


This article presents crucial insights that drive the success of aseptic fill-finish manufacturing, underscoring the necessity of sustaining a sterile environment, ensuring regulatory compliance, and leveraging advanced technologies. The significance of these insights is backed by evidence that illustrates the essential role of cleanroom practices, quality control measures, and innovations in automation. Collectively, these elements enhance operational efficiency and product safety within the pharmaceutical sector, highlighting the critical nature of collaboration in addressing industry challenges.
The aseptic fill-finish manufacturing landscape is rapidly evolving, driven by the increasing demand for biologics and the necessity for stringent quality control measures. As the industry faces mounting challenges—ranging from regulatory compliance to contamination risks—understanding the key principles and innovations in this field becomes crucial for success.
What strategies can organizations implement to navigate these complexities and ensure the safety and efficacy of their products? This article delves into ten essential insights that illuminate the path to achieving excellence in aseptic fill-finish manufacturing.
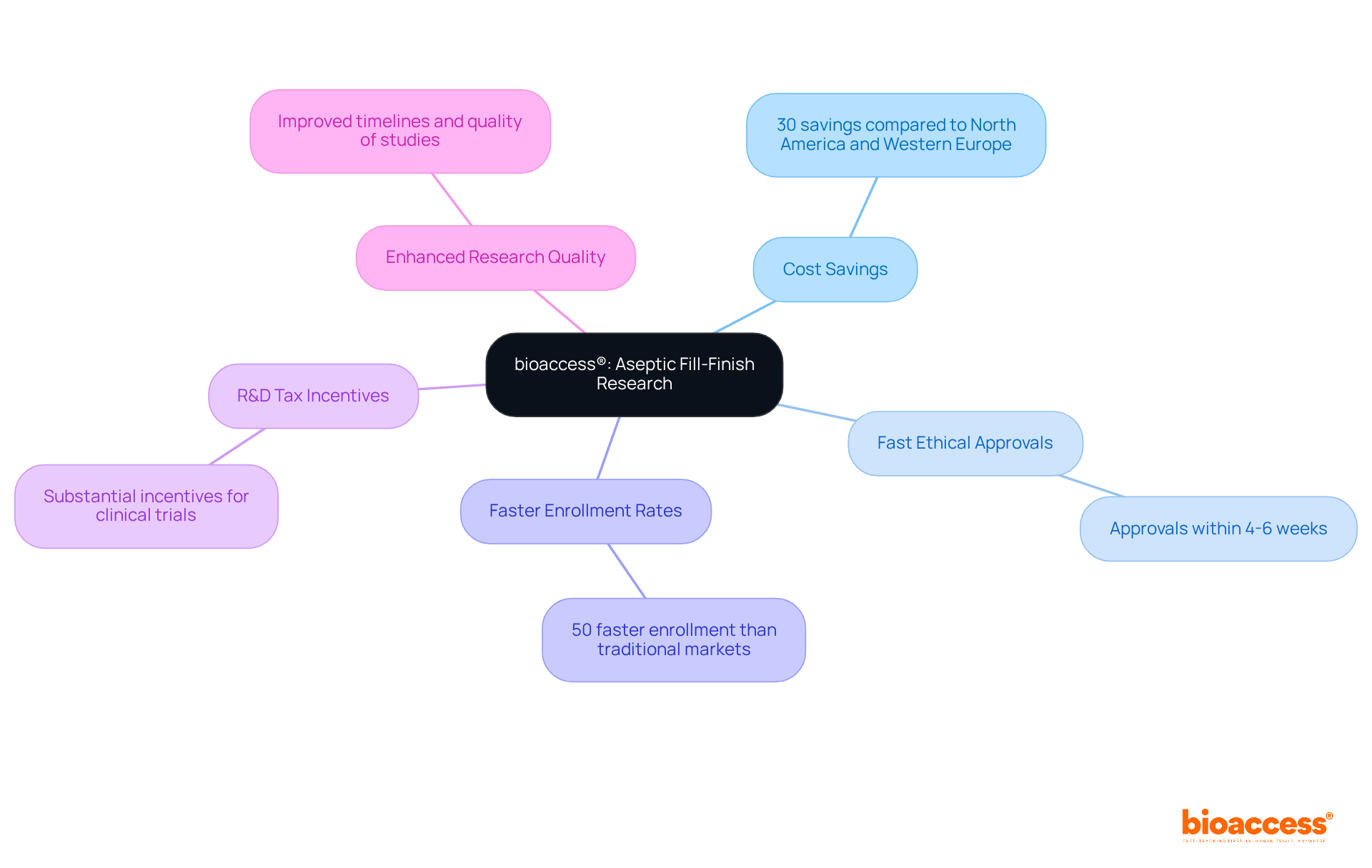
bioaccess® is at the forefront of aseptic fill finish packaging research in Latin America, leveraging Colombia's competitive advantages to facilitate rapid clinical trials.
With cost savings exceeding 30% compared to North America and Western Europe, bioaccess® accelerates ethical approvals within just 4-6 weeks, significantly enhancing regulatory efficiency.
The company boasts enrollment rates that are 50% faster than those in traditional markets, supported by a population of over 50 million, with 95% of individuals covered by universal healthcare.
Furthermore, Colombia presents substantial R&D tax incentives, amplifying its attractiveness for clinical trials.
This strategic positioning not only expedites research timelines but also elevates the overall quality of clinical studies conducted in the region, empowering Medtech, Biopharma, and Radiopharma innovators to achieve aseptic fill finish for their solutions and bring them to market with greater efficiency.
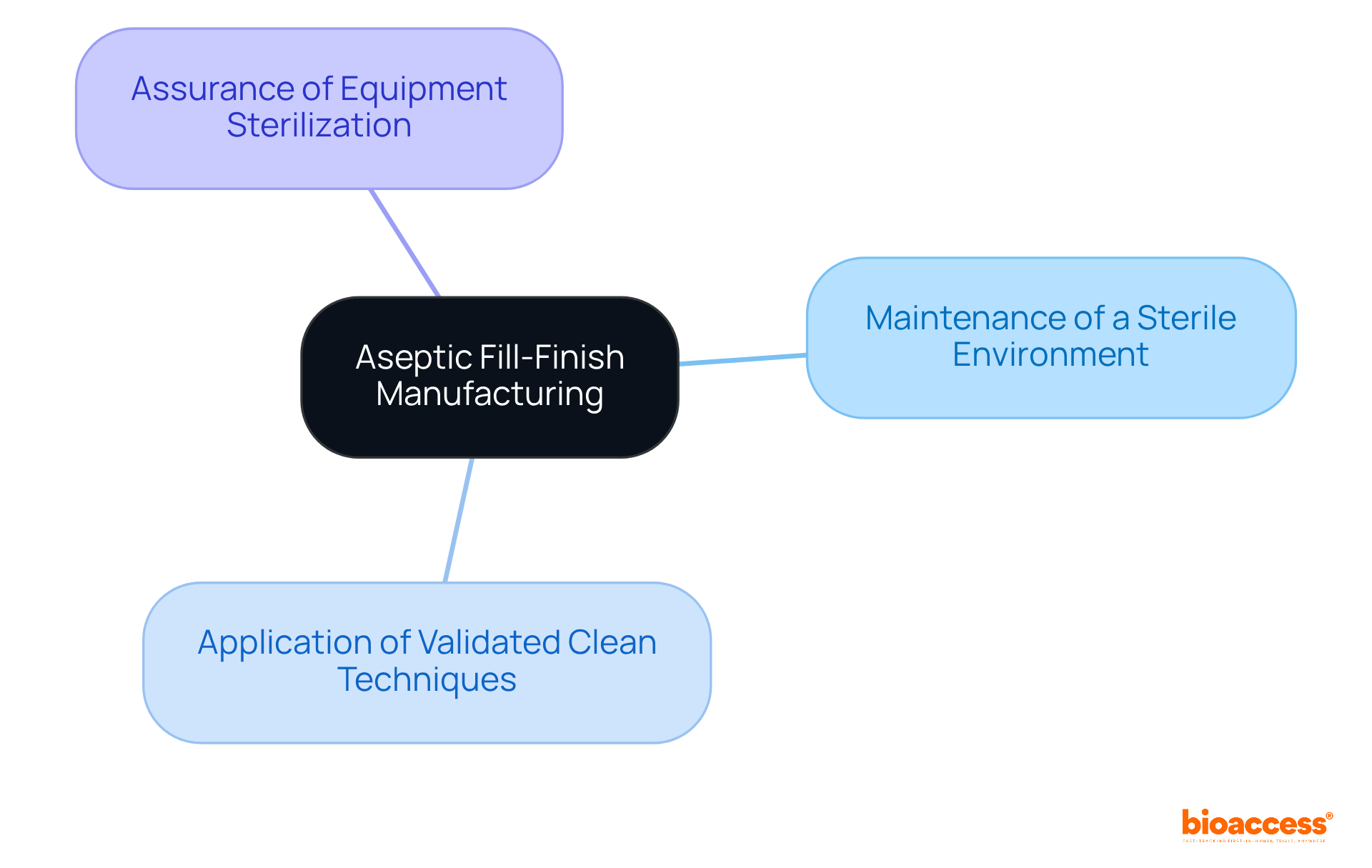
Aseptic fill finish manufacturing serves as a cornerstone operation within the pharmaceutical sector, focusing on the sterile filling of medications into containers under meticulously regulated conditions. This process is underpinned by key principles, including:
The primary objective of this procedure is to prevent contamination during the aseptic fill finish, which is essential for safeguarding the safety and efficacy of the final product—an aspect that is paramount for patient health.
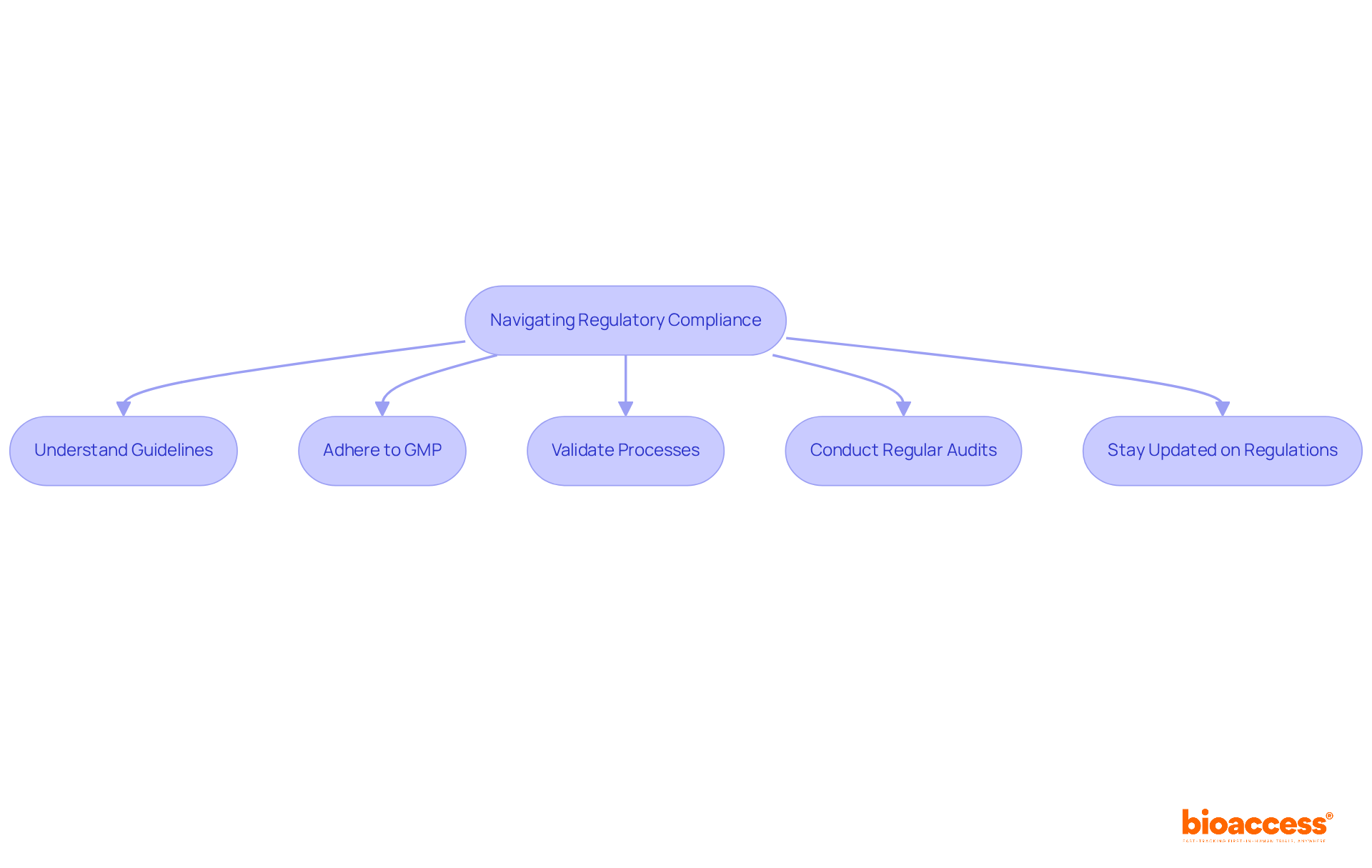
Navigating regulatory compliance in aseptic fill finish operations requires a comprehensive understanding of the guidelines established by regulatory bodies such as the FDA and EMA. Adherence to Good Manufacturing Practices (GMP) is essential, necessitating companies to validate all processes meticulously. Regular audits and inspections are critical in maintaining compliance, helping to avert costly delays in product approvals. Furthermore, staying updated on evolving regulations is vital for upholding operational integrity and ensuring patient safety.
Specialists like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, underscore the significance of regulatory understanding in the aseptic fill finish of the sterile production process, particularly concerning biologics and pharmaceuticals. As the sterile packaging market continues to evolve, businesses must prioritize regulatory adherence to foster trust and reliability in their manufacturing processes.
With projections indicating an increase in the sterile packaging market from USD 7.5 billion in 2025 to USD 13.3 billion by 2035, the importance of compliance becomes increasingly evident. Successful case studies further illustrate the critical role of robust compliance strategies in achieving operational success.
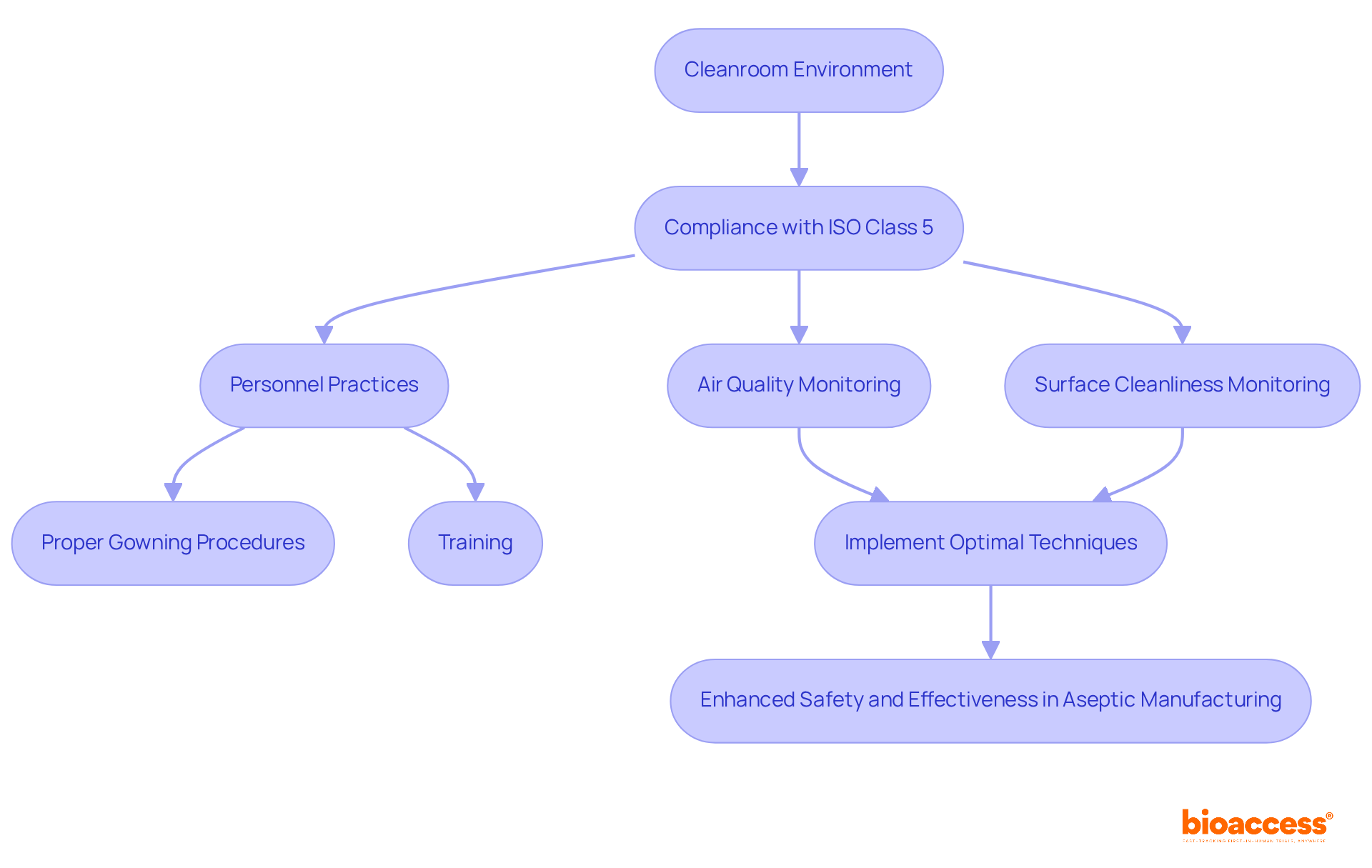
Cleanrooms play a pivotal role in sterile packaging production, providing a meticulously regulated environment that significantly reduces contamination risks. To meet stringent standards such as ISO Class 5, these facilities must limit airborne particle levels to a maximum of 3,520 particles (0.5 microns or larger) per cubic meter of air.
Consistent monitoring of air quality, surface cleanliness, and personnel practices is essential for maintaining the integrity of the cleanroom environment. This includes adhering to air change rates (ACH) between 240 and 480, crucial for ensuring optimal air quality.
Proper gowning procedures and comprehensive training for personnel are imperative, as they directly impact contamination rates. Research indicates that the number of operators in a cleanroom can greatly influence particle loads, highlighting the necessity for effective training and operational protocols.
By implementing these optimal techniques, organizations can ensure successful cleanroom operations, thereby enhancing the safety and effectiveness of aseptic manufacturing activities.
Aseptic fill finish manufacturing presents significant challenges that can critically affect product quality and patient safety. Maintaining sterility, managing complex formulations, and navigating stringent regulatory requirements are among the key issues. Notably, equipment failures pose a considerable risk; nearly 60% of surveyed organizations reported batch failures within the past 3-12 months, with biopharmaceutical facilities experiencing an average batch failure once every 40.6 weeks. This underscores the urgent need for effective maintenance strategies. Contamination risks remain a primary concern, as even minor breaches can result in severe consequences.
To address these challenges, implementing a multifaceted approach that incorporates aseptic fill finish is essential. Establishing robust training programs enhances staff competency, while regular equipment maintenance is crucial for reducing failures. Moreover, a stringent emphasis on quality control throughout the manufacturing cycle is vital. The implementation of cutting-edge technologies, such as automation and isolator systems, is increasingly relevant. There is a notable trend toward enhanced automation in sterile procedures, including the use of robots for transfers between processing stages. This not only ensures compliance with evolving regulatory standards but also aligns with the revised EU GMP Annex 1 guidance, which emphasizes contamination control and the strategic use of technology to minimize human intervention.
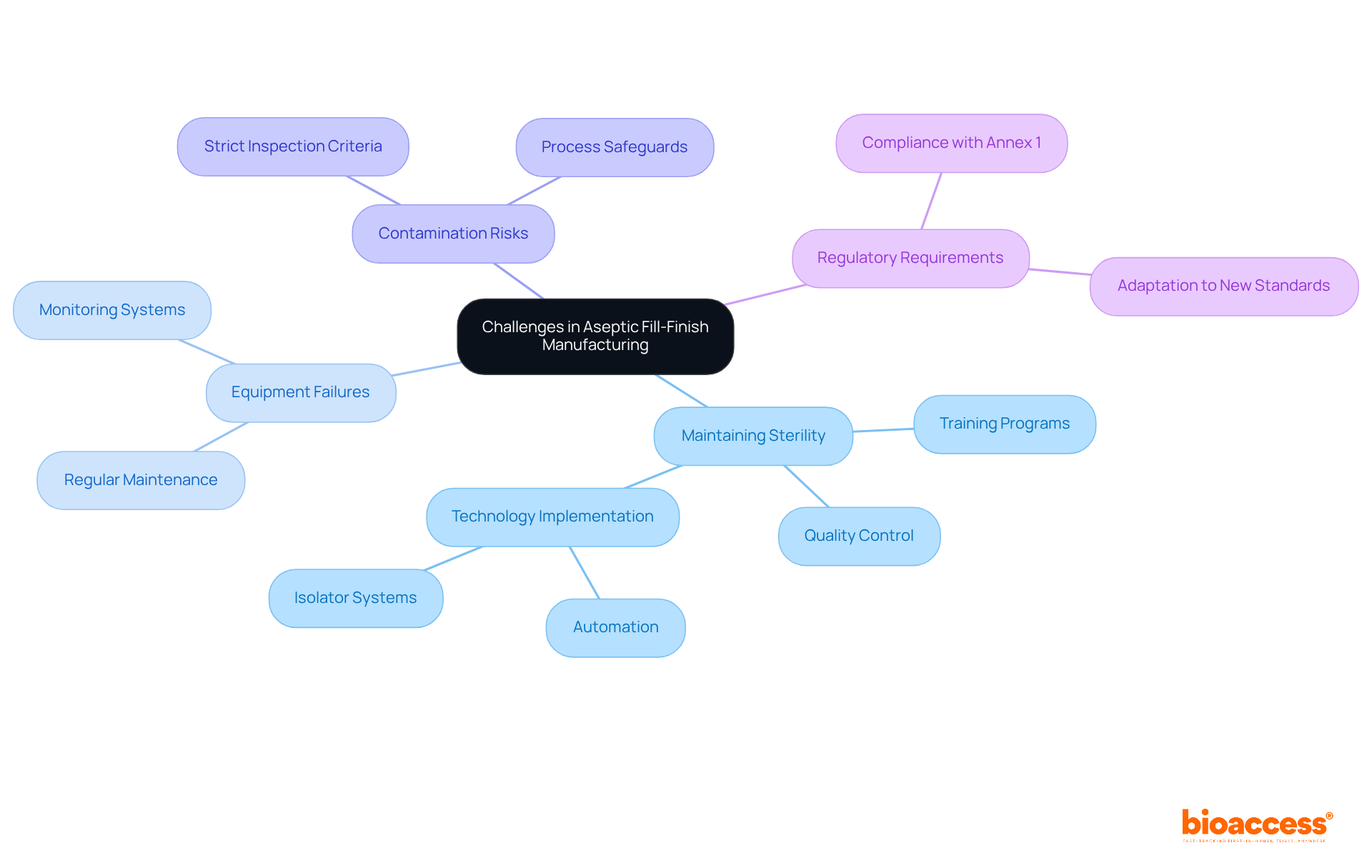
Implementing quality control in aseptic fill finish processes is crucial for ensuring the safety and efficacy of the item. This entails thorough environmental monitoring, in-process testing, and final item testing at every production stage. Environmental monitoring is especially critical, as it aids in identifying potential contamination hazards that could jeopardize quality. Statistics suggest that the sterile fill-finish market is anticipated to expand from USD 6.04 billion in 2024 to USD 17.17 billion by 2034, highlighting the significance of safe and effective pharmaceuticals in tackling public health issues, particularly as noncommunicable diseases (NCDs) represent 71% of all global fatalities.
Quality control teams must be well-trained and equipped with advanced tools to swiftly identify and rectify any deviations from established protocols. Routine evaluations and assessments of quality assurance methods are essential for ensuring adherence to strict regulatory requirements and improving overall quality. Recent advancements in environmental monitoring technologies, including automation and digitalization, have further enhanced the precision and effectiveness of these processes, ensuring that sterile conditions are maintained throughout production.
The impact of robust environmental monitoring on product safety cannot be overstated; it is a fundamental component in preventing contamination and ensuring that products meet the highest safety standards. As Colleen Dixon, CEO of Selkirk Pharma, stated, "Time is of the essence in clinical drug development. Delays in the aseptic fill finish process might result in missed trial milestones and major financial setbacks." As the sterile packaging market continues to expand, fueled by rising demand for biologics and injectable medications, the focus on efficient environmental monitoring will be crucial in protecting public health.
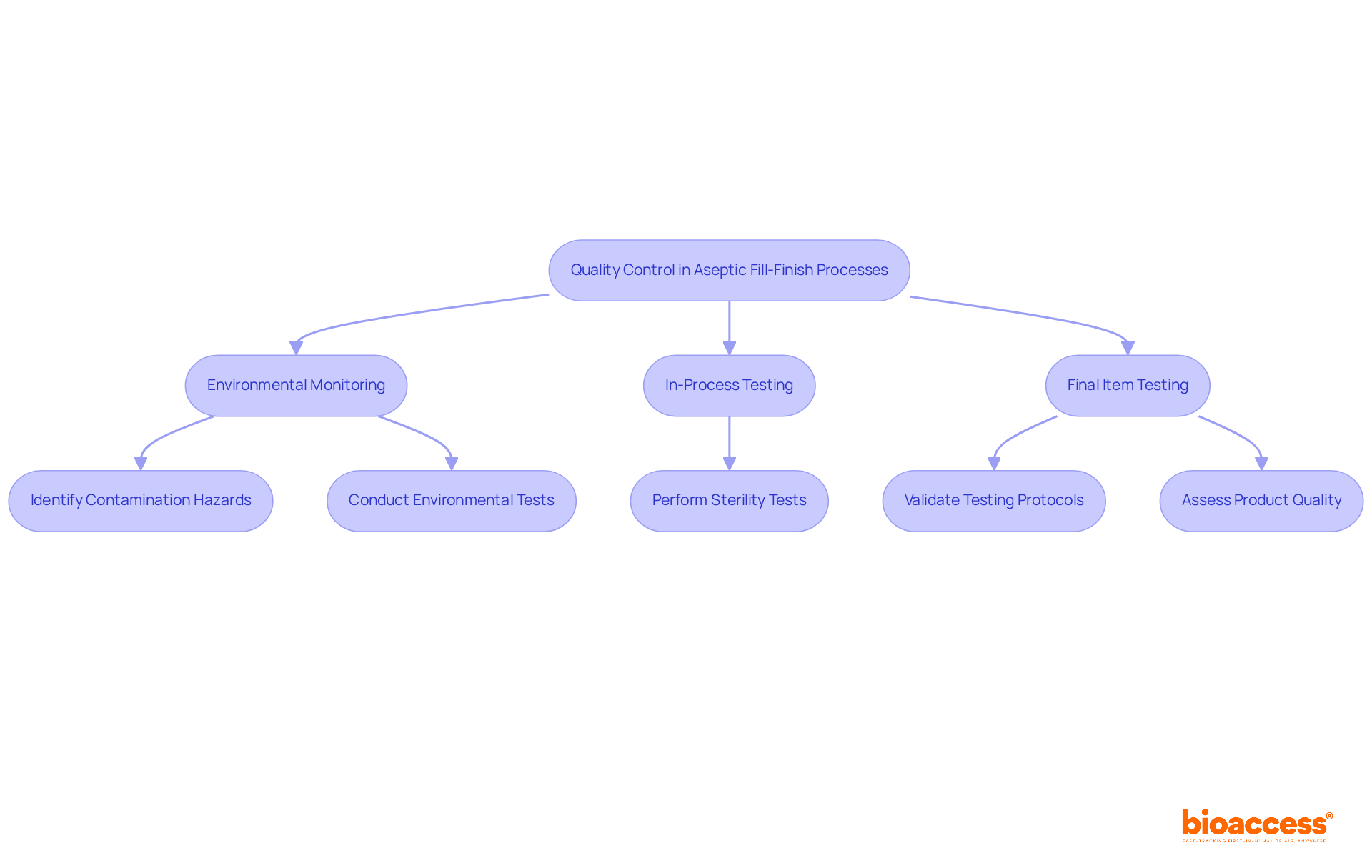
The manufacturing environment for aseptic fill finish is undergoing a significant transformation driven by advancements in automation, robotics, and single-use systems. These innovations not only enhance operational efficiency but also reduce contamination risks associated with the aseptic fill finish, ultimately improving product quality.
For instance, automated sterile filling systems adeptly manage various operational needs, significantly decreasing human involvement and the potential for errors. The market for services related to aseptic fill finish is projected to grow from USD 6.0 billion in 2024 to USD 8.3 billion by 2033, reaching USD 16.9 billion by 2034. This growth underscores the rising demand for aseptic fill finish technologies.
Furthermore, the integration of digital solutions for monitoring and data analysis enables real-time adjustments to manufacturing processes, ensuring compliance with stringent regulatory standards such as ISO 13408 and ISO 22000. This capability is crucial as regulatory bodies like the US FDA and EMA mandate rigorous validation and documentation for sterile filling automation.
Companies that embrace these innovations are strategically positioned to meet the escalating demand for biologics and injectable medications, which require aseptic fill finish processes that are vital in various therapeutic areas, including oncology and immunology.
As the sector evolves, it is imperative for firms to stay informed about technological advancements such as aseptic fill finish to maintain a competitive edge in the sterile packaging market. The adoption rates of automation in sterile manufacturing are steadily rising, driven by the necessity for higher sterility levels and operational efficiency, particularly in the aseptic fill finish process. This trend is expected to continue, with significant funding directed towards automated systems and single-use technologies, further revolutionizing the sterile packaging processes.
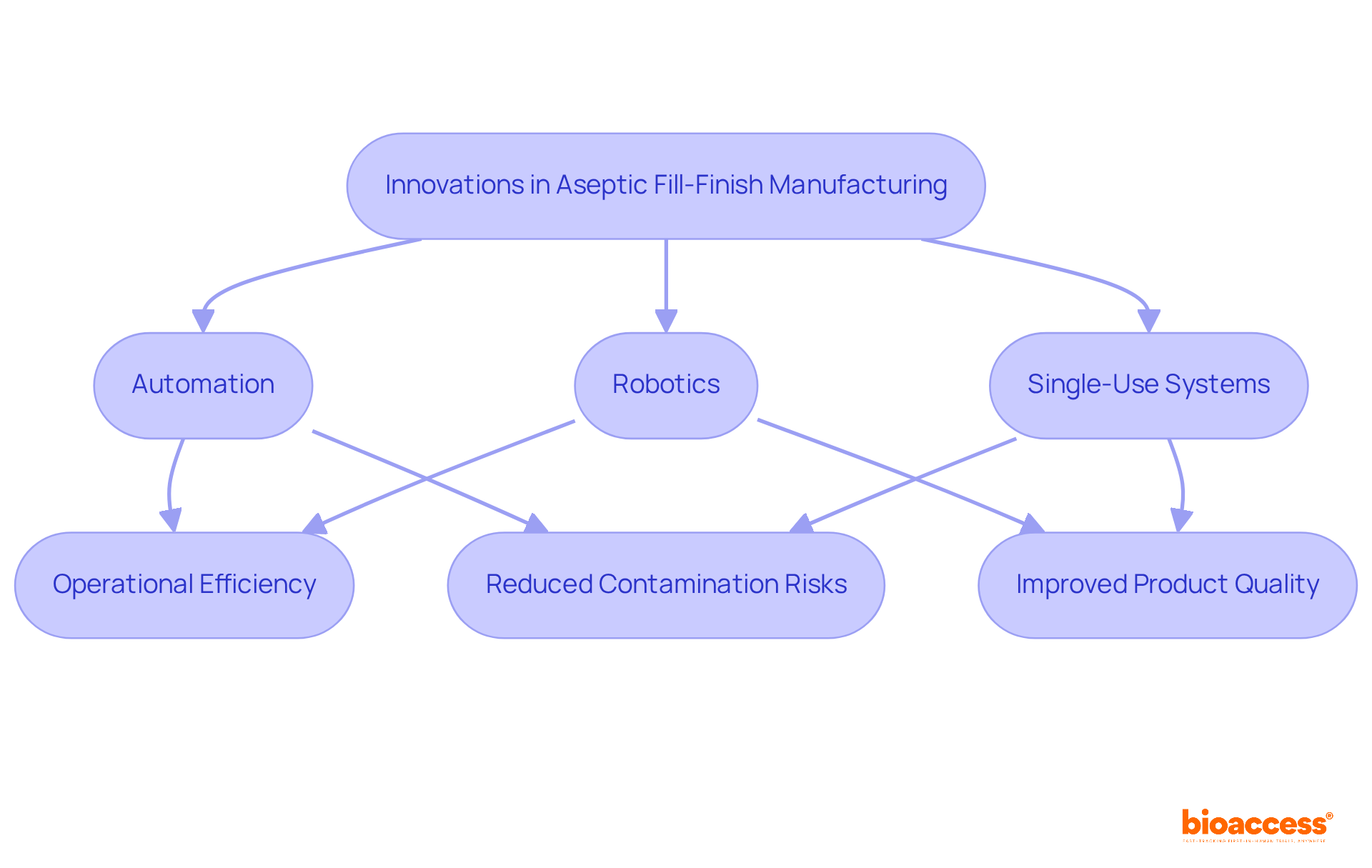
Training cleanroom staff is essential in the realm of aseptic fill finish production. It is imperative that personnel are well-versed in sterile techniques, gowning procedures, and contamination control measures. Regular training sessions and assessments are crucial in ensuring compliance with industry standards and best practices. Furthermore, fostering a culture of continuous learning and improvement not only enhances team performance but also contributes significantly to the success of clean operations.
The future of aseptic fill finish manufacturing is increasingly characterized by a strong emphasis on sustainability, automation, and the incorporation of advanced technologies like artificial intelligence (AI) and machine learning. These innovations enhance operational efficiency while significantly reducing waste and improving product quality.
As the demand for customized medicine and biologics escalates, manufacturers are compelled to adopt more flexible and adaptable methods. Companies that proactively embrace these trends will be strategically positioned to meet evolving market demands, ensuring their competitiveness in a rapidly changing landscape.
The emphasis on sustainability is particularly crucial, as regulatory agencies tighten standards, urging organizations to innovate in their sterile processes, especially in aseptic fill finish, while minimizing environmental impact.
Moreover, the successful adoption of AI in sterile manufacturing is revolutionizing workflows, with statistics indicating that organizations leveraging these technologies can achieve substantial gains in productivity and efficiency.
As the industry evolves, staying ahead of these trends will be essential for success.
Essential insights on sterile production highlight the necessity of maintaining a clean atmosphere and adhering to stringent regulatory standards. Notably, approximately 80% of product recalls are associated with packaging-related issues, emphasizing the critical need for robust quality control measures.
The aseptic fill finish market, which is valued at USD 16.00 billion in 2024, is projected to grow at a CAGR of 8.90% through 2034, with expectations to reach USD 37.53 billion by 2034, driven by the increasing demand for biologics and biopharmaceuticals. Furthermore, advancements in technology—such as single-use systems and AI-driven methods—are essential for enhancing efficiency and sustaining sterility throughout production.
As Vishakha Agrawal notes, 'The aseptic fill finish process involves filling and sealing sterile drug products in containers like vials, syringes, and ampules.' Successful strategies for regulatory compliance encompass thorough training and the implementation of advanced monitoring systems, which are vital for mitigating risks associated with maintaining sterility.
As the industry continues to evolve, it is imperative for organizations to stay informed about emerging trends and regulatory changes to excel in the competitive landscape of aseptic fill finish.
The landscape of aseptic fill-finish manufacturing is shaped by critical insights that emphasize the importance of maintaining sterile environments and adhering to rigorous regulatory standards. This process not only safeguards the efficacy and safety of pharmaceutical products but also plays a vital role in the broader healthcare ecosystem. As the demand for biologics and biopharmaceuticals continues to surge, the need for efficient and compliant aseptic fill-finish operations has never been more pressing.
Key arguments outlined in this article highlight the multifaceted challenges faced by manufacturers, including:
Innovations such as automation and AI-driven solutions are transforming operations, driving efficiency, and reducing contamination risks. Furthermore, the importance of thorough training for personnel cannot be overstated, as it directly influences the success of aseptic processes.
Looking ahead, organizations must remain vigilant and adaptable in the face of evolving market demands and regulatory landscapes. Embracing advancements in technology and fostering a culture of continuous improvement will be essential for maintaining a competitive edge in aseptic fill-finish manufacturing. The commitment to excellence in this field not only enhances product safety but also contributes significantly to public health outcomes, underscoring the critical nature of aseptic manufacturing in today's healthcare environment.
What is bioaccess® and what role does it play in aseptic fill-finish research in Latin America?
bioaccess® is a leader in aseptic fill-finish packaging research in Latin America, utilizing Colombia's advantages to expedite clinical trials and enhance regulatory efficiency.
How does bioaccess® achieve cost savings in clinical trials?
bioaccess® offers cost savings exceeding 30% compared to North America and Western Europe, which contributes to its competitive edge in conducting clinical trials.
What is the timeline for ethical approvals in Colombia as facilitated by bioaccess®?
Ethical approvals in Colombia can be achieved in just 4-6 weeks, significantly improving the regulatory process.
How do enrollment rates for clinical trials in Colombia compare to traditional markets?
Enrollment rates in Colombia are 50% faster than those in traditional markets, aided by a population of over 50 million and a high percentage of universal healthcare coverage.
What are the R&D tax incentives available in Colombia?
Colombia offers substantial R&D tax incentives, making it an attractive location for conducting clinical trials.
What is the primary objective of aseptic fill-finish manufacturing?
The primary objective of aseptic fill-finish manufacturing is to prevent contamination during the sterile filling of medications, which is crucial for ensuring the safety and efficacy of the final product.
What are the key principles of aseptic fill-finish manufacturing?
Key principles include maintaining a sterile environment, applying validated clean techniques, and ensuring all equipment is thoroughly sterilized.
Why is regulatory compliance important in aseptic fill-finish operations?
Regulatory compliance is vital to adhere to guidelines set by regulatory bodies like the FDA and EMA, ensuring Good Manufacturing Practices (GMP) are followed to maintain product safety and efficacy.
What role do audits and inspections play in regulatory compliance?
Regular audits and inspections are critical for maintaining compliance, helping to prevent costly delays in product approvals.
What is the projected growth of the sterile packaging market?
The sterile packaging market is projected to grow from USD 7.5 billion in 2025 to USD 13.3 billion by 2035, highlighting the increasing importance of compliance in the industry.