


The primary objective of the article titled "10 Key Insights on Clinical Trial Participation for Researchers" is to furnish researchers with crucial information concerning individual participation in clinical trials. It delves into several critical aspects, including:
By addressing these elements, the article equips researchers with valuable insights aimed at enhancing recruitment strategies and ensuring ethical practices in clinical trials.
The landscape of clinical trials is evolving rapidly, presenting both opportunities and challenges for researchers and participants alike. As the demand for innovative therapies grows, understanding the intricacies of clinical trial participation becomes paramount. By delving into key insights on this subject, researchers can unlock the potential benefits of streamlined processes, enhanced participant protections, and the critical role of diversity in studies.
However, how can researchers navigate the complexities of trial phases, informed consent, and financial considerations while ensuring ethical standards are upheld? This article explores these vital aspects, offering guidance to optimize the clinical trial experience for all involved.
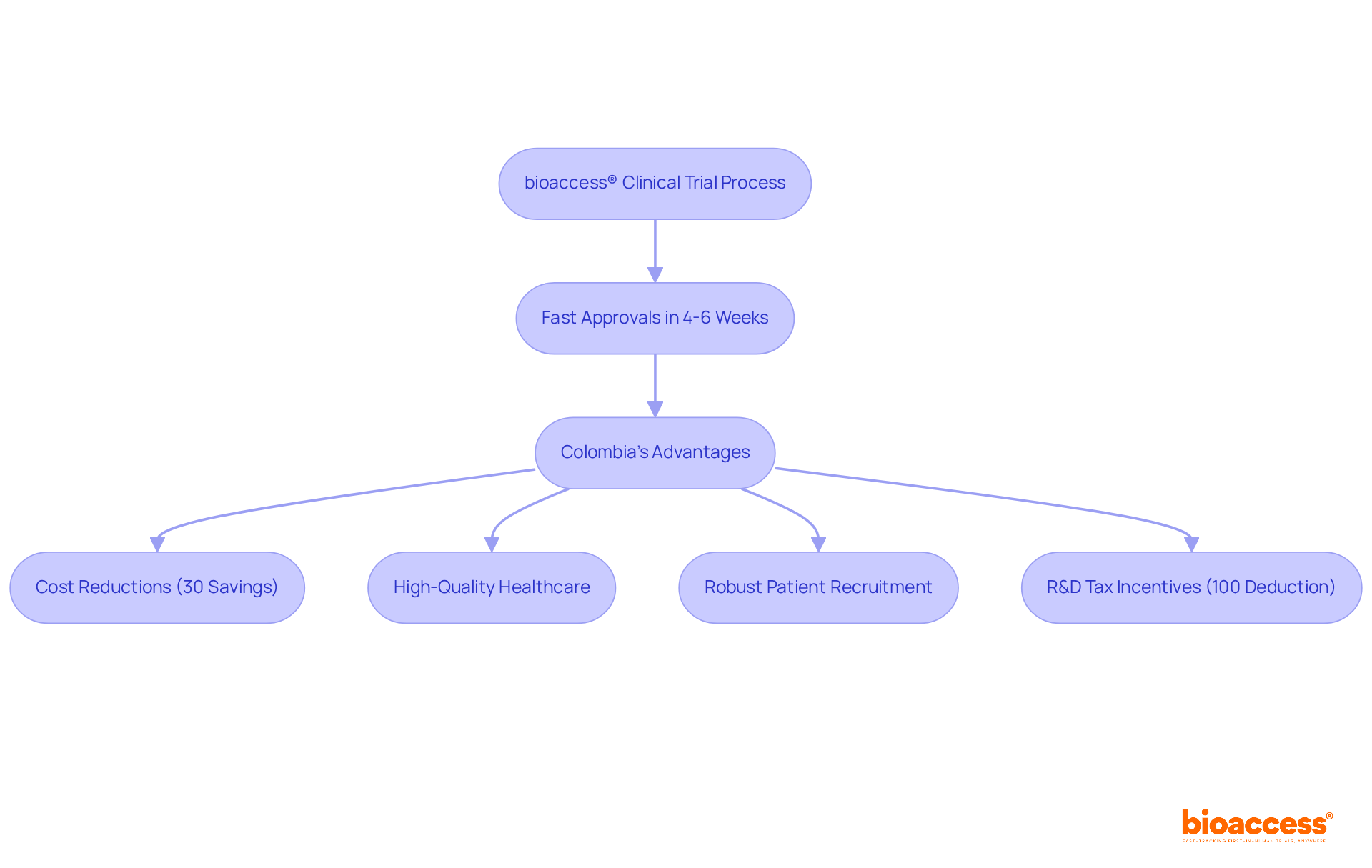
bioaccess® utilizes its extensive expertise in Latin America, the Balkans, and Australia to secure ethical approvals in an impressive 4-6 weeks, a significant improvement over traditional markets. This accelerated process is particularly beneficial in Colombia, where the total IRB/EC and INVIMA review typically spans only 90-120 days.
By capitalizing on Colombia's competitive advantages—such as:
bioaccess® enables researchers to initiate studies without the protracted delays often associated with regulatory processes. Furthermore, Colombia presents appealing R&D tax incentives, including a 100% tax deduction for investments in science and technology, enhancing its attractiveness for medtech and biopharma startups.
This approach not only boosts overall efficiency but also reduces the time to market for new therapies, positioning it as an exceptional choice for companies seeking to streamline their clinical trial participation.
Clinical studies operate under stringent guidelines aimed at safeguarding all participants involved. At the heart of these regulations lies the informed consent process, which mandates that researchers communicate potential risks and benefits with utmost clarity. Participants are to be thoroughly informed and retain the autonomy to withdraw from the study at any moment without incurring penalties.
Additionally, oversight by Institutional Review Boards (IRBs) plays a crucial role in ensuring that ethical standards are rigorously upheld, as they meticulously review study protocols to protect the rights and safety of individuals. Continuous monitoring of participant health is also mandated, reinforcing a steadfast commitment to safety throughout the study.
This proactive approach not only enhances data integrity but also fosters trust between researchers and the community, ultimately encouraging greater volunteer participation in research studies.

Clinical trial participation presents individuals with a unique opportunity to access innovative therapies and procedures that remain unavailable to the general public. These studies frequently provide new treatments or interventions capable of significantly enhancing health outcomes. In addition to receiving cutting-edge therapies, participants benefit from comprehensive medical care and meticulous monitoring throughout the study process, ensuring their safety and well-being.
The importance of medical studies is underscored by evidence indicating that individuals living with Alzheimer's who engage in clinical trial participation tend to experience improved health outcomes compared to those who do not. This highlights the crucial role that research studies play in advancing medical knowledge and enhancing patient care.
Recent trends illustrate that decentralized studies are accelerating enrollment and fostering diversity among participants, a vital factor in developing treatments that cater to a broader population. Furthermore, strategic partnerships, such as the collaboration between bioaccess™ and Caribbean Health Group, are enhancing collaboration and efficiency in research studies, ultimately aiming to expedite the introduction of new therapies to the market.
As the research landscape evolves, experts emphasize the need for patient-centered approaches that prioritize individual choice and experience. This shift not only boosts enrollment rates but also enriches the data collected, leading to more robust findings that can inform future medical advancements. Overall, clinical trial participation not only provides hope for individuals seeking groundbreaking therapies but also contributes to the collective effort of enhancing healthcare solutions.
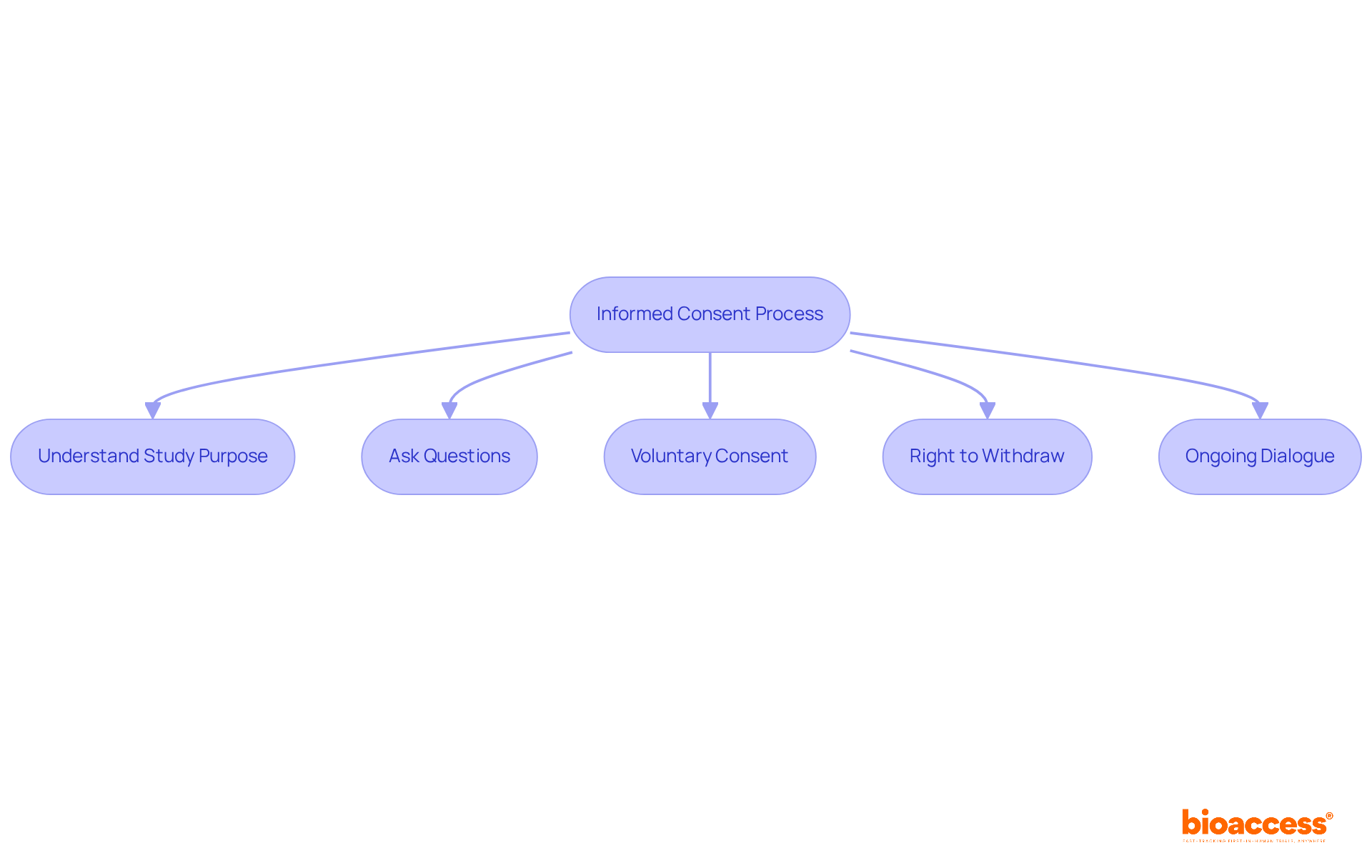
Informed consent is a fundamental process that guarantees individuals fully comprehend the nature of a study, including its purpose, procedures, risks, and benefits. As a potential contributor to clinical trial participation, you possess the right to thoroughly understand the procedures and objectives of a specific study prior to giving your consent.
It is essential for individuals to recognize their right to ask questions and seek clarification on any aspect of the trial before agreeing to participate. This understanding is crucial, as research indicates that individuals demonstrated an accuracy rate of 71-81% in knowledge questions related to electronic informed consent (eIC), reflecting their awareness of their rights.
Participants are required to provide voluntary consent, ensuring their decision is made without coercion. Importantly, they retain the right to withdraw from the study at any time without facing any repercussions, thereby reinforcing the ethical standards of medical research.
This ongoing dialogue between participants and study personnel fosters trust and transparency, empowering individuals to make informed decisions regarding their clinical trial participation in research studies. As James Riddle noted, 'Maintaining informed consent in research studies is an ongoing process,' highlighting the importance of continuous communication throughout the study.
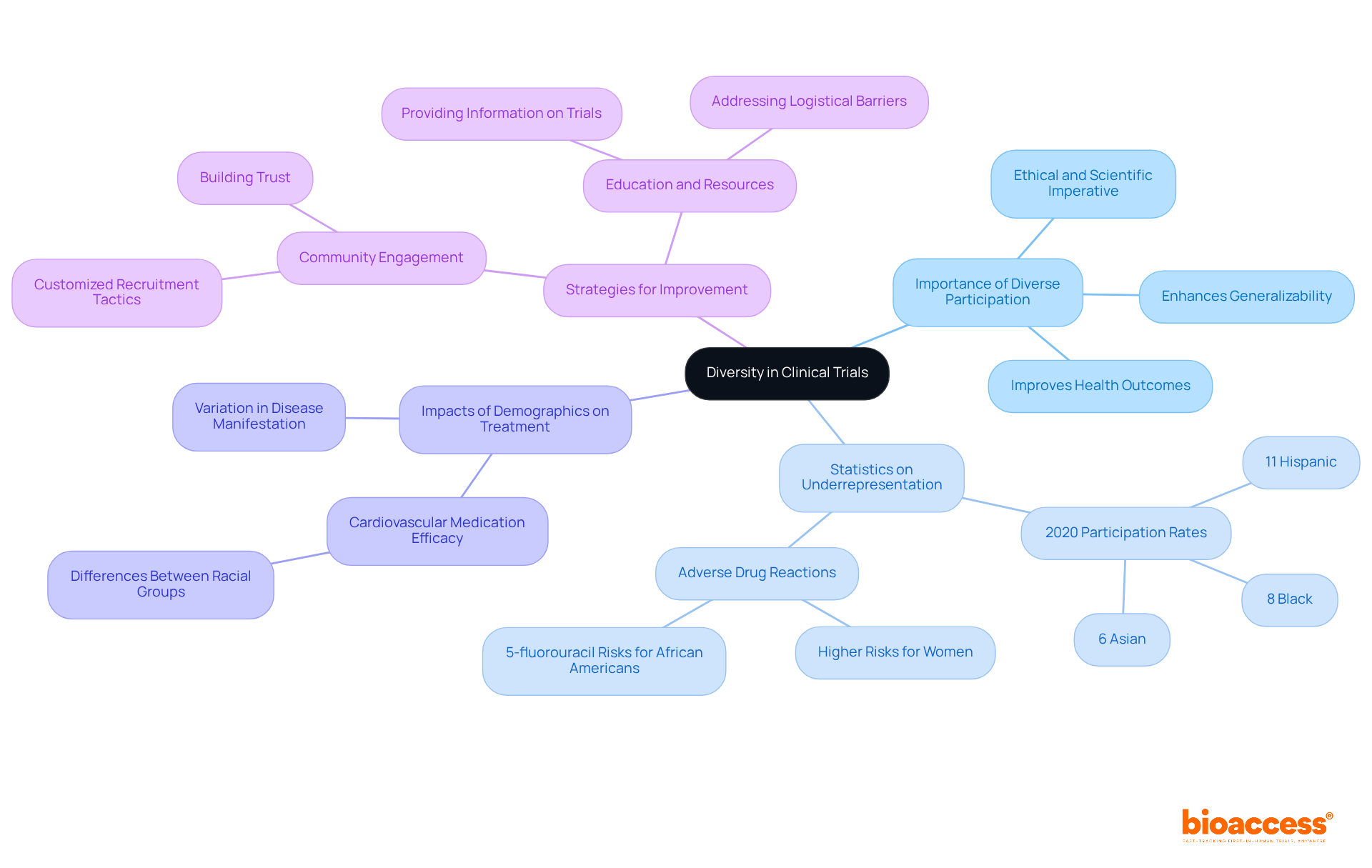
Including varied patient groups in research studies is essential for precisely evaluating the effectiveness and safety of treatments across different demographics. Engaging participants from various backgrounds in clinical trial participation not only enhances the generalizability of research findings but also contributes to improved health outcomes. For instance, studies have shown that certain cardiovascular medications yield different clinical results based on racial backgrounds, particularly between white individuals of European ancestry and African Americans. This highlights the importance of involving varied groups to recognize possible negative impacts and enhance recommendation strategies.
Recent research highlights that women are more likely to have adverse drug reactions than men. A 2020 analysis revealed that only 8% of participants in new medication studies in the U.S. were Black, 6% were Asian, and 11% were Hispanic, indicating significant underrepresentation of these critical demographics. Such disparities can obstruct the recognition of risks related to treatments, as observed with the chemotherapeutic medication 5-fluorouracil, which has been associated with increased adverse effects in African Americans due to inadequate clinical trial participation in research studies.
Community-involved methods have proven successful in attracting underrepresented groups, illustrating that customized tactics can greatly improve diversity in research studies. By promoting trust and understanding within these communities, researchers can enhance clinical trial participation rates, ultimately resulting in more thorough and relevant health outcomes. As the medical community increasingly recognizes the ethical and scientific imperative of diversity, the focus on inclusive recruitment strategies will be vital for advancing medical knowledge and ensuring equitable healthcare solutions.
Participation in clinical trials is essential for the advancement of medical science and the creation of novel therapies. Engaging in these studies through clinical trial participation allows individuals to contribute to the collection of crucial data that can lead to significant breakthroughs in disease understanding and patient care. Research studies have been vital in the advancement of vaccines for contagious illnesses, boasting a success rate of 33.4 percent, the highest among treatment fields. Furthermore, the insights gained from contributor involvement not only enhance current medical practices but also lay the groundwork for future advancements in healthcare.
Current trends indicate that up to 73% of patients seek information about research opportunities through their healthcare providers, underscoring the importance of effective communication in recruitment initiatives. Additionally, the growing complexity of medical studies, with eligibility requirements increasing from an average of 31 to 50 between 2001 and 2015, highlights the necessity for diverse contributions from individuals to ensure comprehensive data gathering.
Moreover, the ongoing research and development pipeline, which includes over 20,109 drugs as of 2022, reflects the extensive efforts to innovate in the medical field. Engagement in clinical trial participation by each participant is a crucial step toward achieving these advancements, ultimately benefiting not only current patients but also future generations who will rely on the treatments developed through these studies. By participating, individuals are not merely subjects; they are integral to the evolution of healthcare.

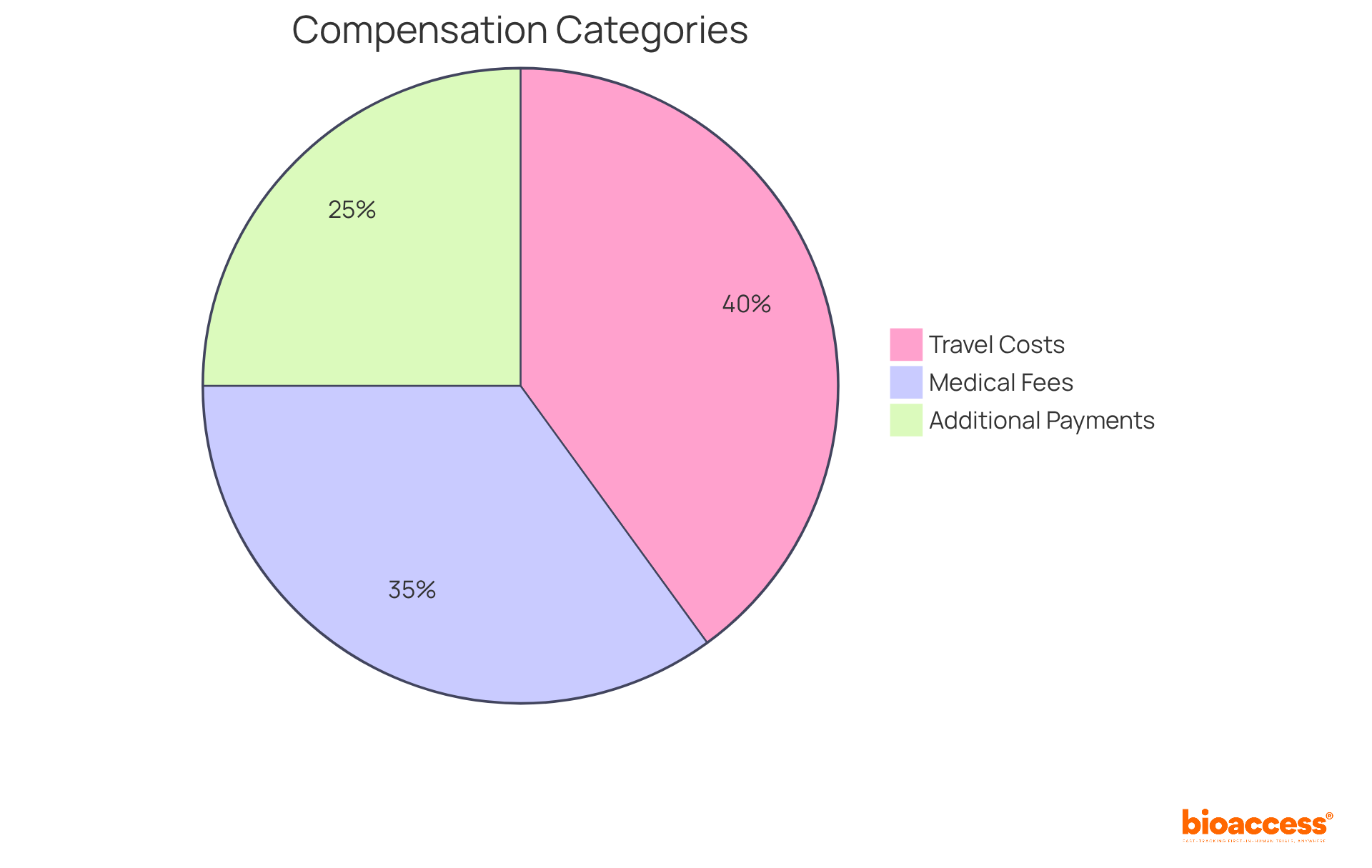
Compensation for their time and any costs incurred during the research may be provided to participants involved in clinical trial participation. This compensation can encompass a variety of expenses, including:
For instance, Phase I studies typically offer compensation ranging from $2,000 to over $5,000 for healthy participants, with extended hospital stays potentially exceeding $7,000. Phase II trials average between $300 and $3,000 for disease-specific efficacy studies. Furthermore, individuals may receive compensation for indirect expenses, such as travel and accommodation, depending on the study's design.
In 2025, updates to reimbursement policies will emphasize the necessity for transparency and fairness in compensation practices, ensuring that individuals are adequately informed about their rights and the financial aspects of their involvement. It is crucial for individuals to engage in discussions with the research team regarding financial considerations prior to clinical trial participation, as this can clarify which expenses will be covered and the overall compensation structure. Additionally, clear communication regarding compensation in informed consent documents is vital to foster trust and understanding. By grasping these financial aspects, individuals can make informed decisions about their clinical trial participation in clinical research.
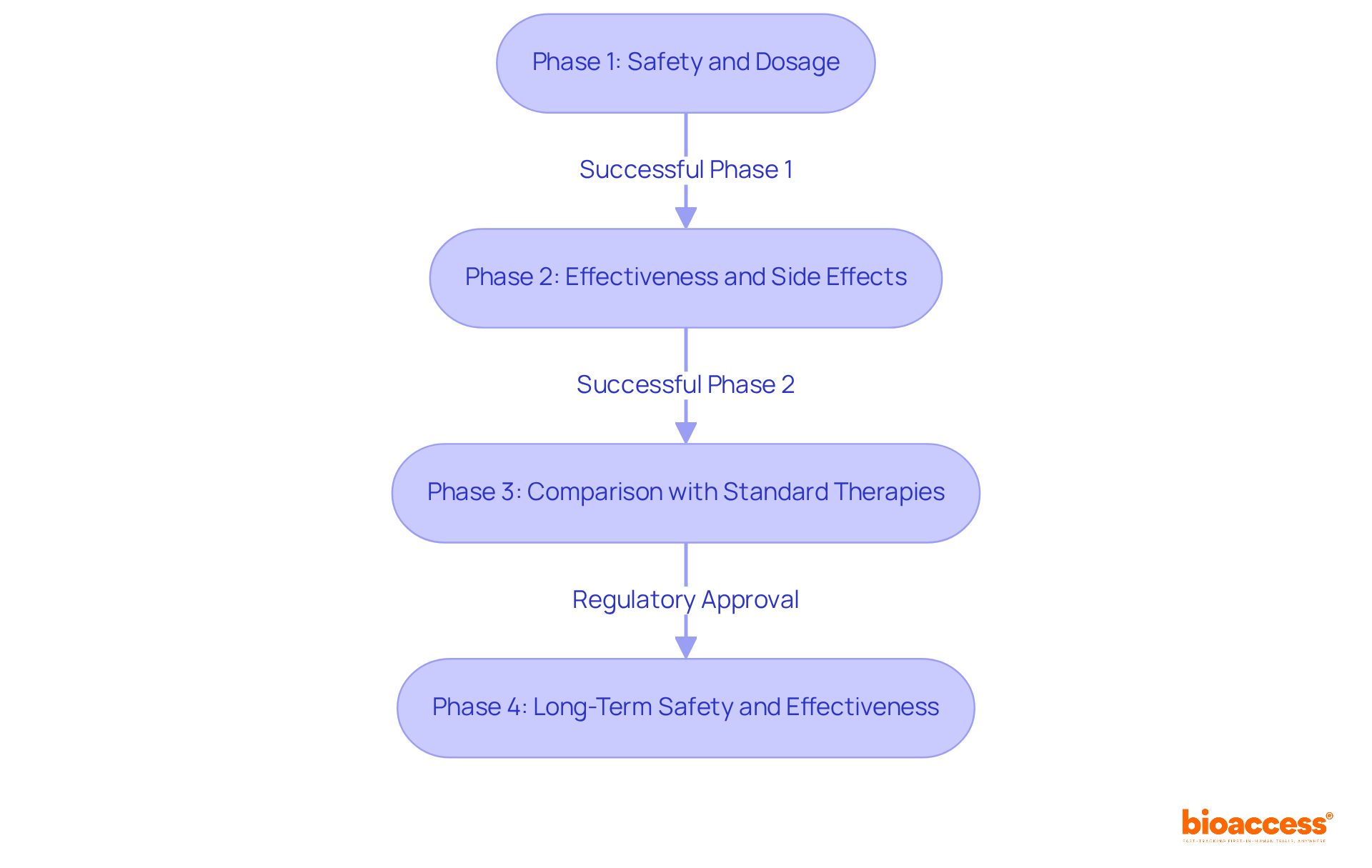
Clinical studies are systematically classified into four distinct phases, each with specific goals and enrollment criteria that are essential for assessing the safety and effectiveness of new therapies.
Phase 1: This initial phase primarily focuses on safety and dosage. Typically involving 20 to 100 healthy participants, Phase 1 studies aim to establish the safety profile of the intervention and determine the optimal dosing. These studies generally last for several months and pose the greatest potential hazard for participants, as they represent the first occasion a novel therapy is evaluated in humans.
Phase 2: Following successful Phase 1 evaluations, Phase 2 investigates the effectiveness of the intervention while monitoring side effects. This phase usually includes 100 to 300 individuals with the specific condition being treated. Phase 2 studies can extend from several months to two years, providing crucial information on the intervention's effectiveness and safety, which informs decisions about advancing to Phase 3.
Phase 3: This extensive phase encompasses 300 to 3,000 participants and compares the new approach against existing standard therapies. Phase 3 studies typically span from 1 to 4 years and are conducted across multiple sites, such as community hospitals and physicians' offices. They yield comprehensive evidence regarding the intervention's value and safety, which is vital for regulatory approval. bioaccess® offers professional services to support this phase, including feasibility studies and site selection, ensuring effective setup and compliance with regulatory requirements.
Phase 4: After obtaining regulatory authorization, Phase 4 studies concentrate on long-term safety and effectiveness in real-world conditions. These studies involve ongoing monitoring and can last indefinitely, helping to identify rare side effects and confirm the treatment's long-term benefits.
Understanding these stages is critical for researchers and participants alike during clinical trial participation, as they navigate the complex landscape of medical studies aimed at safeguarding patient safety while advancing medical knowledge. bioaccess® provides comprehensive clinical study management services, including feasibility assessments, site selection, and project oversight, to assist researchers in executing successful studies.
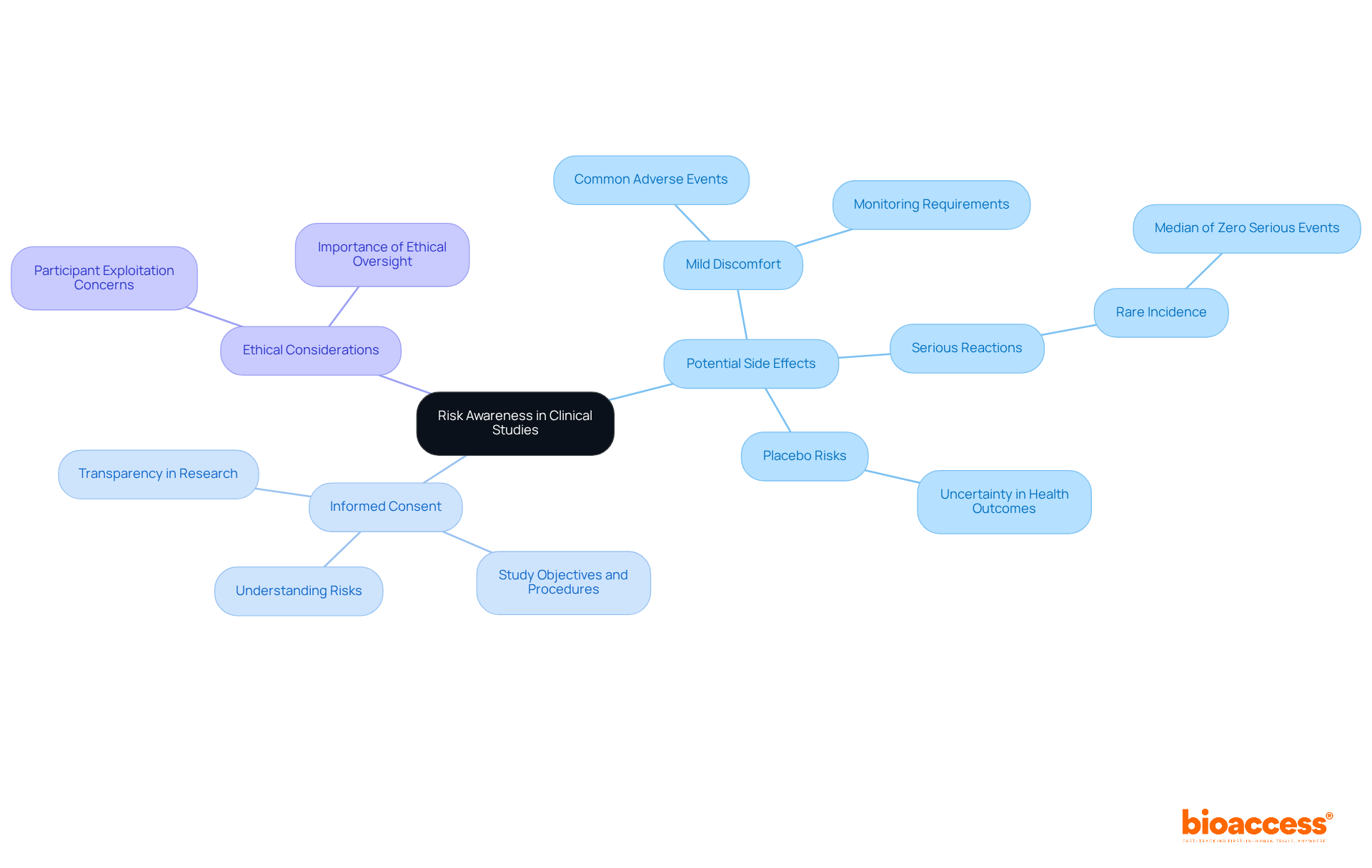
Clinical studies present numerous advantages; however, participants must remain vigilant about potential risks, particularly concerning side effects from experimental treatments. These side effects can range from mild discomfort to more significant reactions, necessitating careful medical monitoring throughout the study. For instance, a systematic review of phase I trials involving over 27,000 participants demonstrated that while mild adverse events were common, serious adverse events were infrequent, with a median incidence of zero serious events per 1,000 subjects per day of observation. This underscores that, although individuals may experience mild side effects, the likelihood of serious harm remains remarkably low.
Moreover, participants may face the possibility of receiving a placebo instead of an actual treatment, potentially leading to uncertainty regarding their health outcomes. Recognizing these risks is vital for making informed decisions about participation. Recent findings emphasize that informed consent is crucial, ensuring that individuals comprehend the study's objectives, procedures, and potential discomforts. This level of transparency fosters a more ethical approach to research involving human subjects, enabling participants to effectively weigh the benefits against the risks.
In conclusion, while the advancement of innovative therapies through research studies is imperative, individuals must be thoroughly informed about the potential discomforts and side effects linked to their participation, empowering them to engage in the process with clarity and confidence.
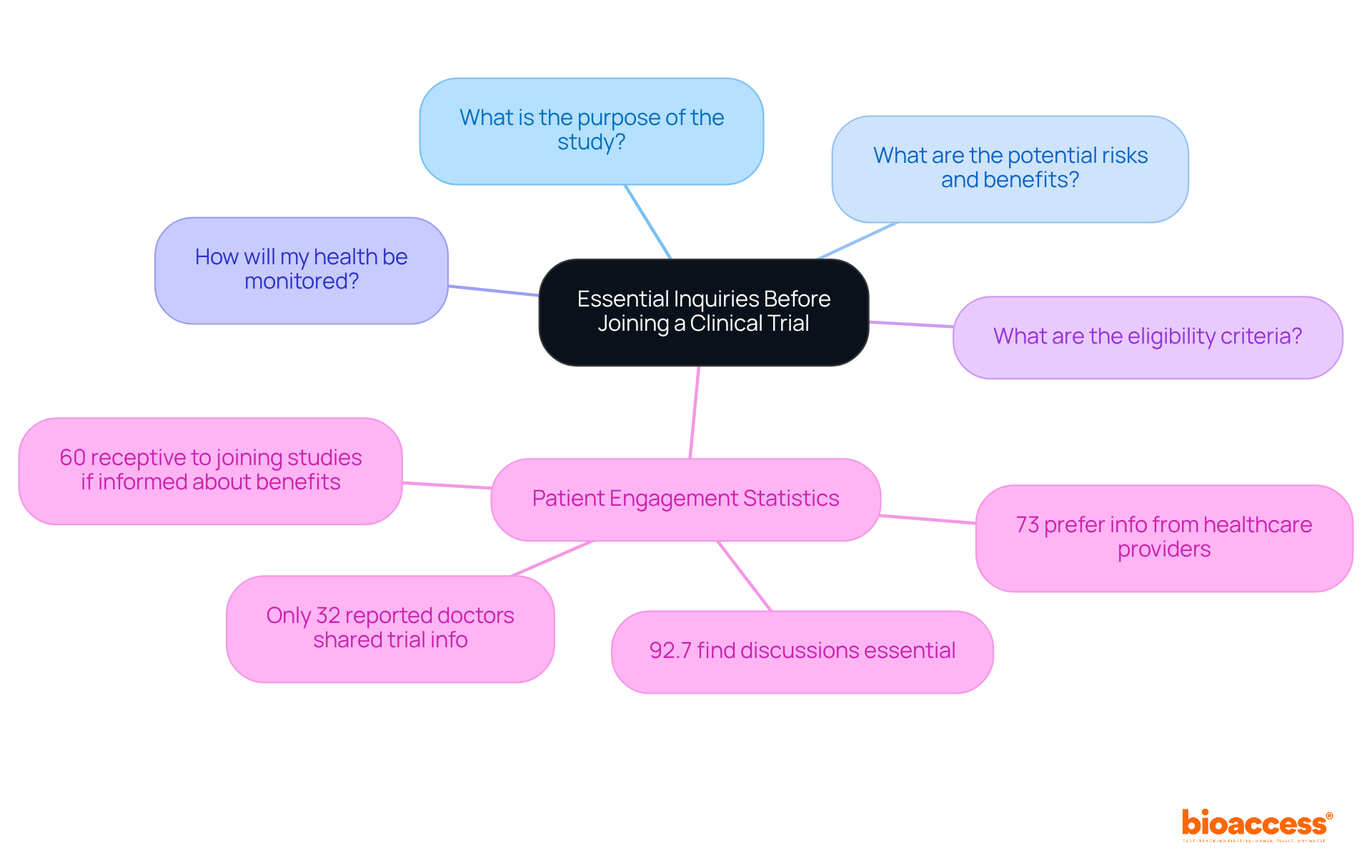
Before signing up for a research study, prospective individuals should pose crucial questions to the research team to gain a comprehensive understanding of the investigation. Key inquiries include:
Engaging in this dialogue not only clarifies uncertainties but also fosters a sense of trust and collaboration between participants and researchers. Statistics reveal that 73% of patients prefer to obtain information regarding research studies from their healthcare providers; however, only 32% of patients reported that their doctors had ever shared details about research studies with them, highlighting a significant communication gap. Furthermore, studies indicate that 92.7% of oncology patients find it essential to discuss their participation with doctors, underscoring the necessity for open dialogue. Additionally, 60% of patients are receptive to joining research studies if informed about potential advantages. By actively engaging in these discussions, individuals can make informed decisions regarding their clinical trial participation, ultimately contributing to the advancement of medical research.
Engaging in clinical trials is a pivotal aspect of advancing medical research and innovation. This article highlights the multifaceted benefits of clinical trial participation for researchers, including:
By understanding the intricacies of informed consent and the rights of participants, researchers can foster a more transparent and trustworthy environment that encourages greater involvement in studies.
Key insights outlined in the article underscore the importance of informed dialogue between researchers and potential participants. Participants gain access to innovative therapies while contributing to significant medical advancements, making their involvement crucial for the development of effective treatments. The emphasis on financial considerations and compensation illustrates the need for transparency in clinical trials, further empowering individuals to make educated decisions about their participation.
Ultimately, the collective effort of researchers and participants in clinical trials is fundamental to shaping the future of healthcare. By prioritizing inclusivity and addressing the diverse needs of patient populations, the medical community can enhance the relevance and efficacy of research outcomes. Engaging in clinical trials not only benefits individual participants but also contributes to the broader goal of improving health outcomes for future generations.
What is bioaccess® and how does it benefit clinical trials?
bioaccess® is a company that accelerates the clinical trial experience by securing ethical approvals in 4-6 weeks, significantly faster than traditional markets. This is particularly beneficial in Colombia, where the total review process typically takes 90-120 days.
What advantages does Colombia offer for clinical trials?
Colombia offers several advantages for clinical trials, including cost reductions exceeding 30% compared to North America and Western Europe, a high-quality healthcare system, robust patient recruitment capabilities, and appealing R&D tax incentives, such as a 100% tax deduction for investments in science and technology.
How does bioaccess® enhance the efficiency of clinical trials?
By leveraging Colombia's competitive advantages and reducing the delays associated with regulatory processes, bioaccess® enables researchers to initiate studies more quickly, ultimately reducing the time to market for new therapies.
What safety measures are in place for participants in clinical trials?
Clinical trials operate under stringent guidelines to protect participants, including the informed consent process, oversight by Institutional Review Boards (IRBs), and continuous monitoring of participant health to ensure safety and ethical standards are upheld.
What are the benefits of participating in clinical trials for individuals?
Participants in clinical trials have access to innovative therapies and procedures not available to the general public, receive comprehensive medical care, and experience meticulous monitoring throughout the study, which can lead to improved health outcomes.
How do clinical trials contribute to medical advancements?
Clinical trials play a crucial role in advancing medical knowledge and enhancing patient care. Evidence suggests that individuals with conditions like Alzheimer's who participate in trials often have better health outcomes, highlighting the importance of research in developing effective treatments.
What trends are influencing clinical trial participation?
Recent trends include decentralized studies that accelerate enrollment and promote diversity among participants, along with strategic partnerships that enhance collaboration and efficiency in research studies, ultimately aiming to expedite the introduction of new therapies.
Why is a patient-centered approach important in clinical trials?
A patient-centered approach prioritizes individual choice and experience, which boosts enrollment rates and enriches the data collected, leading to more robust findings that can inform future medical advancements.