


The article delivers essential insights for Clinical Research Directors concerning IND (Investigational New Drug) sites, underscoring the critical need to:
By illuminating the swift approval timelines in Latin America, alongside the necessity for meticulous preparation and ethical considerations, the article delineates how these elements can profoundly elevate the success rates of clinical studies.
The landscape of clinical research is evolving, with Latin America emerging as a pivotal hub for Investigational New Drug (IND) studies. As regulatory frameworks become more agile and patient populations more diverse, clinical research directors face both exciting opportunities and significant challenges in navigating the IND application process.
What strategies can directors employ to optimize recruitment, ensure compliance, and ultimately enhance study outcomes in this dynamic environment? This article delves into ten key insights that will empower clinical research directors to leverage IND sites effectively, ensuring their studies are not only successful but also ethically sound.
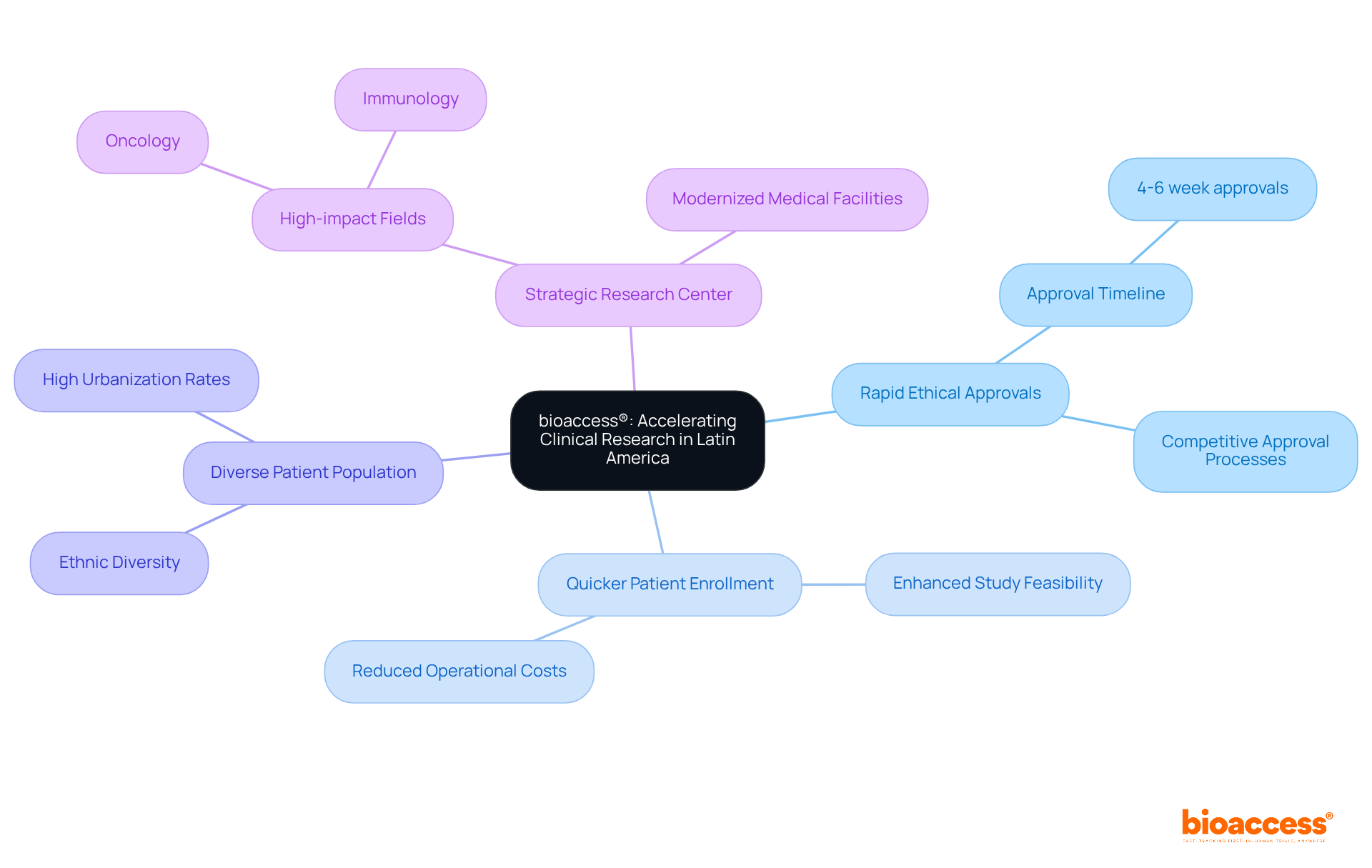
bioaccess® stands out as a leader in accelerating medical studies in Latin America, leveraging the region's rapid regulatory frameworks and diverse patient population. With ethical approvals obtained in an impressive 4-6 weeks, trial directors can significantly reduce timelines compared to conventional markets. This rapid approval not only enhances study feasibility but also facilitates quicker patient enrollment at the ind site, which is crucial for accelerating product development and market entry for innovations in Medtech, Biopharma, and Radiopharma.
The capability to enlist substantial patient groups effectively establishes Latin America as an appealing center for research, particularly in high-impact fields like oncology and immunology. As the region continues to enhance its medical facilities, the potential for successful studies rises, making it a strategic option for sponsors aiming to optimize their development budgets while ensuring high-quality standards.
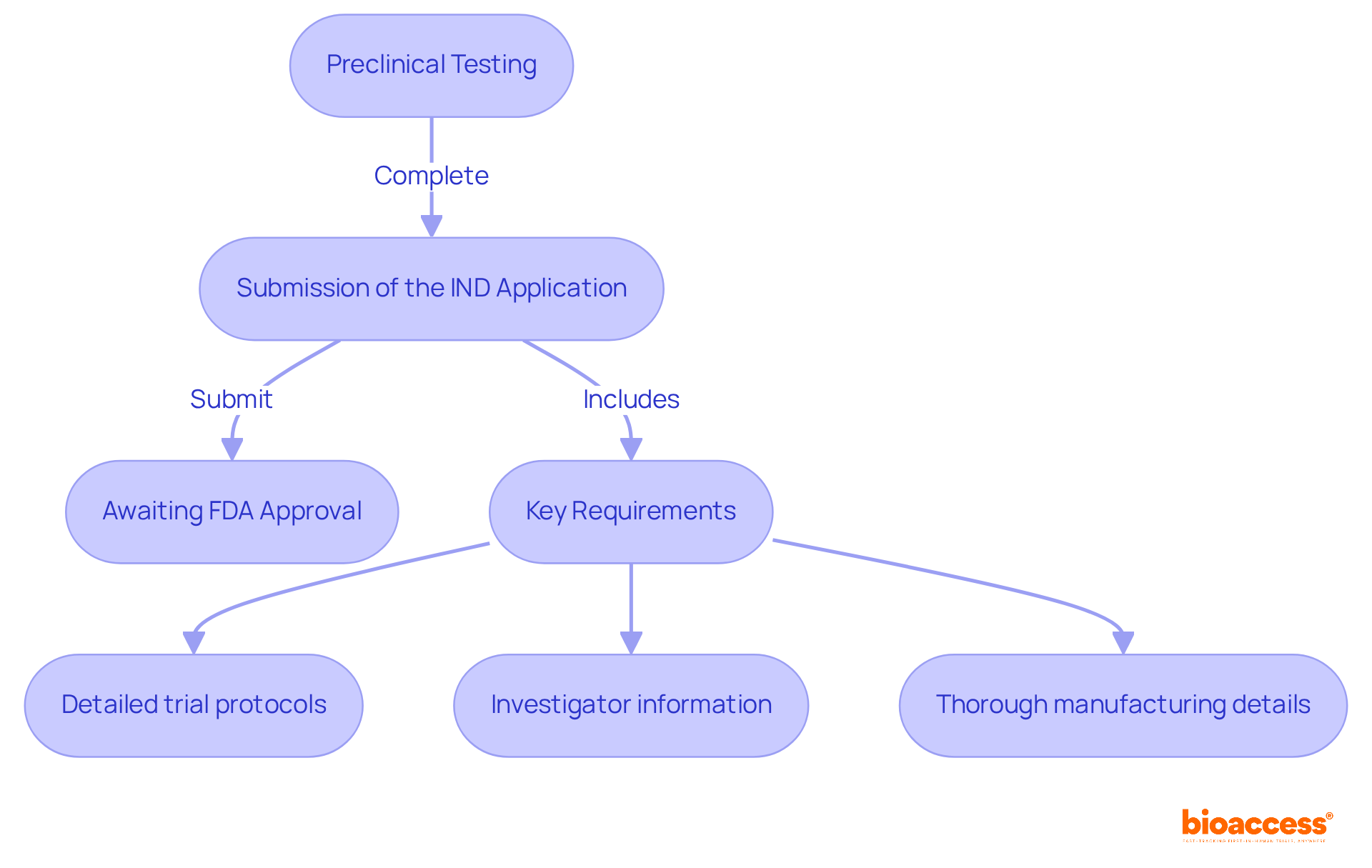
The IND site application process is a pivotal phase in drug development, comprising essential steps such as:
Directors must ensure that all required data, including comprehensive safety and efficacy information, is meticulously compiled. Key requirements for a successful IND site application include:
Notably, approximately 70% of IND applications are approved on the first attempt, underscoring the importance of careful preparation and adherence to FDA guidelines. Effective communication with regulatory experts can significantly enhance understanding of necessary documentation, such as FDA Form 1571, thereby improving compliance rates. By prioritizing these elements, directors in the medical field can facilitate a smoother transition into testing, reducing the risk of delays or holds.
IND site activities are pivotal in the execution of clinical trials, significantly impacting patient recruitment, data collection, and adherence to protocols. The selection of an IND site can profoundly affect study outcomes, particularly regarding patient retention rates and data integrity. Organizations that actively involve patients in the investigation process experience up to a 40% improvement in recruitment and a 30% increase in retention rates. A notable example is GlobalCare Clinical Studies, which, through its partnership with bioaccess™, has expanded its ambulatory services in Colombia, achieving over a 50% reduction in subject recruitment time and a retention rate exceeding 95%.
As highlighted by the Patient-Centered Outcomes Research Institute, "The best studies occur when patients and researchers collaborate closely, each contributing vital knowledge to the discussion." Therefore, clinical study directors must prioritize locations with a proven record of successful studies and robust patient involvement strategies.
Effective patient recruitment strategies at an IND site include:
This approach not only enhances the likelihood of achieving study goals but also fosters a collaborative environment where patient insights shape the study design, ultimately leading to more relevant and significant outcomes.
To implement these insights, directors should conduct thorough evaluations of potential IND sites, focusing on their patient engagement practices and historical performance in recruitment and retention.

The 30-day IND hold represents a critical regulatory checkpoint, enabling the FDA to evaluate the proposed study for safety concerns before initiation. This mandatory waiting period can significantly delay trial commencement, impacting overall project timelines. For project directors, it is essential to anticipate this potential obstacle by incorporating buffer periods into timelines. Furthermore, ensuring that all safety data is meticulously prepared can mitigate the likelihood of an IND hold.
During this hold, activities such as advertising, eligibility screening, and seeking informed consent are prohibited, highlighting the necessity of strategic planning. Once an IND is active, sponsors can swiftly ship investigational products to designated investigators without needing further IRB approval, thus streamlining the process. However, investigation activities cannot commence until the IND is effective, underscoring the importance of thorough preparation.
To alleviate delays caused by IND holds, directors should consider proactive communication with regulatory bodies and consult with IRBs to clarify permissible activities during the hold period. As Lauren Hartsmith, Director of Regulatory Affairs, articulates, "Before the FDA permits a pharmaceutical drug product to be lawfully marketed, sponsors are required to submit information about the product’s safety and efficacy so the FDA can determine whether the drug is safe and effective in its proposed use(s)." By understanding the nuances of the IND procedure and preparing comprehensively, study teams can navigate these regulatory demands more efficiently, ultimately minimizing disruptions to study schedules.
At bioaccess, we deliver focused management services for studies that directly address the challenges posed by the IND site holds. Our expertise encompasses compliance reviews and project management, ensuring that all necessary documentation is prepared to facilitate a seamless transition once the IND is active. By connecting Medtech, Biopharma, and Radiopharma startups with leading-ranked facilities, we enable studies to commence 40% faster, allowing for a more effective response to the challenges of IND holds. Effective management of the IND site can lead to a streamlined process for shipping investigational products, ensuring timely progress in research studies.

To ensure adherence to regulations at the IND site, study directors must prioritize ongoing training for their personnel on the latest regulatory updates. This training is crucial, as the frequency of regulatory changes in medical studies can significantly affect trial conduct and compliance. For instance, the ICH E6(R3) Good Clinical Practice guidelines, set to be finalized in 2025, will introduce new responsibilities for ethics committees, investigators, and sponsors, emphasizing the need for robust systems and practices.
Effective regulatory training programs, including workshops, online courses, and simulation exercises that focus on real-world scenarios, enhance staff knowledge and foster a culture of compliance within the IND site of the organization. Additionally, a compliance checklist for the IND site can streamline processes, ensuring that all study activities are thoroughly documented and meet regulatory standards.
Proactive communication with regulatory bodies represents another best practice that can protect the integrity of studies. By establishing strong relationships with these entities, medical investigation teams can navigate updates more effectively, transforming compliance challenges into strategic advantages. Moreover, utilizing Bioaccess's extensive research management services—such as feasibility studies, site selection, compliance reviews, and project management—can significantly improve adherence efforts. These services not only streamline processes but also ensure that all aspects of the study align with regulatory standards. As the landscape of medical research evolves, investing in thorough regulatory education and leveraging Bioaccess's expertise will be vital for ensuring successful study outcomes.

Optimizing recruitment strategies during the ind site process necessitates a multifaceted approach that leverages digital marketing, community outreach, and collaborations with local healthcare providers. Digital marketing is pivotal, as it enables targeted engagement with potential participants through platforms that reach over 3 billion users globally. Successful campaigns utilize social media advertising on an ind site to connect with diverse demographics, thereby enhancing visibility and participation rates.
Furthermore, employing patient engagement tools—such as personalized communication and educational content—can significantly improve recruitment outcomes. Clear communication regarding the benefits of participation on the ind site is essential; it not only attracts candidates but also fosters trust, ensuring a diverse and representative participant pool. Notably, with 73% of patients preferring to obtain information about research opportunities from their physician's office, collaborations with healthcare professionals are critical in recruitment strategies.
In Colombia, the partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a prominent location for medical studies, supported by the Minister of Health. This initiative enhances recruitment efficiency, contributes to local economic growth, and improves healthcare.
By incorporating these strategies—including bioaccess™'s extensive services such as feasibility studies, site selection, compliance reviews, setup for testing, import permits, project management, and reporting—research directors can enhance recruitment efficiency and promote successful study outcomes.

The FDA plays a pivotal role in reviewing applications at the IND site, assessing the safety and efficacy of proposed studies. In 2025, the FDA is projected to receive approximately 1,500 submissions at the IND site, with around 9% facing holds for studies. It is crucial for directors to be cognizant of the review timeline, as the FDA typically conducts its review within a 30-day window.
Essential information highlighted by the FDA includes:
Maintaining open lines of communication with the FDA can facilitate a smoother review process and help address any concerns that may arise. As emphasized by industry experts, "Preclinical data is critical in establishing a safety profile that supports the transition to human testing." Proactive follow-up can address any deficiencies early, thereby streamlining the review process and minimizing delays.
To enhance your chances of a favorable review outcome, ensure that all components of your application for the IND site are complete and formatted correctly prior to submission. Furthermore, leveraging bioaccess®'s expert services—including patient recruitment, regulatory approval, and trial data delivery—can accelerate clinical trial processes, from feasibility studies and site selection to compliance reviews and project management, ensuring that your IND application is well-prepared and positioned for success. Katherine Ruiz's expertise in regulatory affairs further underscores the importance of thorough preparation in navigating the IND application process.

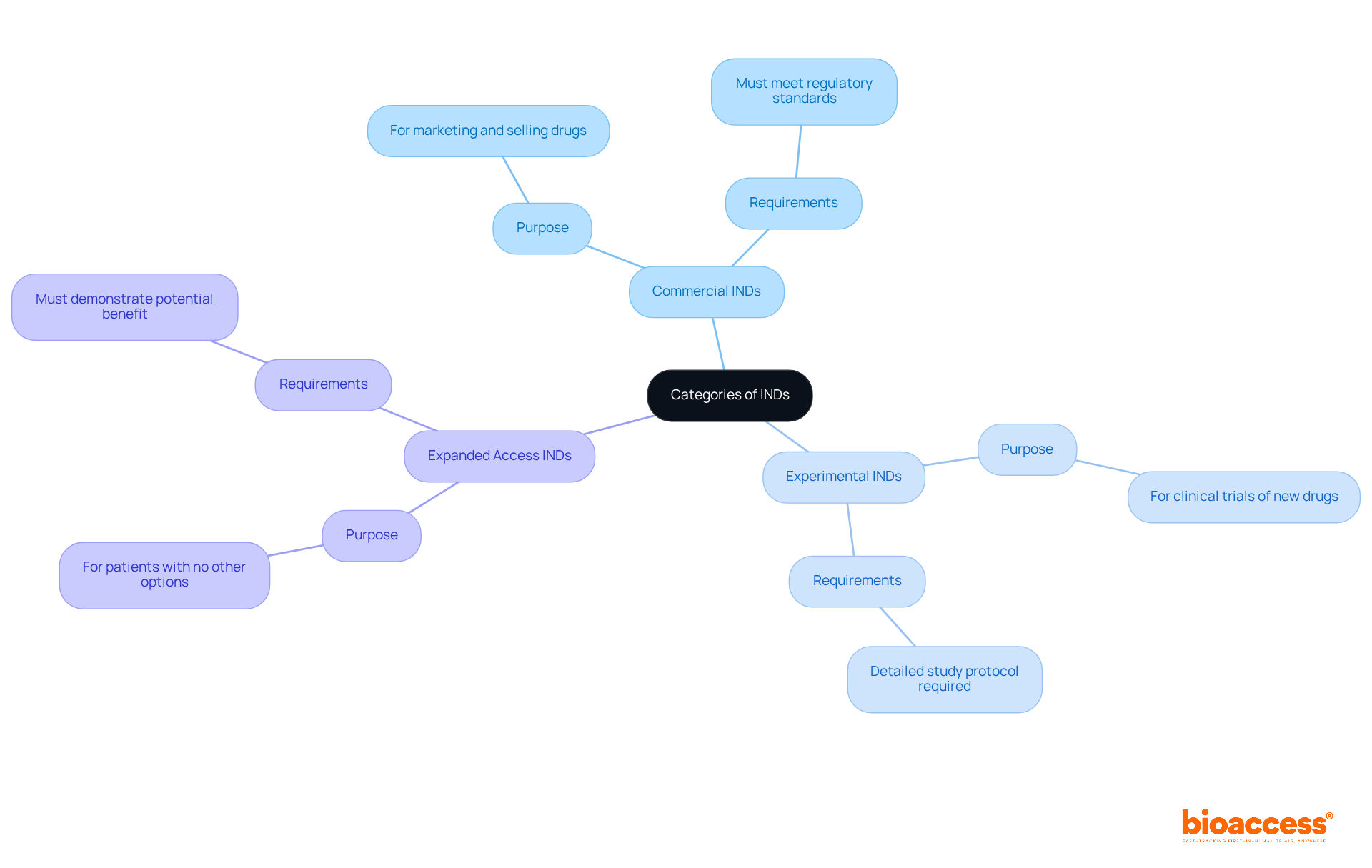
There are several types of INDs:
Each category serves a distinct purpose and has specific requirements. Understanding these distinctions is crucial for Directors of Clinical Research, particularly in the context of regulatory oversight by authorities such as INVIMA in Colombia, which is an important site.
INVIMA, the Colombia National Food and Drug Surveillance Institute, plays a vital role in ensuring compliance with health regulations. It oversees the marketing and manufacturing of health products, including medical devices. Its classification as a Level 4 health authority by PAHO/WHO underscores its competence in safeguarding the safety, efficacy, and quality of medicines.
Directors must ensure that their chosen IND type aligns with these regulatory standards and their study objectives.
Informed consent stands as a cornerstone of ethical considerations in IND site studies, fundamentally encompassing participant safety and data confidentiality. Clinical study directors must prioritize obtaining informed consent at the IND site, ensuring that participants fully grasp the study's nature, risks, and benefits. This process not only safeguards participants' rights but also cultivates trust, which is essential for participant retention.
Research indicates that approximately 75% of study participants comprehend basic elements of informed consent; however, many encounter difficulties in understanding complex concepts such as randomization and placebo. To enhance understanding, simplifying consent forms and utilizing audio-visual aids can significantly improve the consent process.
Additionally, establishing an ethics committee to oversee study protocols addresses ethical issues, bolstering the credibility of the inquiry and ensuring adherence to ethical standards. As Naveen Dha aptly states, 'Informed consent is essential to the ethical practice of medical studies and is a vital element of the investigative process.'
By implementing effective informed consent strategies, including innovative communication methods, study directors can enhance participant involvement and retention at the IND site, ultimately contributing to the success of their projects.

Clinical research directors must prioritize a thorough understanding of the IND site application process, as it is crucial for the success of clinical studies. Acquaintance with the different types of IND site and the FDA's evaluation process is vital; these factors directly impact study results. Regulatory compliance plays an important role. Studies indicate that adherence to regulations at the IND site can significantly improve success rates in clinical studies. For instance, complete genotoxicity testing should be finalized before advancing to Phase 2, ensuring that potential risks are adequately assessed.
Moreover, optimizing recruitment strategies is essential. By leveraging diverse patient pools, such as those found in Latin America and the Balkans, directors can expedite enrollment, achieving rates that are 50% faster than traditional markets. Addressing ethical considerations is equally important, as it fosters trust and transparency, which are vital for participant retention and overall study integrity.
Effective strategies for trial directors involve performing comprehensive studies at the IND site, which include pharmacokinetics assessments and toxicity evaluations. These studies not only forecast possible safety issues but also assist in calculating safe initial doses for medical experiments. By implementing these insights and remaining informed about regulatory developments, directors can navigate the complexities of clinical research more effectively, ultimately leading to more successful trial outcomes.
Understanding the intricacies of IND sites is essential for clinical research directors aiming to enhance the success of their studies. Recognizing the critical role these sites play in the IND application process enables directors to navigate regulatory requirements more effectively, ultimately leading to improved study outcomes. The insights presented emphasize the importance of strategic planning and compliance, which are vital in ensuring that clinical trials proceed smoothly and efficiently.
Key arguments highlighted throughout the article include:
By leveraging these insights, directors can optimize recruitment strategies and enhance the overall quality of their clinical trials.
As the landscape of clinical research continues to evolve, it is imperative for directors to remain proactive and informed. Embracing the strategies outlined—such as fostering strong communication with regulatory bodies, prioritizing ethical considerations, and utilizing advanced recruitment techniques—will not only streamline the IND application process but also contribute to the advancement of medical research. The commitment to adopting these best practices will ultimately lead to more efficient clinical trials and better health outcomes for patients.
What is bioaccess® and its role in clinical research in Latin America?
bioaccess® is a leader in accelerating medical studies in Latin America, utilizing the region's rapid regulatory frameworks and diverse patient population to enhance study feasibility and facilitate quicker patient enrollment.
How quickly can ethical approvals be obtained for clinical trials in Latin America?
Ethical approvals can be obtained in an impressive 4-6 weeks, significantly reducing timelines compared to conventional markets.
Why is Latin America considered an appealing center for research?
Latin America is appealing for research due to its capability to enlist substantial patient groups, especially in high-impact fields like oncology and immunology, and its improving medical facilities.
What are the key steps in the IND application process?
The key steps in the IND application process include preclinical testing, submission of the IND application, and awaiting FDA approval.
What are the key requirements for a successful IND site application?
Key requirements include detailed trial protocols, investigator information, and thorough manufacturing details.
What is the approval rate for IND applications on the first attempt?
Approximately 70% of IND applications are approved on the first attempt.
How can effective communication with regulatory experts benefit IND site applications?
It enhances understanding of necessary documentation, such as FDA Form 1571, thereby improving compliance rates and facilitating a smoother transition into testing.
What impact do IND site activities have on clinical trials?
IND site activities significantly impact patient recruitment, data collection, and adherence to protocols, affecting study outcomes, patient retention rates, and data integrity.
How can patient involvement improve clinical trial outcomes?
Organizations that involve patients in the investigation process experience up to a 40% improvement in recruitment and a 30% increase in retention rates.
What strategies can enhance patient recruitment at an IND site?
Effective strategies include utilizing patient advisory boards to inform study design and implementing outreach programs that resonate with diverse populations.
Why is it important for clinical study directors to evaluate potential IND sites?
Evaluating potential IND sites is crucial to assess their patient engagement practices and historical performance in recruitment and retention, leading to more relevant study outcomes.