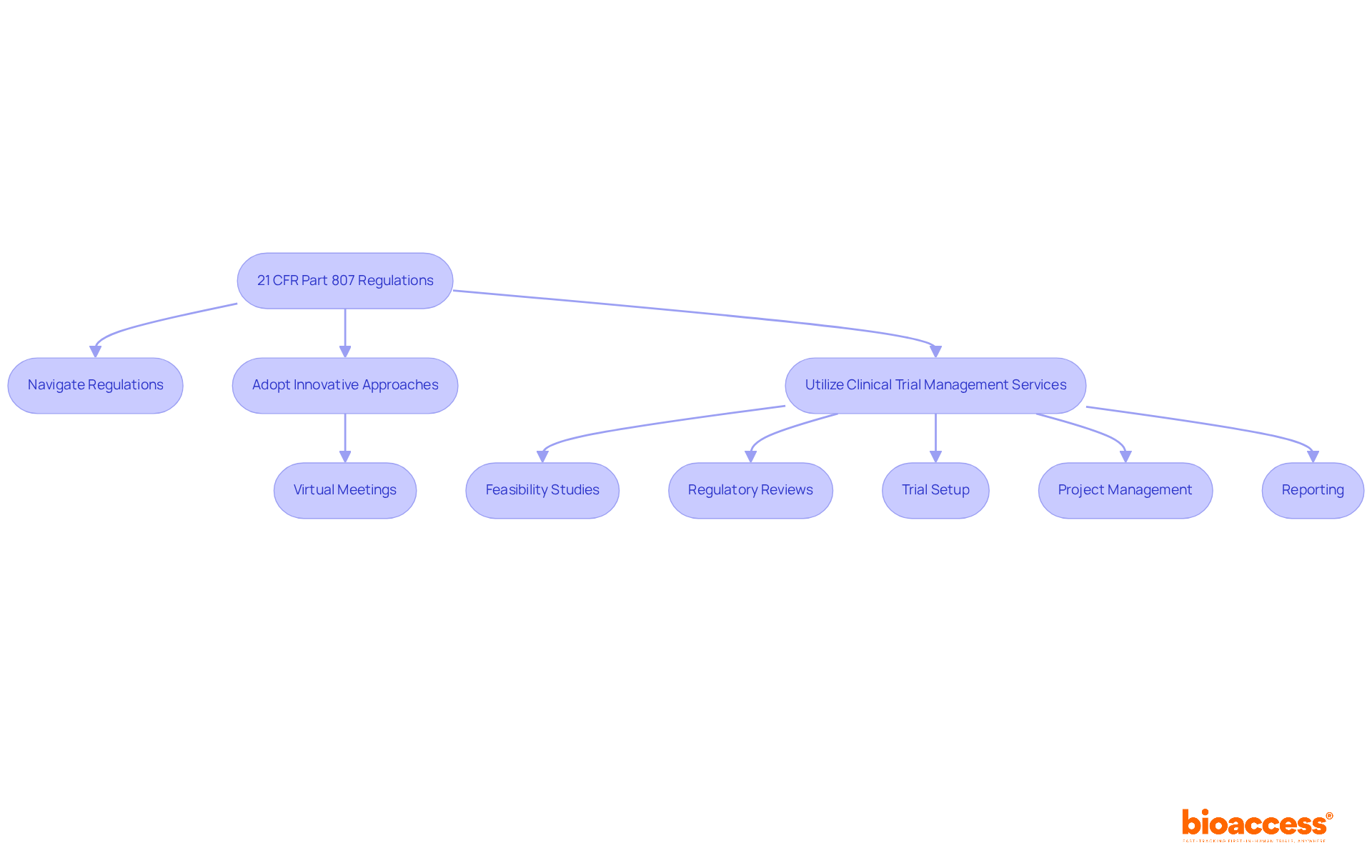
To navigate these complexities, bioaccess offers thorough clinical trial management services that directly address these regulatory requirements. For instance:
Regulatory professionals emphasize that a well-structured compliance strategy not only streamlines processes but also enhances the likelihood of successful market entry. It's also crucial to consider potential conflicts of interest when appointing a distributor or importer as a U.S. agent. Therefore, fostering a culture of adherence to 21 CFR Part 807 is essential for optimizing operational efficiency and achieving favorable outcomes in the U.S. market.
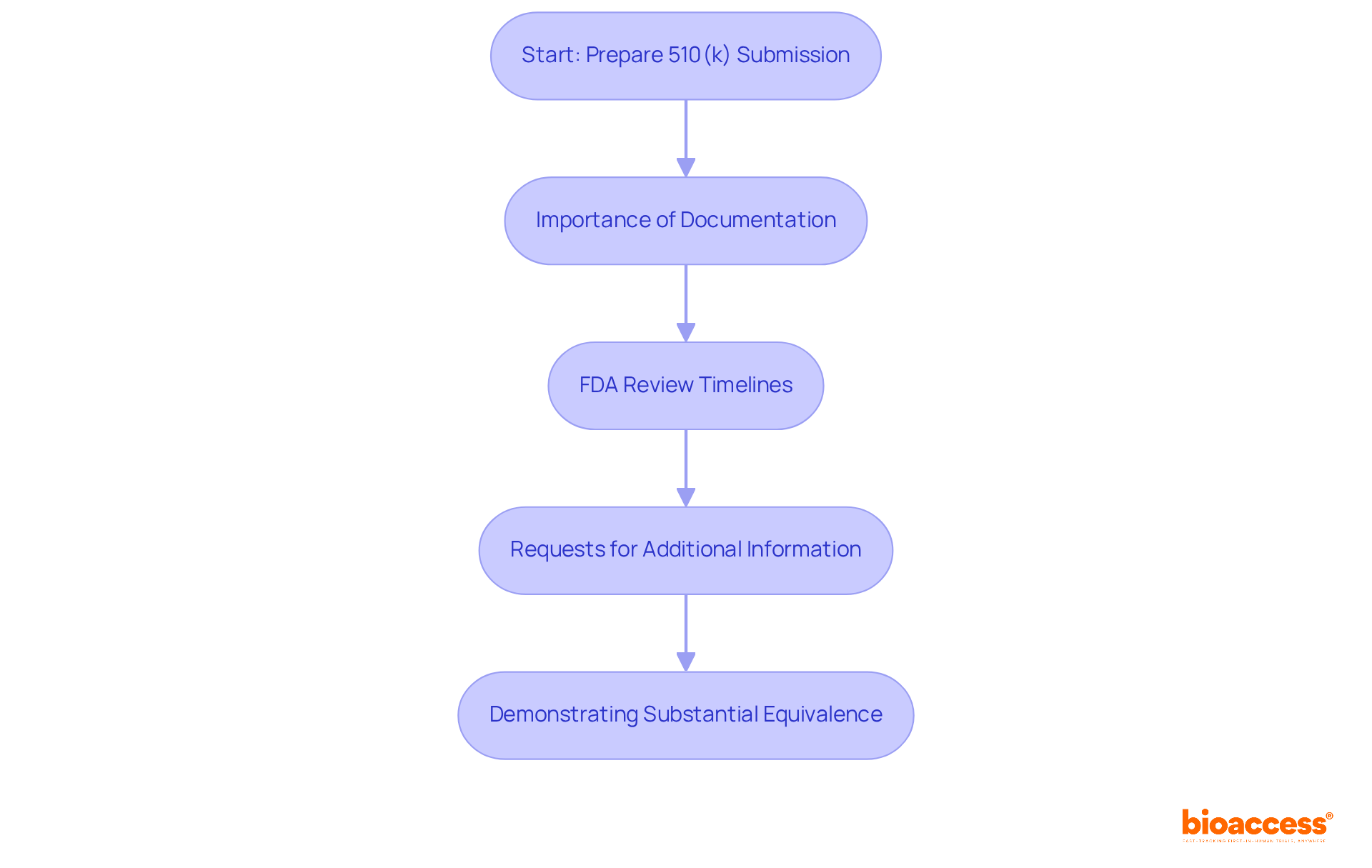
Premarket notifications, commonly referred to as 510(k) submissions, play a vital role for medical products that aren't exempt from premarket review. Under 21 CFR Part 807, it is required for manufacturers to demonstrate that their product is substantially equivalent to an existing legally marketed item. Understanding this process is essential for navigating the complexities of medical product approval.
Importance of Documentation: Thorough documentation is paramount for successful 510(k) submissions. The FDA mandates complete, accurate, and well-organized data to demonstrate substantial equivalence. Incomplete submissions can lead to rejection, resulting in costly delays and the need for resubmissions.
FDA Review Timelines: The FDA strives to review traditional 510(k) submissions within 90 days. However, the average time to receive a decision can extend to approximately five months due to requests for additional information. In 2021, the average review time was reported at 147 days, underscoring the complexities involved in the process.
Requests for Additional Information: A significant portion of submissions—67% in the year ending September 2022—resulted in requests for additional information during the review process. These requests can prolong timelines and increase costs, highlighting the necessity for meticulous preparation.
Demonstrating Substantial Equivalence: Manufacturers must provide evidence that their product is as safe and effective as the predicate product. This includes detailed comparisons of intended use and technological characteristics. The FDA emphasizes that 'substantially equivalent' does not imply identicality, necessitating a careful selection of predicate products to avoid rejection.
Grasping these components is crucial for maneuvering through the 510(k) submission process efficiently, ensuring prompt market entry and compliance with legal standards.
Certain products may qualify for exemptions under 21 CFR Part 807, which significantly reduce regulatory burdens. Class I products, which account for nearly 50% of healthcare instruments in the U.S. market, are often exempt from premarket notification requirements. This exemption enables a more streamlined approval process, making it crucial for clinical research directors to understand the specific criteria involved. By doing so, they can facilitate quicker market entry for qualifying products.
Regulatory experts emphasize that while many Class I products enjoy exemptions, they still require compliance with general controls as specified in 21 CFR Part 807. This includes appropriate labeling and adherence to Good Manufacturing Practices (GMP). Common examples of Class I exempt devices include:
These exemptions not only expedite access to essential medical tools but also encourage innovation within the industry.
The impact of these exemptions on clinical research is profound. By reducing the time and complexity associated with approvals, clinical research directors can focus on advancing their studies and introducing innovative solutions to the market more effectively. Services offered by Bioaccess, such as feasibility studies and compliance reviews, are designed to support this process, ensuring that clinical trials are conducted smoothly and effectively. As the FDA continues to refine its oversight framework, staying informed about these exemptions will empower professionals to navigate the evolving landscape of medical device regulations effectively.

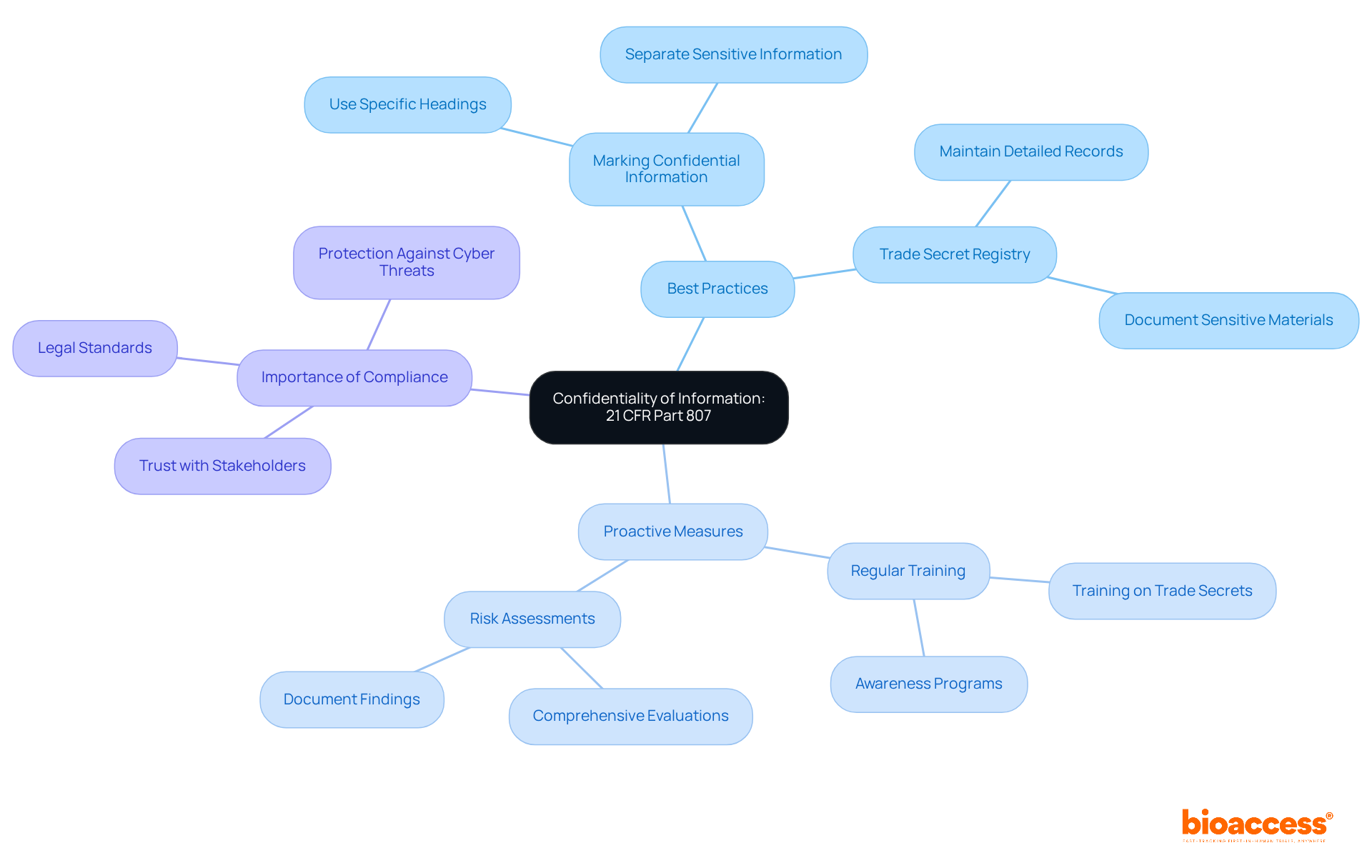
Protecting the confidentiality of information submitted to the FDA is critical for manufacturers under 21 CFR Part 807. Safeguarding proprietary data, including trade secrets and sensitive business information, is essential. Companies should adopt several best practices to achieve this. For instance:
Understanding the FDA's policies on data disclosure is crucial, especially since the agency processes thousands of FOIA requests annually, which can inadvertently expose proprietary information. Compliance experts emphasize that upholding confidentiality not only meets legal standards but also fosters trust with stakeholders. As industry experts point out, proactive measures such as:
are vital in navigating the complexities of FDA submissions.
By embedding these practices into their operational strategies, manufacturers can effectively protect their proprietary data while ensuring compliance with FDA guidelines, specifically 21 CFR Part 807. This approach not only safeguards their interests but also enhances their credibility in the Medtech landscape.
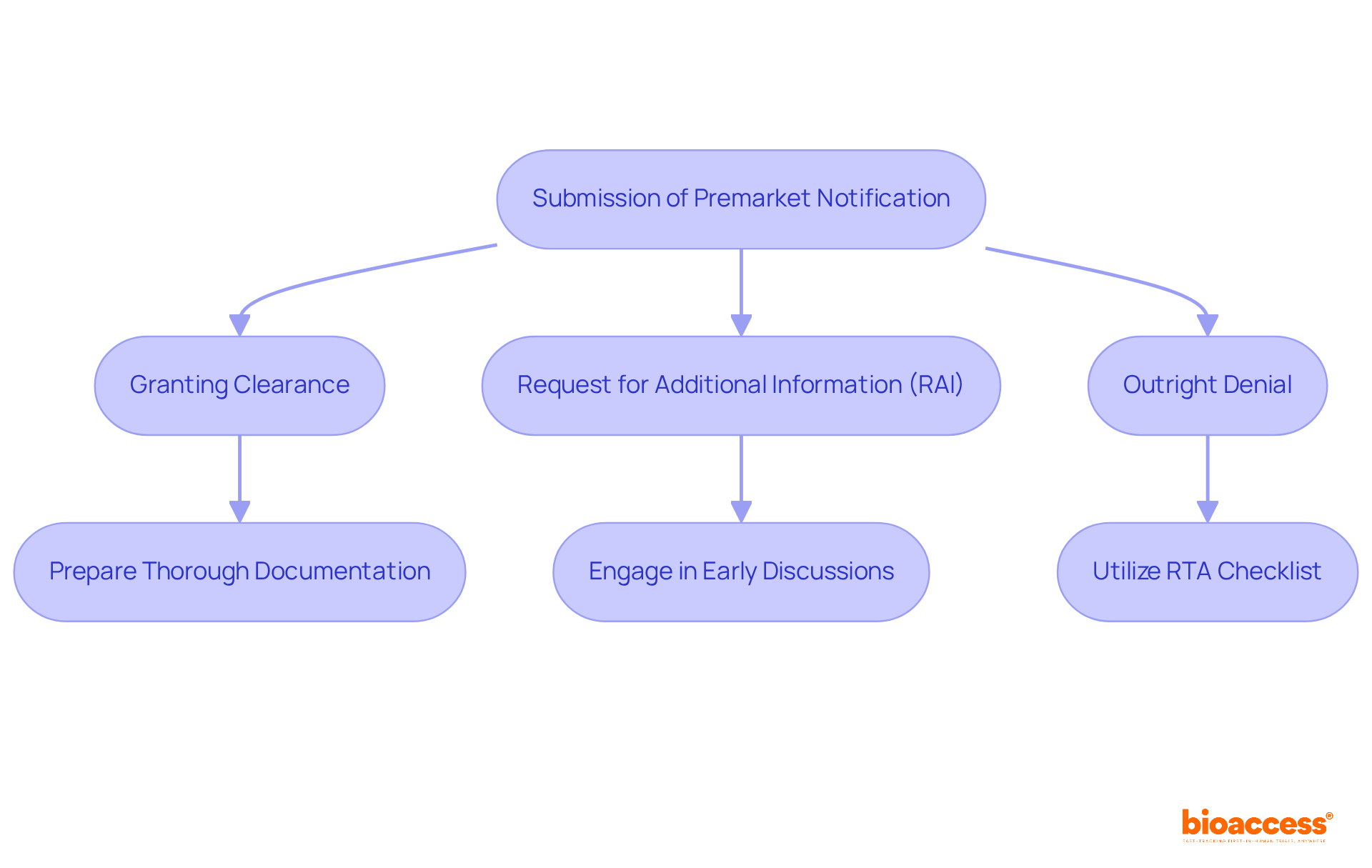
The FDA's actions regarding premarket notifications play a pivotal role in shaping the approval process for medical products, especially given the intricate regulatory landscape that startups face. These actions can encompass:
Clinical research directors must be proactive in preparing for these outcomes, ensuring their submissions are both thorough and meticulously documented. Notably, the average denial rate for 510(k) submissions hovers around 32%, underscoring the critical need for comprehensive preparation to sidestep common pitfalls.
To effectively manage FDA requests for additional information, directors should implement strategies that streamline communication and enhance the clarity of their submissions. Engaging in early discussions with the FDA can yield valuable insights into the specific information required, thereby minimizing the risk of delays. Regulatory experts, such as Ana Criado, Director of Regulatory Affairs at bioaccess®, stress that grasping the implications of FDA responses is essential for clinical research directors, as it empowers them to navigate the complexities of the approval process more adeptly.
For instance, leveraging the FDA's Refuse to Accept (RTA) checklist as a final review tool can ensure that all necessary documentation is included prior to submission, thereby boosting the chances of acceptance. Furthermore, maintaining organized documentation and preparing for potential RAIs can facilitate a smoother response process, ultimately leading to quicker approvals. By applying these strategies, clinical research directors can significantly enhance their organizations' prospects for success in the competitive realm of medical innovation. As a practical tip, directors should routinely review and refine their submission processes to incorporate lessons learned from previous submissions, fostering continuous improvement in their approach.
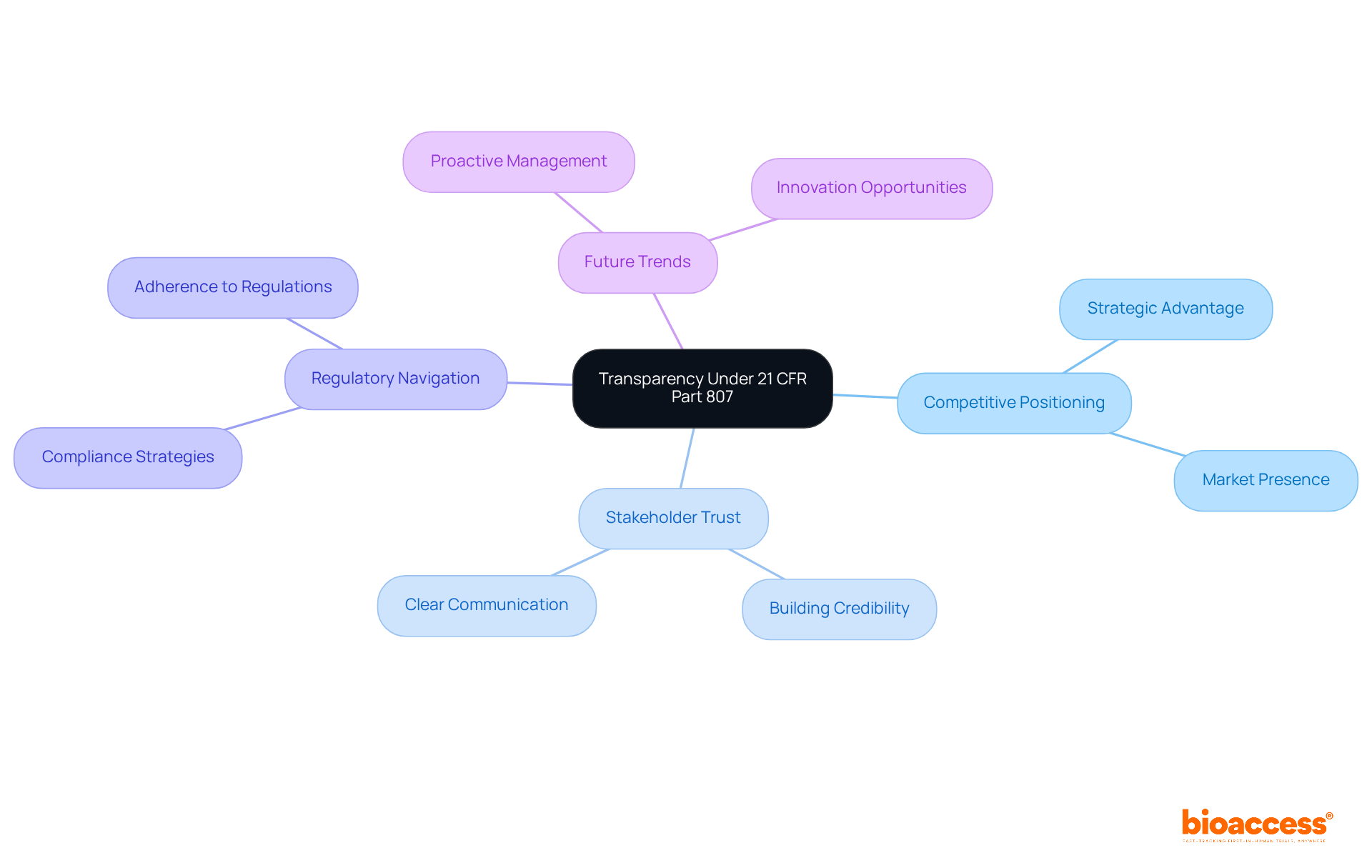
Under 21 CFR Part 807, the registration and device listing information is made publicly accessible, fostering transparency within the medical device industry. This transparency significantly influences competitive positioning and stakeholder trust. Companies that prioritize transparent registration practices gain a strategic advantage, building credibility and fostering stronger relationships with regulators and the public. For instance, organizations that maintain clear communication about their adherence efforts are better equipped to navigate regulatory environments and enhance their market presence.
As we look ahead to 2025, the focus on transparency is likely to intensify, with industry leaders advocating for proactive management of public information to uphold a positive reputation and ensure compliance. Experts emphasize that viewing transparency as a strategic component can transform potential challenges into opportunities for innovation and growth. This approach not only strengthens trust but also positions companies to thrive in an evolving landscape.
The regulations outlined in 21 CFR Part 807 are pivotal in shaping clinical research practices, offering a structured framework for device registration, listing, and premarket notifications. Clinical research directors must adeptly navigate these regulations to ensure compliance while pursuing their research objectives. By understanding the nuances of these regulations, directors can align their strategies effectively, enhancing the efficiency and success rates of their clinical trials.
Current trends indicate a growing emphasis on governance flexibility, with directors increasingly adopting innovative approaches to streamline adherence procedures. For example, many are utilizing virtual meetings to expedite protocol reviews, significantly reducing timelines. As one clinical research director noted, aligning our strategies with 21 CFR Part 807 not only ensures compliance but also speeds up our capability to bring innovative solutions to the market. Research shows that organizations prioritizing compliance comprehension can reduce their time to market by as much as 30%.
At bioaccess, we offer comprehensive clinical trial management services, including:
Our goal is to assist directors in navigating these complex regulations. Our team, featuring specialists like Ana Criado and Katherine Ruiz, brings extensive expertise in compliance matters, ensuring our services not only support adherence to 21 CFR Part 807 but also enhance the overall success of clinical trials. By prioritizing regulatory understanding, directors can position their organizations for success in an evolving clinical landscape.
Navigating the complexities of 21 CFR Part 807 is crucial for clinical research directors who seek to ensure compliance and enhance their organizations' operational efficiency. This regulatory framework lays out essential guidelines for device registration, listing, and premarket notifications, all vital for advancing medical innovations and expediting the clinical trial process.
Key insights throughout this article underscore the importance of:
Directors must also recognize exemptions that can streamline the approval process and the significance of safeguarding confidential information in line with regulatory standards. These insights highlight the need for a proactive approach to regulatory adherence, emphasizing that a thorough understanding of these regulations can significantly reduce time to market and boost success rates in clinical trials.
As the landscape of medical device regulations evolves, it is imperative for clinical research directors to stay informed and adaptable. By prioritizing compliance strategies and leveraging resources like bioaccess, organizations can navigate the complexities of 21 CFR Part 807 while enhancing their credibility and fostering innovation in the medical field. Embracing this regulatory framework ultimately empowers directors to propel their clinical research efforts forward, ensuring they effectively meet both current and future challenges.
What is bioaccess® and how does it assist clinical research directors?
Bioaccess® helps clinical research directors achieve compliance with 21 CFR Part 807 by offering tailored solutions that meet compliance standards, thereby accelerating the clinical trial process and boosting the success rates of medical innovations.
How quickly can ethical approvals be obtained through bioaccess®?
Ethical approvals can be obtained in just 4-6 weeks with the help of bioaccess®, enabling organizations to initiate studies more swiftly.
What are the key requirements for medical equipment establishments under 21 CFR Part 807?
Medical equipment establishments must register with the FDA, providing essential information such as the establishment's name, address, and a comprehensive list of items produced. This registration must be updated annually and whenever there are operational changes.
What are the consequences of non-compliance with 21 CFR Part 807 registration requirements?
Non-compliance can lead to penalties and hinder the legal promotion of products, affecting an organization's market presence.
Why is a robust quality management system (QMS) important for medical equipment companies?
A robust QMS is crucial as it helps navigate the registration process under 21 CFR Part 807, ensuring that all necessary documentation is meticulously maintained for smoother registration experiences.
What best practices should be followed for updating device listings under 21 CFR Part 807?
It is important to promptly update device listings whenever there are changes in specifications or manufacturing processes. Establishing a routine review process and utilizing the FDA's electronic registration system can enhance accuracy.
How often should organizations review equipment listings for compliance?
Organizations should ideally schedule regular evaluations of equipment listings every three months to ensure ongoing precision and compliance with legal obligations.
What is the significance of educating team members about device listings?
Educating team members on the importance of maintaining precise listings fosters a culture of adherence and vigilance, which mitigates the risk of non-compliance and streamlines approval processes.