


Understanding the complex world of antibodies is essential for advancing clinical research, especially regarding the distinct roles of the Fab and Fc regions. These two components not only determine how antibodies recognize and bind to antigens but also shape their interactions with the immune system, ultimately influencing therapeutic outcomes. As immunotherapy continues to evolve, researchers must consider how to leverage the unique properties of Fab and Fc to enhance the efficacy of antibody-based treatments. This article explores ten key insights that clarify the differences between Fab and Fc, examining their implications for antibody functionality and the future of clinical research.
bioaccess® stands at the forefront of accelerating clinical research for immune-based treatments, capitalizing on its unique geographical advantages, particularly in Colombia. By harnessing Colombia's cost efficiency-offering savings exceeding 30% compared to trials in North America and Western Europe-bioaccess® ensures a swift regulatory process, with IRB/EC and MoH (INVIMA) reviews completed within 90-120 days. This strategic advantage is vital for Medtech, Biopharma, and Radiopharma innovators eager to fast-track their products to market while upholding ethical standards.
Moreover, Colombia boasts a high-quality healthcare system, recognized among the best globally, and a diverse patient population, with over 95% covered by universal healthcare. This environment facilitates rapid patient recruitment, essential for timely clinical trials. The rigorous ICH/GCP certification process that hospitals in Colombia must undergo further enhances the credibility of the clinical research conducted there.
By streamlining enrollment and approvals, bioaccess® significantly improves patient access to groundbreaking therapies, ultimately leading to better health outcomes. Recent successful clinical trials for antibody therapeutics in Latin America highlight the effectiveness of this model, showcasing how geographical factors can profoundly impact the speed and success of clinical research. As the landscape evolves, collaboration becomes increasingly important-bioaccess® invites innovators to explore how they can leverage these advantages for their clinical research needs.
Antibodies play a pivotal role in clinical research, which can be divided into two primary areas known as fab vs fc:
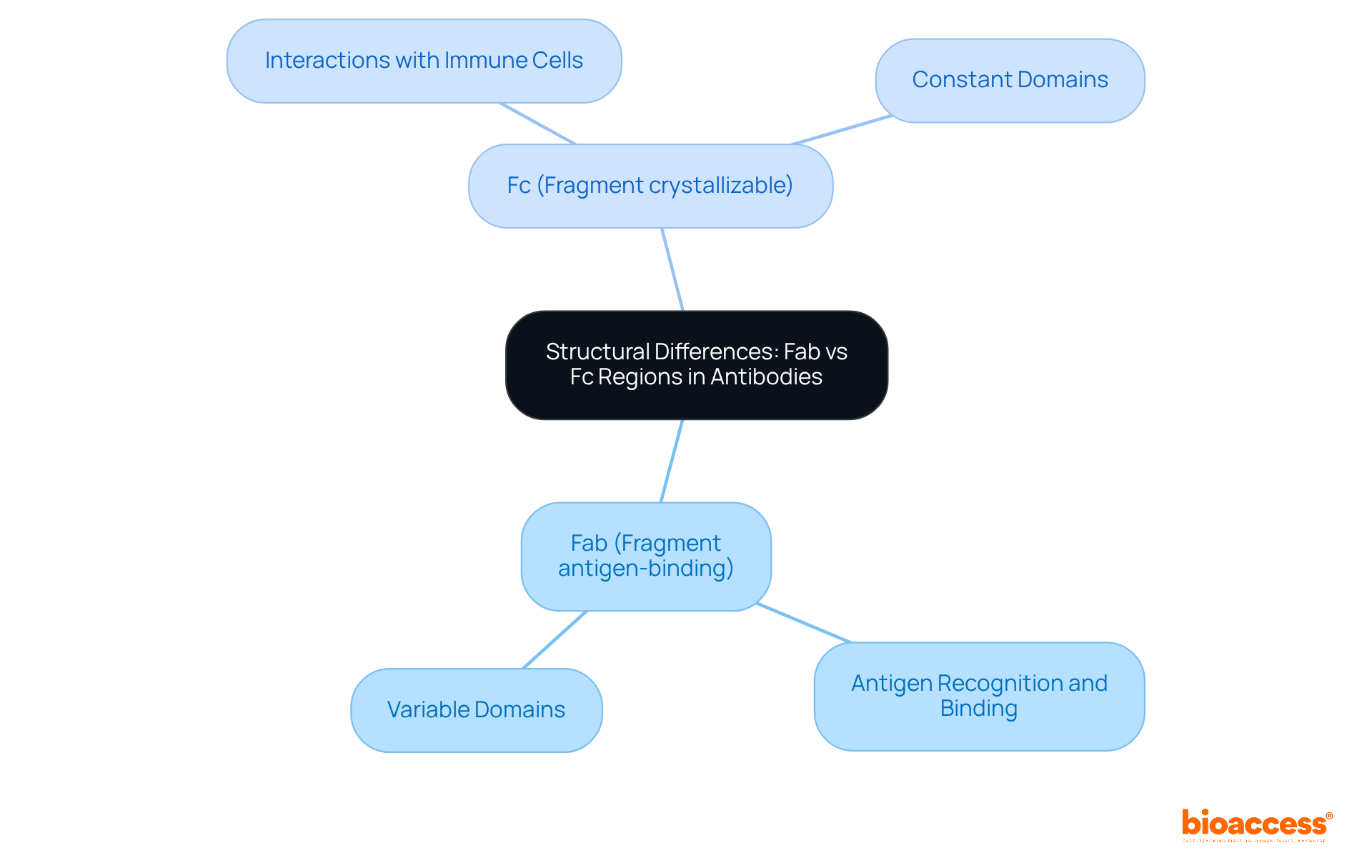
The distinction between fab vs fc is crucial for antigen recognition and binding, featuring variable and constant domains that form the antigen-binding site. In contrast, the differences in fab vs fc sections mediate interactions with immune cells and effector functions, composed solely of constant domains. This structural distinction is not just a detail; it is essential for the protein's functionality, influencing how these molecules engage with their targets and elicit immune responses. Understanding these components is vital for addressing key challenges in the Medtech landscape.
The distinction between fab vs fc areas plays a crucial role in the specificity of immunoglobulins, encompassing the variable domains that identify and bind to specific antigens. This binding is made possible by the unique structure of the Fab, which ensures high affinity and specificity toward target molecules. The ability of the Fab segment to adapt and recognize various antigens is vital for developing effective therapeutic proteins, making it a central focus in protein engineering and design.
Recent advancements in immunoglobulin engineering have concentrated on refining the Fab component to enhance binding affinity. Techniques such as CDR clustering illustrate how modifications can significantly improve therapeutic effectiveness. For instance, engineered proteins have demonstrated improved interactions with Fc receptors, which are essential for initiating immune responses. Experts in the field emphasize that strengthening the structural integrity of the Fab region is critical for maintaining its functionality throughout the lifecycle of the immune protein.
This focus on the fab vs fc area not only highlights its significance in treatment development but also mirrors current trends in protein engineering aimed at maximizing therapeutic potential. As the landscape of clinical research evolves, collaboration and innovation in this area will be key to overcoming challenges and advancing treatment options.
The Fc portion plays a crucial role in engaging the immune system by binding to Fc receptors on immune cells. This interaction triggers essential effector functions, including:
These functions are vital for eliminating pathogens and infected cells. In the realm of therapeutic proteins, there is a concentrated effort to refine the Fc part to enhance these immune responses, ultimately boosting the overall effectiveness of antibody-based therapies.
Understanding the significance of the Fc portion is paramount in clinical research, particularly as it relates to the Medtech landscape. By addressing key challenges in immune engagement, researchers can pave the way for innovative solutions that improve patient outcomes. Collaboration among experts in this field is essential to drive advancements and ensure that therapeutic strategies are both effective and accessible.

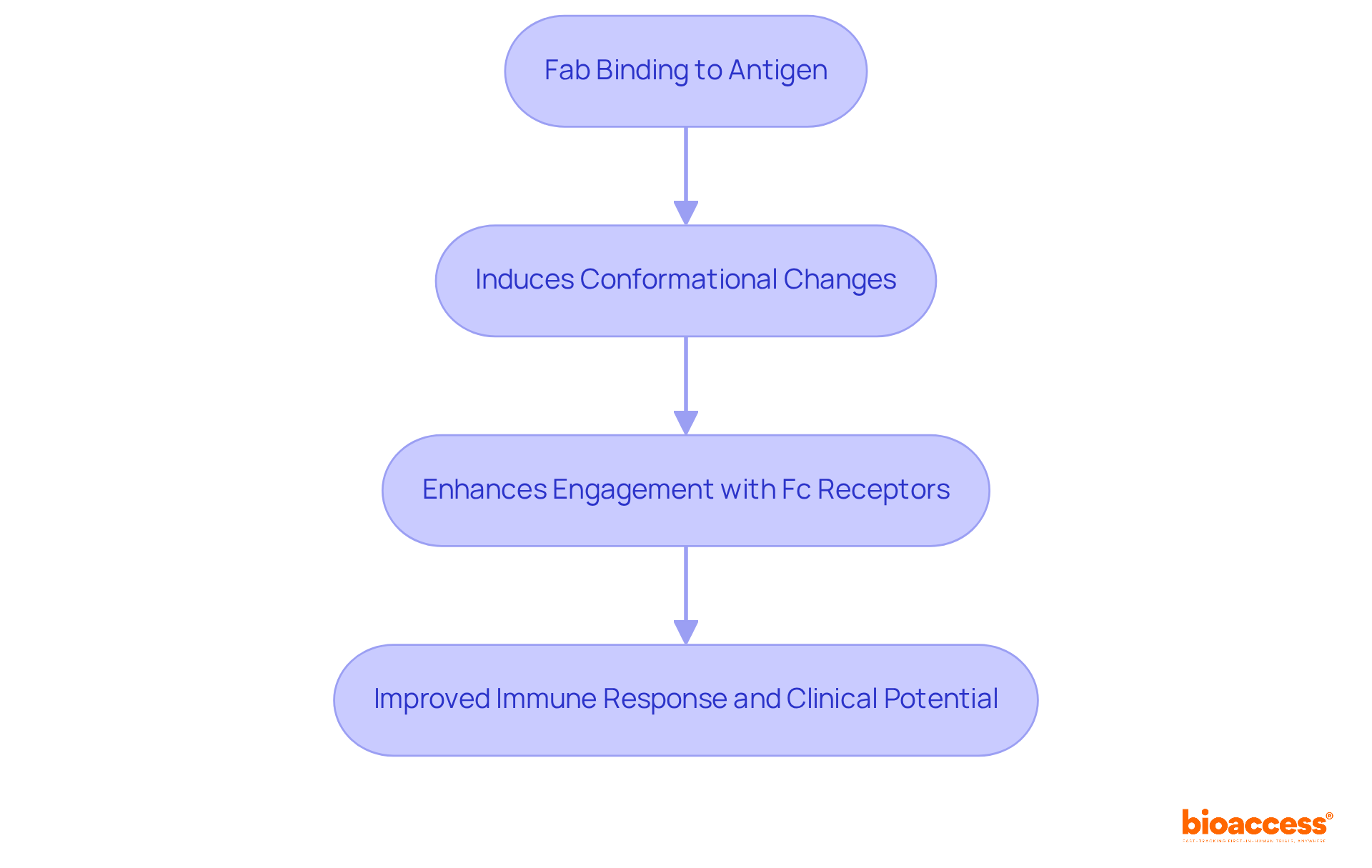
The functionality of immunoglobulins is essential and is influenced by the dynamic interplay of Fab vs Fc parts, which significantly enhances overall efficacy. When the Fab binds to an antigen, it induces conformational changes in the region related to Fab vs Fc, which may improve its ability to engage with Fc receptors. This allosteric effect holds particular importance in medical applications, as it can influence the immune response and elevate the clinical potential of monoclonal proteins.
For instance, specialized proteins designed with an understanding of Fab vs Fc interactions have demonstrated enhanced effectiveness in clinical settings. This underscores the necessity of considering these interactions in protein design. Studies have shown that the structural and functional connectivity in Fab vs Fc domains is vital for improving treatment outcomes, highlighting the critical role these interactions play in developing effective antibody-based therapies.
In the realm of immunoglobulin engineering, the comparison of Fab vs Fc segments plays a crucial part in developing more effective treatment agents. By modifying the Fab region, researchers can significantly enhance specificity and binding affinity, which are vital for targeting diseases accurately. Meanwhile, adjustments to the Fc segment can lead to improved effector functions and pharmacokinetics, ensuring that these agents work efficiently within the body. This dual approach not only fosters the creation of proteins that are highly specific to their targets but also empowers them to elicit robust immune responses, ultimately maximizing therapeutic efficacy.
As we navigate the complexities of the Medtech landscape, it becomes evident that collaboration is essential in addressing key challenges in clinical research. The integration of innovative strategies in immunoglobulin engineering exemplifies how targeted therapies can revolutionize treatment options. By harnessing the unique properties of the Fab vs Fc segments, we can pave the way for advancements that not only meet current medical needs but also anticipate future demands.
In conclusion, the importance of collaboration in this field cannot be overstated. As we move forward, it is imperative to engage with experts and stakeholders to explore the next steps in enhancing therapeutic strategies through immunoglobulin engineering.

The manifestation of fab vs fc segments presents significant challenges in the realm of immunoglobulin engineering. Key factors, including:
can greatly influence both the yield and functionality of the antibodies produced. It is essential to enhance these expression systems to ensure that both Fab and Fc components are generated in a manner that maintains their structural integrity and functional capabilities, particularly when considering fab vs fc. This preservation is crucial for their therapeutic applications, underscoring the need for focused efforts in this area.
The distinction between fab vs fc sections plays a pivotal role in various diagnostic applications, particularly in immunoassays and imaging techniques. The specificity of the Fab part enables precise detection of target antigens, which is essential for accurate diagnostics. Meanwhile, modifications to the Fc section can significantly enhance signal amplification and detection sensitivity, making these applications crucial in clinical diagnostics. This capability not only facilitates early disease detection but also allows for effective monitoring of therapeutic responses, ultimately improving patient outcomes.
In the evolving Medtech landscape, the integration of these technologies addresses key challenges faced in clinical research. By leveraging the strengths of the fab vs fc sections, researchers can enhance the reliability of their diagnostic tools. This innovation fosters collaboration among professionals, driving advancements that are vital for patient care. As we look ahead, it is imperative to recognize the importance of these developments and the role they play in shaping the future of diagnostics.
In summary, the collaboration between various stakeholders in the Medtech field is essential for overcoming challenges and enhancing diagnostic capabilities. The next steps involve continued innovation and partnership to ensure that these advancements translate into improved health outcomes for patients.

Allosteric interactions between the fab vs fc regions of immunoglobulins are pivotal in shaping their functionality, particularly in clinical research. For example, when the Fab binds to an antigen, it can trigger conformational changes in the Fc region, thereby enhancing its interaction with Fc receptors. This dynamic interplay is crucial for modulating immune responses and can be strategically harnessed in the development of therapeutic proteins, ultimately improving their effectiveness and safety profiles.
Recent studies underscore the importance of understanding IgG structure-function relationships, as these insights can drive advancements in immune protein engineering. Additionally, the neonatal Fc receptor (FcRn) is vital for serum immunoglobulin recycling, which is essential for achieving optimal treatment outcomes. However, the interdependence of immune protein domains introduces challenges that must be navigated during the design process. To ensure optimal treatment efficacy, empirical testing of immune protein design effects on Fc function is imperative.
In summary, grasping the complexities of fab vs fc interactions is essential for the progression of next-generation therapeutic proteins. As we delve deeper into this field, collaboration and innovative approaches will be key to overcoming existing challenges and enhancing clinical outcomes.
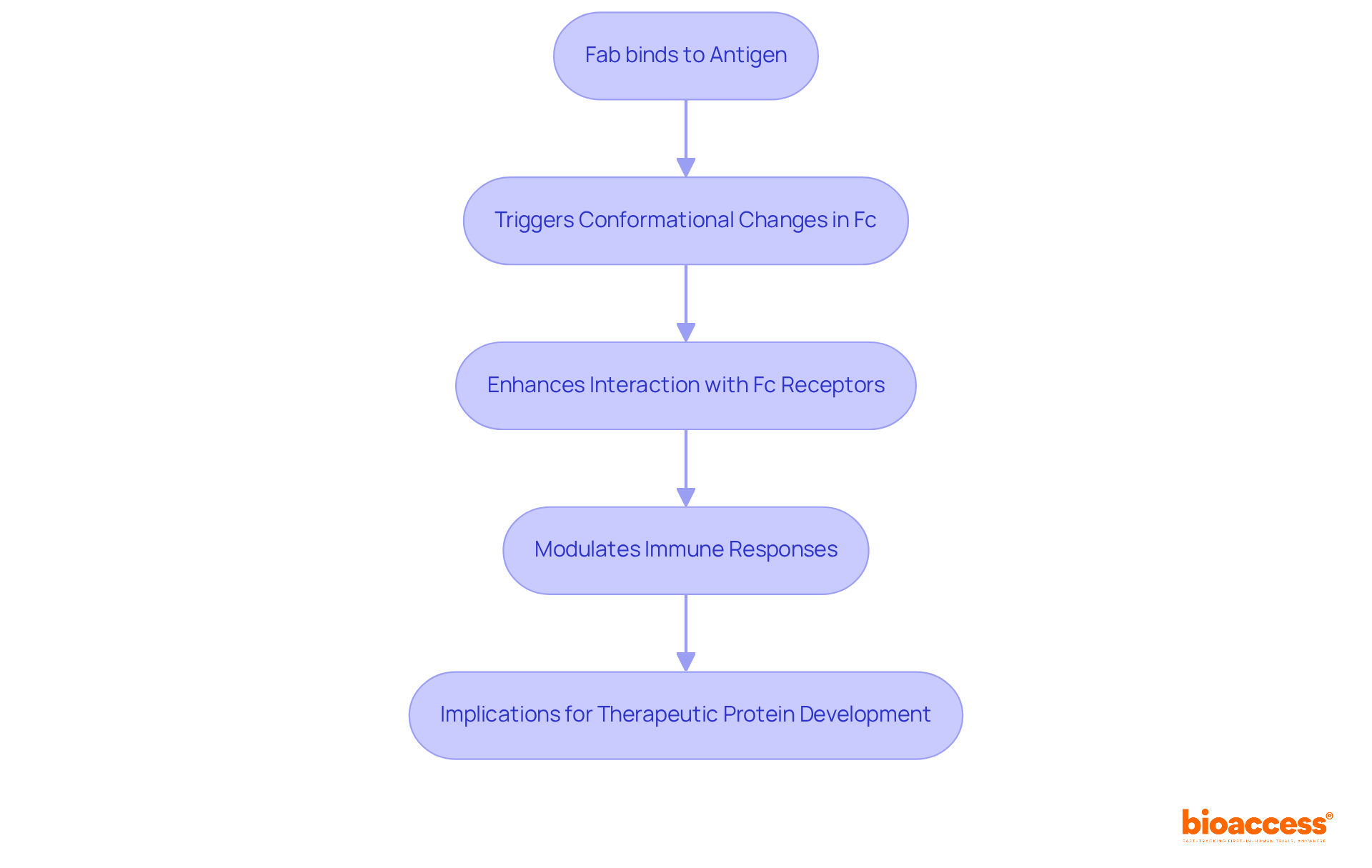
Understanding the differences between the Fab vs Fc components of immune proteins is essential for advancing clinical research. The Fab portion is primarily responsible for antigen specificity, enabling immunoglobulins to effectively bind to their targets. Conversely, the Fc region is pivotal in mediating immune responses by interacting with various immune cells and complement systems. This dual functionality significantly influences the effectiveness of immune protein treatments, as it directly impacts their ability to elicit a robust immune response.
Optimizing these differences is crucial for the development of antibody-based therapies. For instance, engineered proteins that have undergone Fc modifications demonstrate enhanced interactions with Fc receptors, leading to improved effector functions such as antibody-dependent cellular cytotoxicity (ADCC) and an extended half-life in circulation. Recent studies indicate that alterations in the Fc area can substantially boost the therapeutic potential of these proteins, making them more effective in fighting diseases.
Moreover, enhancing the Fab area can lead to improved binding affinity and specificity, which are vital for the progress of targeted therapies. By thoroughly understanding the functions of both regions, researchers can design proteins that not only bind effectively to antigens but also activate the immune system more efficiently.
Insights from recent advancements underscore that a holistic approach to optimizing both Fab vs Fc can yield significant improvements in immunotherapy outcomes. As the field evolves, integrating these optimization strategies will be critical for developing next-generation antibody therapies that provide enhanced efficacy and safety profiles for patients.
Understanding the distinctions between the Fab and Fc regions of antibodies is crucial for advancing clinical research and developing effective immunotherapies. These two components not only serve different functions but also play interdependent roles in how antibodies interact with antigens and elicit immune responses. The Fab region is primarily responsible for binding specificity, while the Fc region mediates crucial immune functions, making their functional interplay essential for therapeutic efficacy.
Key insights from the exploration of Fab and Fc differences highlight their significance in antibody engineering and therapeutic applications. Innovations in modifying these regions can lead to enhanced binding affinity and improved immune engagement, which are vital for the success of antibody-based treatments. Furthermore, understanding these components allows researchers to address challenges in clinical research and optimize therapeutic strategies, ultimately improving patient outcomes.
As the field of immunotherapy continues to evolve, collaboration among researchers, clinicians, and industry stakeholders will be imperative. Embracing the complexities of Fab and Fc interactions can drive advancements that not only meet current medical needs but also pave the way for future innovations in antibody therapeutics. Engaging in this collaborative effort will ensure that the full potential of antibody engineering is realized, leading to more effective treatments and better health outcomes for patients worldwide.
What is bioaccess® and what role does it play in clinical research for antibody therapeutics?
bioaccess® is an organization that accelerates clinical research for immune-based treatments, particularly antibody therapeutics, by leveraging Colombia's unique geographical advantages, including cost efficiency and a swift regulatory process.
What are the cost advantages of conducting clinical trials in Colombia through bioaccess®?
Clinical trials in Colombia can offer savings exceeding 30% compared to trials in North America and Western Europe.
How quickly can bioaccess® complete regulatory reviews for clinical trials?
bioaccess® can complete IRB/EC and MoH (INVIMA) reviews within 90-120 days.
What benefits does Colombia's healthcare system provide for clinical trials?
Colombia has a high-quality healthcare system recognized globally, with over 95% of the population covered by universal healthcare, facilitating rapid patient recruitment for clinical trials.
What is the significance of the ICH/GCP certification process for hospitals in Colombia?
The ICH/GCP certification process enhances the credibility of clinical research conducted in Colombia, ensuring adherence to international standards.
How does bioaccess® improve patient access to new therapies?
By streamlining enrollment and approvals, bioaccess® significantly improves patient access to groundbreaking therapies, leading to better health outcomes.
What are the structural differences between the Fab and Fc regions of antibodies?
The Fab (Fragment antigen-binding) region is responsible for antigen recognition and binding, while the Fc (Fragment crystallizable) region mediates interactions with immune cells and effector functions.
Why is the distinction between Fab and Fc regions important in clinical research?
The distinction is crucial for understanding how antibodies engage with their targets and elicit immune responses, which is essential for addressing challenges in the Medtech landscape.
What role does the Fab region play in antigen specificity?
The Fab region contains variable domains that identify and bind to specific antigens, ensuring high affinity and specificity toward target molecules.
What advancements are being made in immunoglobulin engineering related to the Fab region?
Recent advancements focus on refining the Fab component to enhance binding affinity, with techniques such as CDR clustering improving therapeutic effectiveness.
How does the focus on the Fab region reflect trends in protein engineering?
The emphasis on strengthening the structural integrity of the Fab region is critical for maintaining functionality and maximizing therapeutic potential, mirroring current trends in the field.