


The article titled "10 Key Insights on Annual Report Guidance from the FDA" serves as a crucial resource for organizations in the Medtech and Biopharma industries. It underscores the significance of adhering to the FDA's annual report guidance, which is essential for ensuring regulatory compliance, enhancing transparency, and ultimately improving public health outcomes. This guidance outlines critical areas that organizations must prioritize, including:
to maintain credibility and facilitate timely approvals.
In the ever-evolving landscape of Medtech, understanding these insights is vital. Organizations that align their practices with the FDA's guidance not only navigate regulatory challenges more effectively but also position themselves as leaders in their fields. By prioritizing these components, they can foster trust with stakeholders and the public, ultimately driving better health outcomes.
Collaboration is key in this endeavor. As organizations work together to implement these insights, they can share best practices and learn from one another's experiences. This collective effort not only enhances compliance but also contributes to the overall advancement of the industry.
In conclusion, the importance of the FDA's annual report guidance cannot be overstated. Organizations must take proactive steps to integrate these insights into their operations, ensuring they remain at the forefront of regulatory compliance and public health improvement.
In the complex world of Medtech and Biopharma, following FDA annual report guidance is not just a regulatory obligation; it’s a vital element that upholds the integrity and transparency of clinical research. Organizations can gain a competitive advantage by grasping the key components outlined in the FDA's guidance for 2024, which aims to improve compliance and public health outcomes. Yet, as the regulatory landscape evolves, how can companies adeptly navigate the challenges these requirements present to secure timely approvals and achieve successful product commercialization?
bioaccess® harnesses its extensive experience in navigating FDA regulations to streamline clinical research processes. By ensuring strict adherence to FDA guidelines, bioaccess® accelerates the approval and enrollment stages, allowing Medtech and Biopharma innovators to bring their offerings to market more swiftly. Our comprehensive services encompass:
All of which enhance the integrity of clinical trials. This unwavering commitment to regulatory compliance not only fosters trust with stakeholders and regulatory bodies but also establishes bioaccess® as the premier CRO in Latin America for expedited clinical research services.
In the rapidly evolving Medtech landscape, the challenges of clinical research can be daunting. However, bioaccess® stands ready to address these hurdles, providing tailored solutions that meet the unique needs of each client. By collaborating with us, innovators can navigate the complexities of clinical trials with confidence, knowing they have a partner dedicated to their success.
As we look to the future, the importance of collaboration in clinical research cannot be overstated. Together, we can drive innovation and improve patient outcomes. Reach out to bioaccess® today to explore how we can support your clinical research endeavors.
The annual report guidance FDA for 2024 outlines several critical components that organizations must address to ensure compliance and effective communication with the agency. This guidance is essential for maintaining the integrity of clinical research and enhancing public health outcomes.
These components are not only fundamental for compliance but also play a significant role in enhancing public health outcomes by ensuring that critical information is readily accessible to the FDA, in accordance with the annual report guidance FDA. By addressing these areas, organizations can contribute to a more transparent and effective clinical research environment.
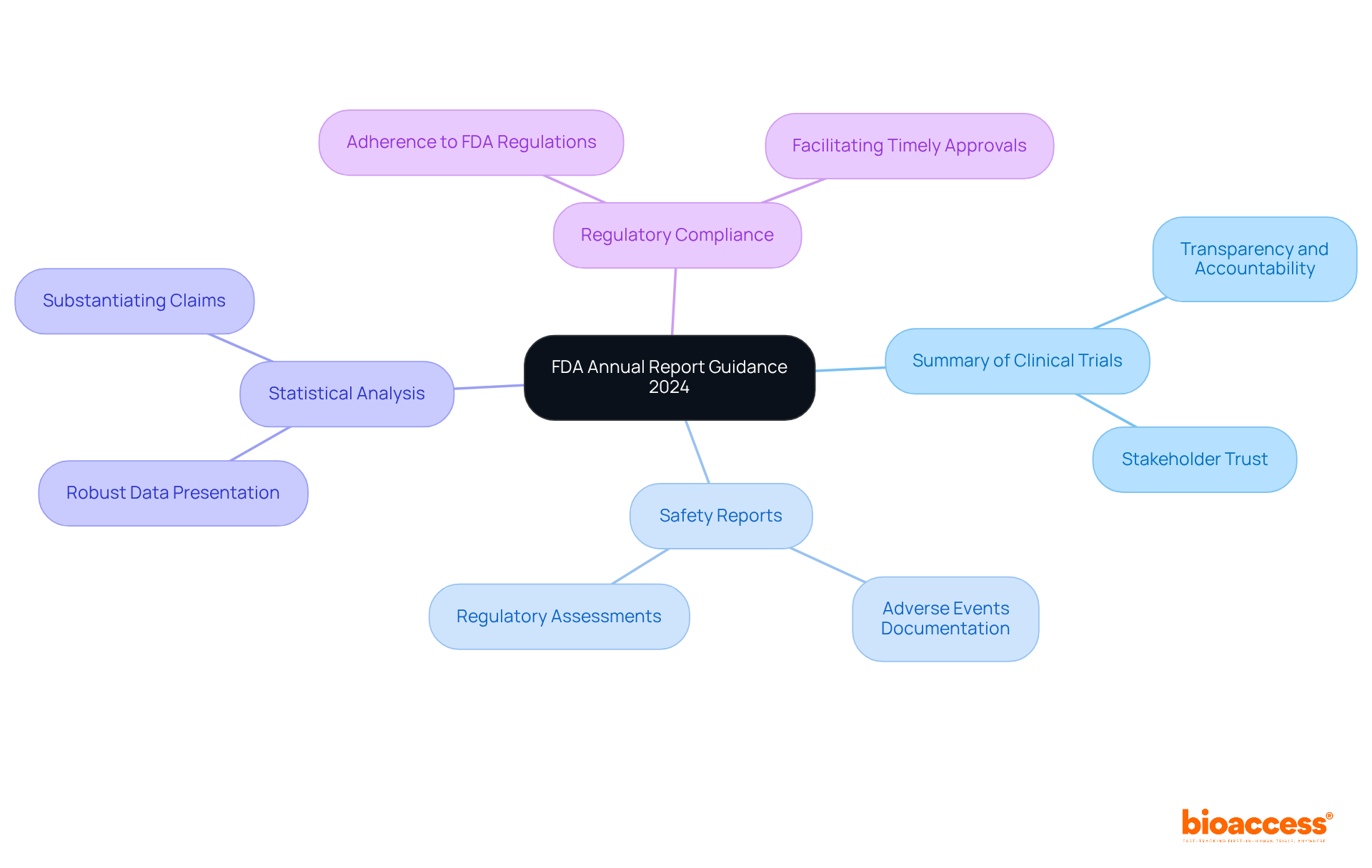
The implications of the annual report guidance FDA on the Medtech and Biopharma industries are significant and multifaceted. Businesses must navigate an evolving regulatory environment that directly affects development timelines and market strategies. Key implications include:
In this context, partnering with bioaccess® can significantly enhance your clinical trial management capabilities. With over 20 years of experience in Medtech, bioaccess® specializes in managing a range of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Their customized method and adaptability, along with extensive services like feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, and reporting, are intended to assist companies in managing the intricacies of FDA guidance efficiently. These factors collectively shape the strategic direction of companies within these sectors, compelling them to align their development processes with the FDA's annual report guidance.

Organizations face significant challenges in adhering to the annual report guidance set by the FDA, particularly in data management. The sheer volume of data generated during larger clinical trials can be overwhelming, complicating collection and management processes. Additionally, resource distribution is crucial; ensuring adequate resources for compliance can strain budgets and timelines, potentially jeopardizing overall project success.
Regulatory changes present another obstacle, as the FDA frequently updates its guidance. This requires ongoing training and adaptation within organizations to maintain compliance. Moreover, effective interdepartmental coordination is essential. Communication between clinical, regulatory, and quality assurance teams is vital, yet achieving this synergy can be difficult.
To navigate these complexities, organizations must engage in strategic planning and invest in a robust regulatory framework. Implementing tailored data management solutions in line with annual report guidance FDA compliance can streamline processes, enhance data integrity, and ultimately facilitate adherence to regulatory requirements. By prioritizing these strategies, organizations can better position themselves for success in the evolving landscape of clinical research.

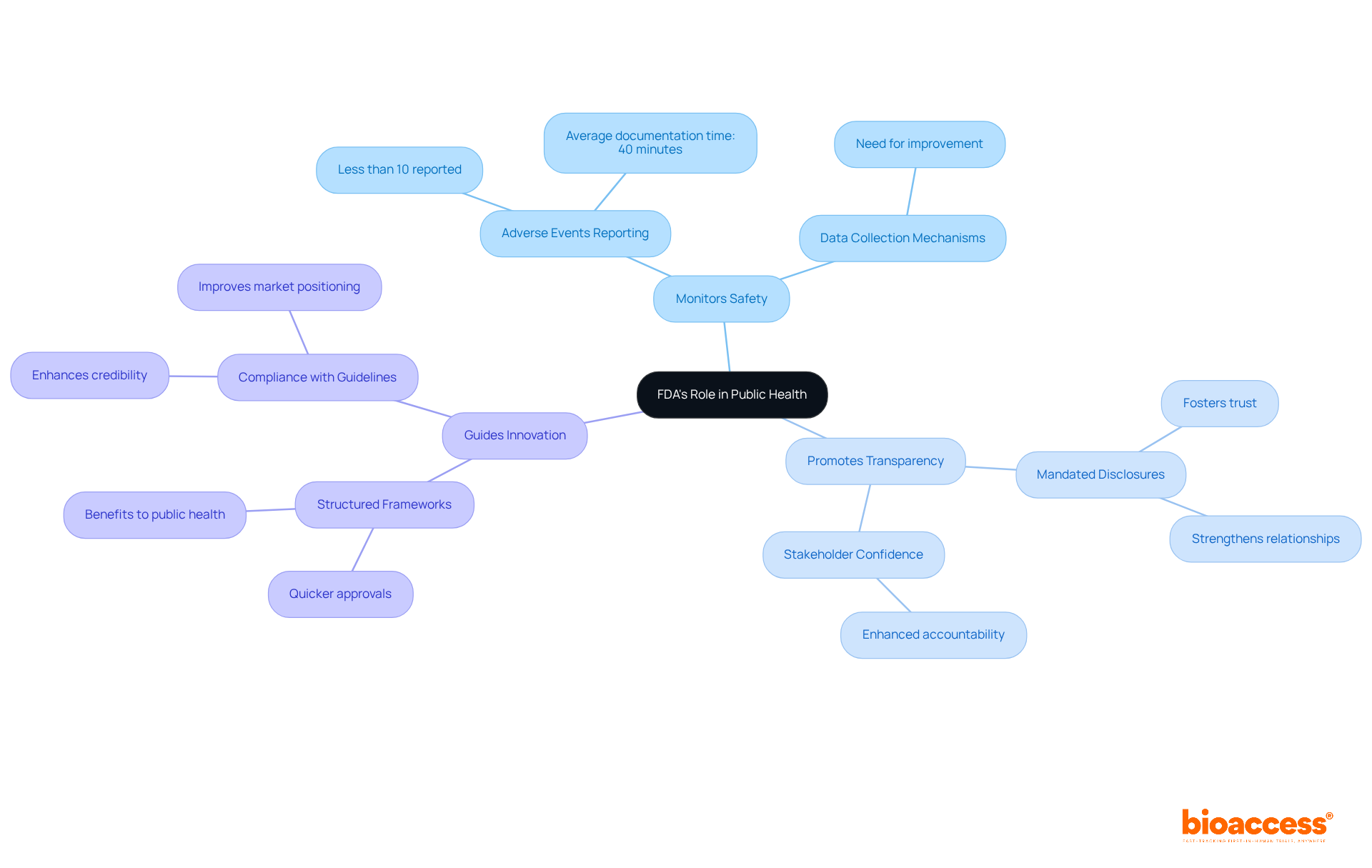
The FDA plays a crucial role in safeguarding public well-being by ensuring the safety and effectiveness of medical products. This regulatory body is essential for maintaining trust in healthcare systems and promoting public health initiatives.
Monitors Safety: The FDA systematically collects data on adverse events, which is vital for identifying potential safety issues. Alarmingly, statistics show that less than 10% of significant adverse events are reported by healthcare professionals. This highlights the urgent need for improved data collection mechanisms to enhance patient safety.
Promotes Transparency: The FDA mandates that companies disclose extensive information about their products, fostering trust within the healthcare system. This transparency is crucial for boosting stakeholder confidence and strengthening relationships with regulatory authorities.
Guides Innovation: By providing a structured framework for developing new therapies, the FDA prioritizes patient safety. Companies that adhere to these guidelines not only enhance their credibility but also position themselves for quicker approvals, ultimately benefiting public health outcomes.
This regulatory oversight is vital for sustaining public trust in medical goods and advancing public health efforts, as it cultivates a culture of adherence and responsibility within the sector.
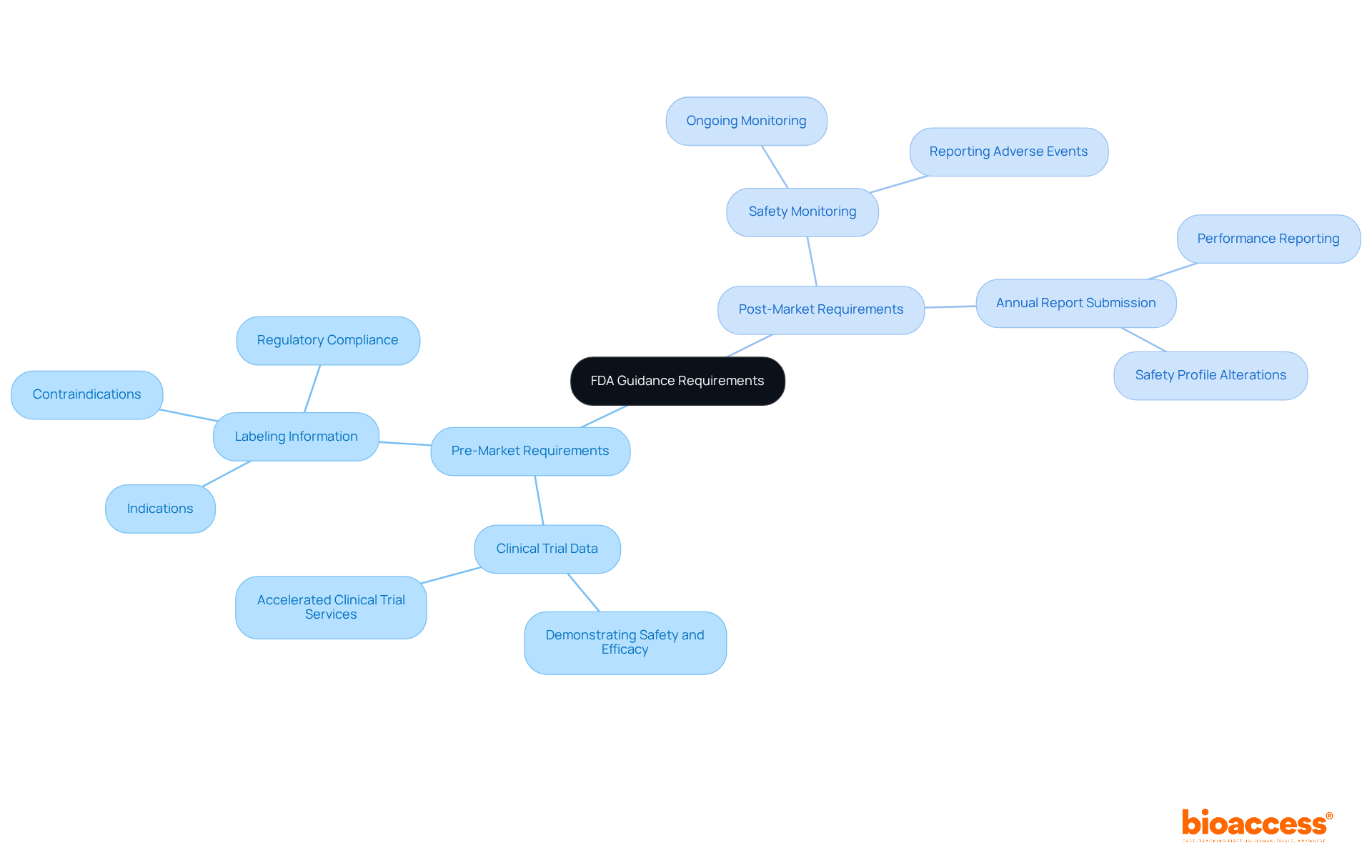
FDA guidance outlines essential information requirements for both pre-market and post-market phases, which bioaccess® expertly facilitates for Medtech, Biopharma, and Radiopharma startups in Latin America and beyond.
Pre-Market Requirements:
Post-Market Requirements:
These requirements are crucial for ensuring that products remain safe and effective throughout their lifecycle. Bioaccess® is dedicated to helping clients navigate these complexities efficiently, reinforcing the importance of collaboration in clinical research.
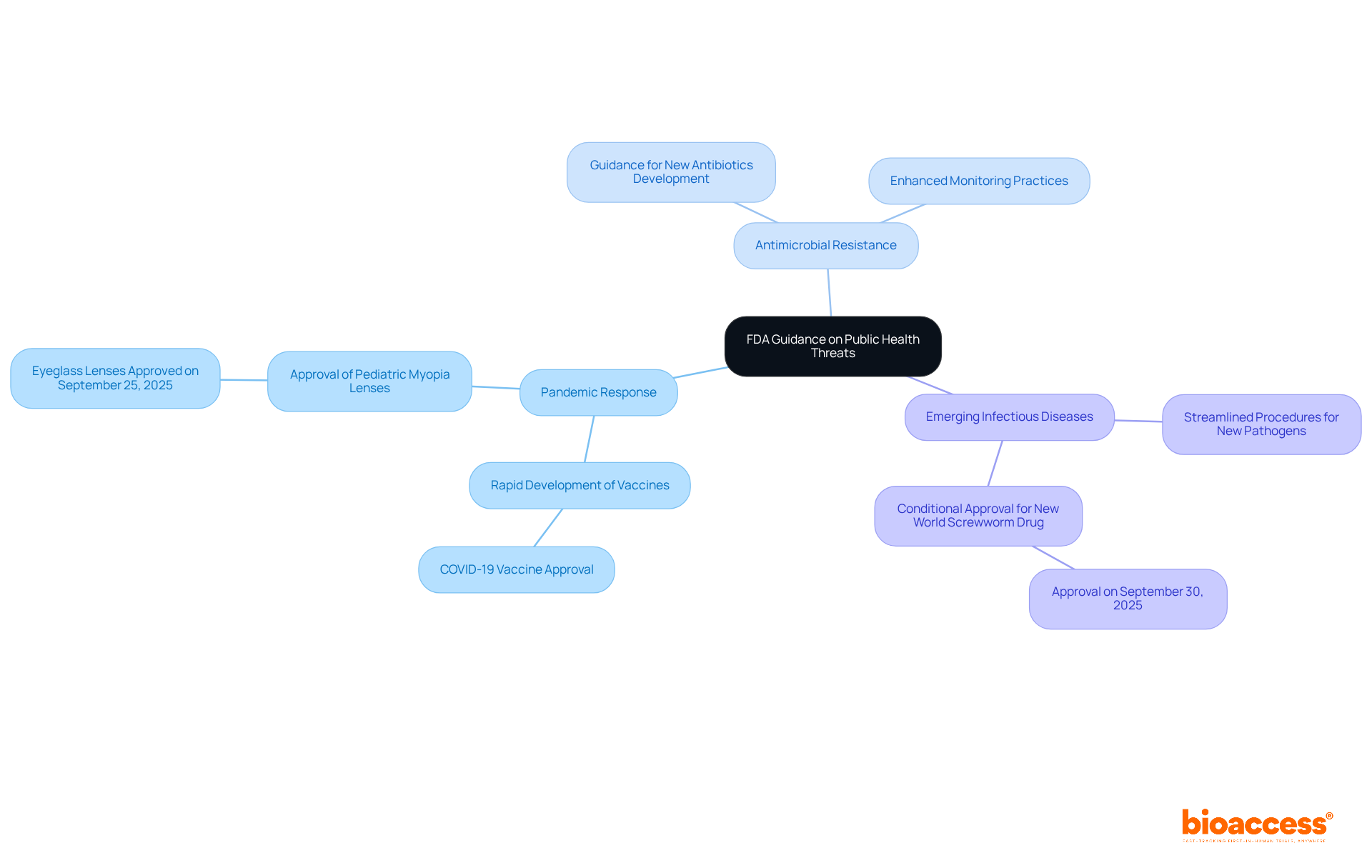
The FDA continually evolves its guidance to effectively tackle emerging public health threats, focusing on several key areas:
Pandemic Response: The agency has demonstrated remarkable agility in the rapid development and approval of vaccines and therapeutics during health crises, significantly shortening the time from research to market. For example, the rapid approval of COVID-19 vaccines illustrates this dedication to public well-being. Furthermore, on September 25, 2025, the FDA approved the sale of the first eyeglass lenses created to slow the advancement of pediatric myopia, highlighting its continuous efforts to tackle various medical issues.
Antimicrobial Resistance: In response to the growing concern of antimicrobial resistance, the FDA has issued guidance aimed at fostering the development of new antibiotics and enhancing monitoring practices. This initiative is crucial for ensuring that effective treatments remain available as resistance patterns evolve.
Emerging Infectious Diseases: The FDA has established streamlined procedures for items targeting newly identified pathogens, ensuring that innovative solutions can be quickly deployed in response to outbreaks. For instance, the conditional endorsement of the initial medication for the prevention and management of New World Screwworm infestations in cattle on September 30, 2025, emphasizes the FDA's proactive strategy for tackling particular wellness threats.
These proactive strategies highlight the FDA's commitment to public welfare, enabling timely access to essential medical products and strengthening its role as a vital participant in protecting well-being during crises. As FDA Commissioner Marty Makary stated on September 25, 2025, "Encouraging retailers to stop selling illegal vapes" reflects the agency's commitment to addressing emerging public welfare threats comprehensively. Moreover, the FDA's reaction to evidence indicating a potential link between acetaminophen use during pregnancy and autism demonstrates its involvement with important public welfare issues. Overall, these measures ensure that the FDA remains attentive to public health requirements, facilitating timely access to essential medical items.
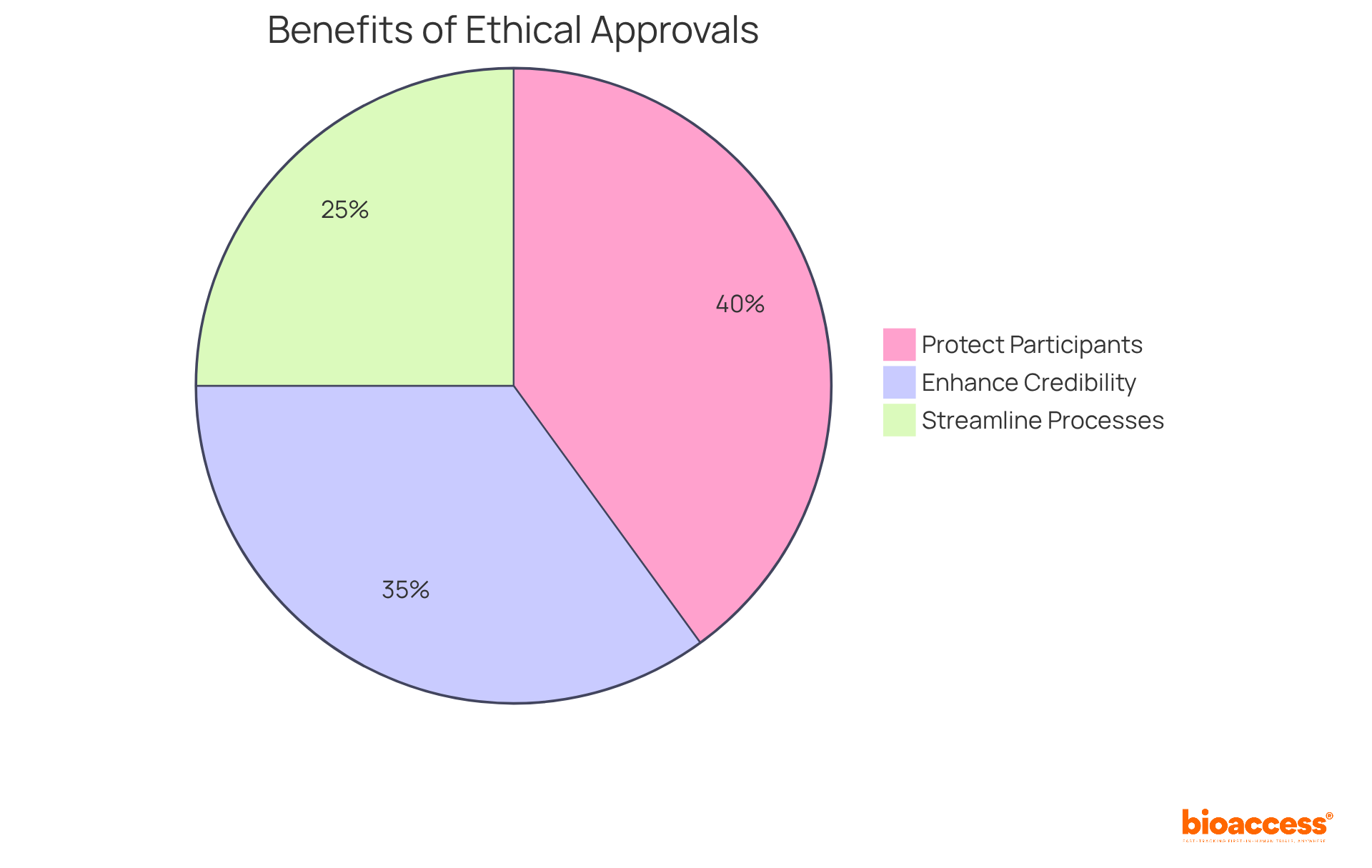
Ethical approvals are essential for accelerating clinical trials. They protect participants by ensuring that the rights and welfare of trial participants are prioritized. Furthermore, they enhance credibility; ethical oversight builds trust with stakeholders and the public, facilitating smoother trial operations. Additionally, they streamline processes; efficient ethical review processes can significantly reduce timelines for trial initiation. By prioritizing ethical considerations, organizations can enhance the overall quality and acceptance of their clinical research.
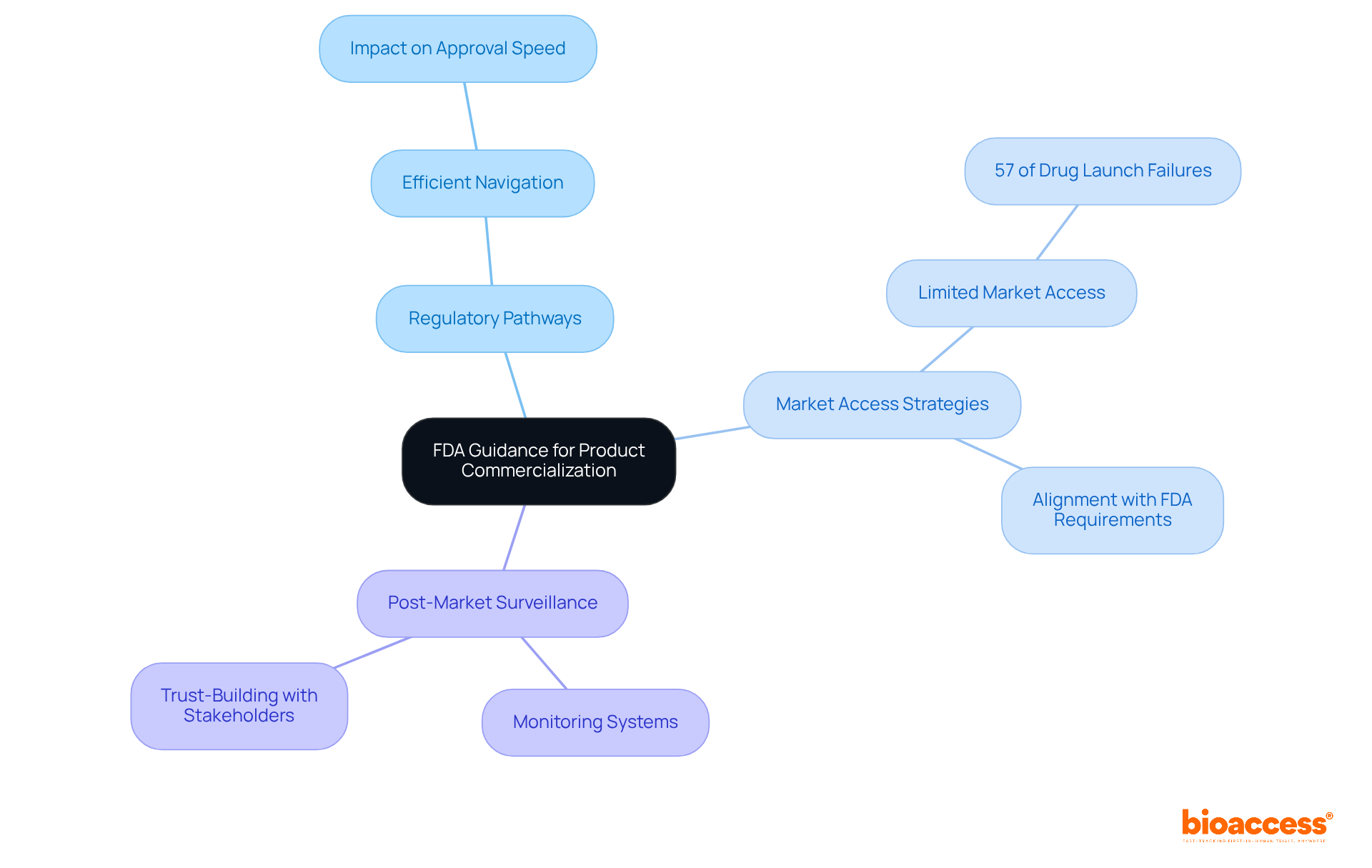
Successful product commercialization hinges on a thorough understanding of FDA guidance, which includes several critical elements:
By aligning commercialization strategies with the annual report guidance FDA, companies can greatly enhance their chances of success in a competitive marketplace. Effective stakeholder engagement, especially with payers, should commence 18-24 months before product launch to refine value messaging and improve market access outcomes. This strategic foresight is essential, as 34% of drugs launched between 2012 and 2021 failed to meet their launch forecasts, often due to a lack of understanding of market and customer needs. Therefore, a rigorous approach to developing and evolving market access strategies is paramount for achieving sustainable success.
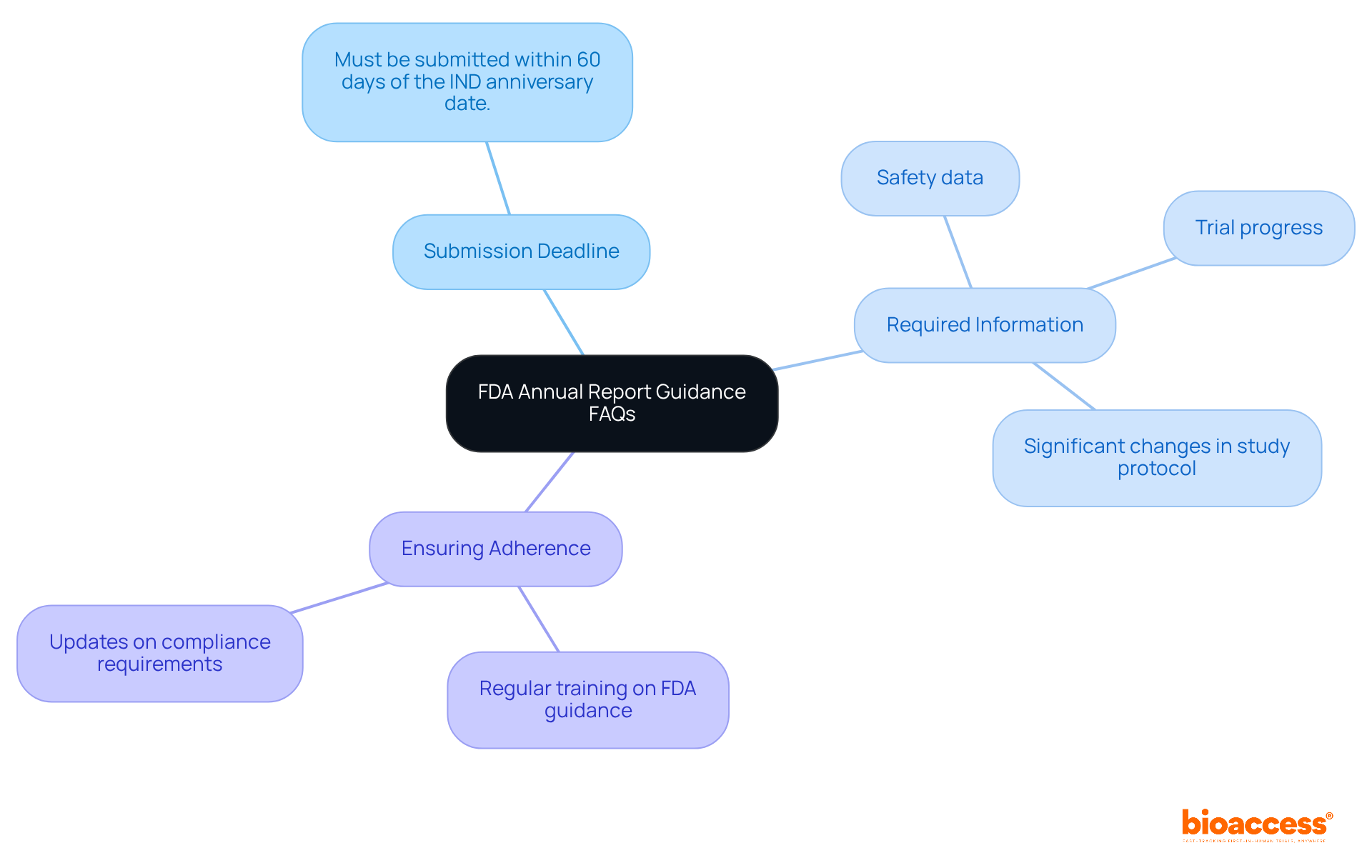
Common questions regarding FDA annual report guidance include:
By addressing these FAQs, organizations can better navigate the complexities of FDA reporting requirements.
The annual report guidance from the FDA is essential for organizations striving to ensure compliance and enhance communication within the clinical research landscape. By following these guidelines, companies not only uphold regulatory integrity but also play a vital role in improving public health outcomes through transparency and accountability in their clinical trials.
Key insights from the guidance underscore the necessity of comprehensive documentation, including:
These components are crucial for navigating the intricate regulatory environment, speeding up product approvals, and fostering innovation in the Medtech and Biopharma sectors. As organizations grapple with challenges in data management and regulatory compliance, strategic planning and interdepartmental coordination become indispensable for success.
Ultimately, the FDA's guidance acts as a pivotal framework that safeguards public health while driving innovation in medical products. By adopting these guidelines, companies can boost their operational efficiencies and ensure timely access to life-saving therapies. Collaborating with experienced partners, such as bioaccess®, can further streamline compliance and accelerate the journey from research to market, reinforcing the essential role of collaboration in advancing healthcare solutions.
What is bioaccess® and what services does it provide?
bioaccess® is a clinical research organization (CRO) that specializes in navigating FDA regulations to streamline clinical research processes. Its services include feasibility assessments, investigator selection, and meticulous project management, all aimed at enhancing the integrity of clinical trials.
How does bioaccess® help accelerate clinical research?
By ensuring strict adherence to FDA guidelines, bioaccess® accelerates the approval and enrollment stages of clinical research, allowing Medtech and Biopharma innovators to bring their products to market more swiftly.
Why is regulatory compliance important in clinical research?
Regulatory compliance is crucial for fostering trust with stakeholders and regulatory bodies, maintaining the integrity of clinical trials, and facilitating timely approvals, which ultimately enhances public health outcomes.
What are the key components of the FDA annual report guidance for 2024?
The key components include a summary of clinical trials, safety reports detailing adverse events, robust statistical analysis to support efficacy and safety claims, and comprehensive documentation of regulatory compliance throughout the trial process.
How does the annual report guidance impact the Medtech and Biopharma industries?
The guidance leads to increased transparency, faster product approvals, and a pressure for innovation while ensuring adherence to regulations, which affects development timelines and market strategies.
What types of studies does bioaccess® manage?
bioaccess® manages a range of studies including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
How can partnering with bioaccess® benefit clinical trial management?
Partnering with bioaccess® provides access to over 20 years of experience in Medtech, along with customized methods, adaptability, and extensive services to efficiently manage the complexities of FDA guidance in clinical trials.