


The article titled "10 Key Insights on ISO 10993 18 for Clinical Research Success" delineates the critical aspects of ISO 10993-18 and its implications for achieving success in clinical research within the medical device sector. It underscores the significance of:
in safeguarding patient safety and ensuring regulatory adherence. By highlighting how these elements collectively bolster the effectiveness and credibility of clinical research efforts, the article establishes a compelling case for their importance in the Medtech landscape.
The landscape of clinical research is undergoing rapid evolution, with regulatory standards such as ISO 10993-18 playing a pivotal role in ensuring the safety and efficacy of medical devices. This standard underscores the importance of chemical characterization of materials, a process that not only protects patient health but also facilitates compliance for manufacturers. As the demand for innovative medical solutions escalates, organizations must consider: how can they effectively navigate the complexities of ISO 10993-18 to achieve success in clinical research? This article delves into ten key insights that illuminate the path to compliance, highlighting best practices and strategies for overcoming the inherent challenges within this critical regulatory framework.
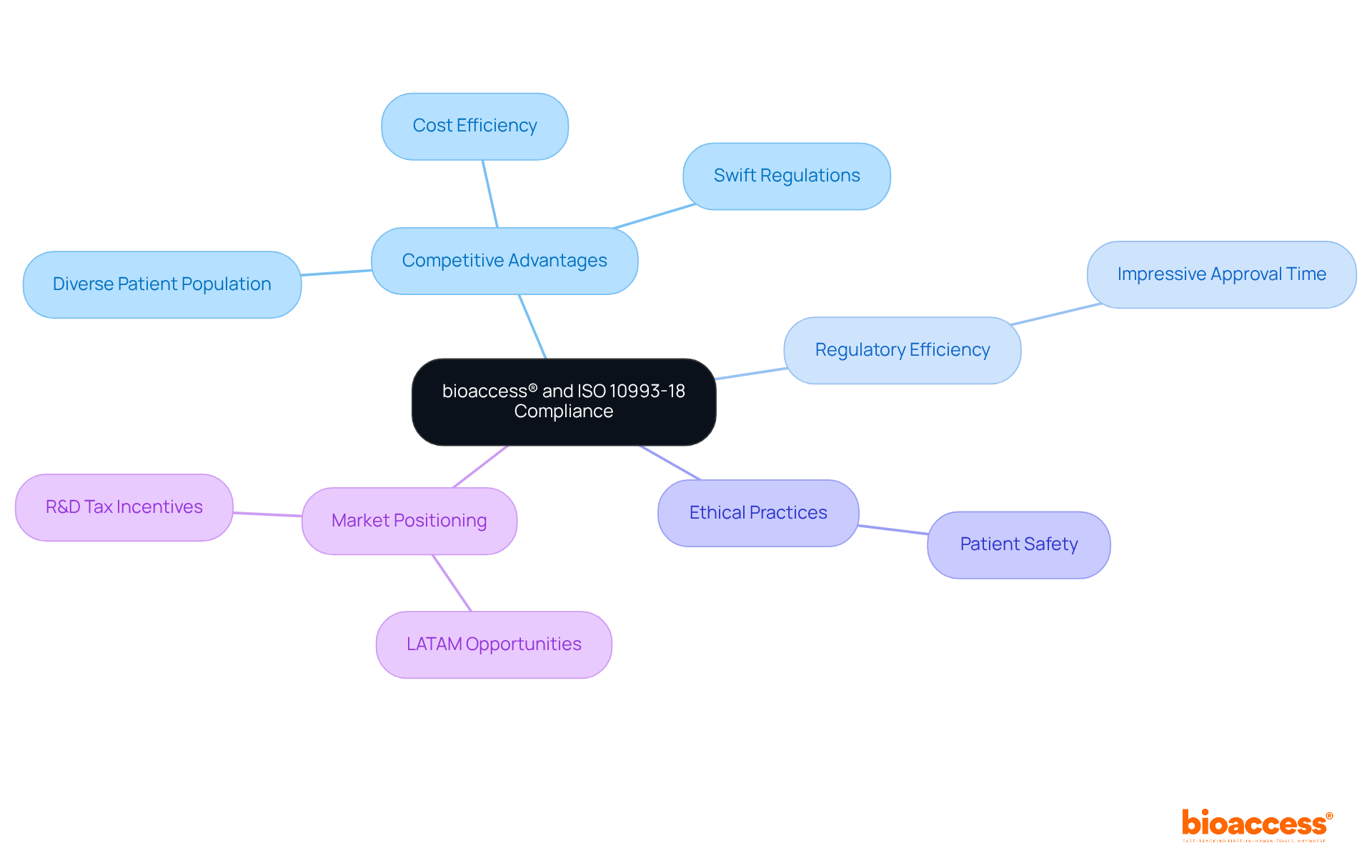
bioaccess® leverages its extensive expertise in early-phase clinical research to ensure compliance with ISO 10993 18, thereby capturing the attention of innovators in Medtech, Biopharma, and Radiopharma. By harnessing Colombia's competitive advantages—swift regulations, cost efficiency with savings exceeding 30% compared to North America, and a diverse patient population—bioaccess® secures ethical approvals within an impressive 90-120 days. This rapid turnaround is essential for innovators striving to meet the rigorous standards set by ISO 10993 18, which focuses on the chemical characterization of materials used in medical devices.
Dedicated to ethical practices and patient safety, bioaccess® empowers clients to navigate the complexities of compliance effectively, thereby supporting successful clinical research projects that align with ISO 10993 18 standards. In the context of LATAM, where regulatory reliance pathways are increasingly relevant, bioaccess® emerges as a leader in accelerating clinical research while ensuring adherence to evolving standards. This is further bolstered by Colombia's cost-effective, high-quality healthcare system and R&D tax incentives, which enhance the appeal of conducting trials in the region.
The collaboration with bioaccess® not only streamlines the compliance process but also positions clients to capitalize on the unique opportunities within the LATAM market. As the landscape of clinical research continues to evolve, engaging with a partner that prioritizes both speed and ethical standards is crucial for success.
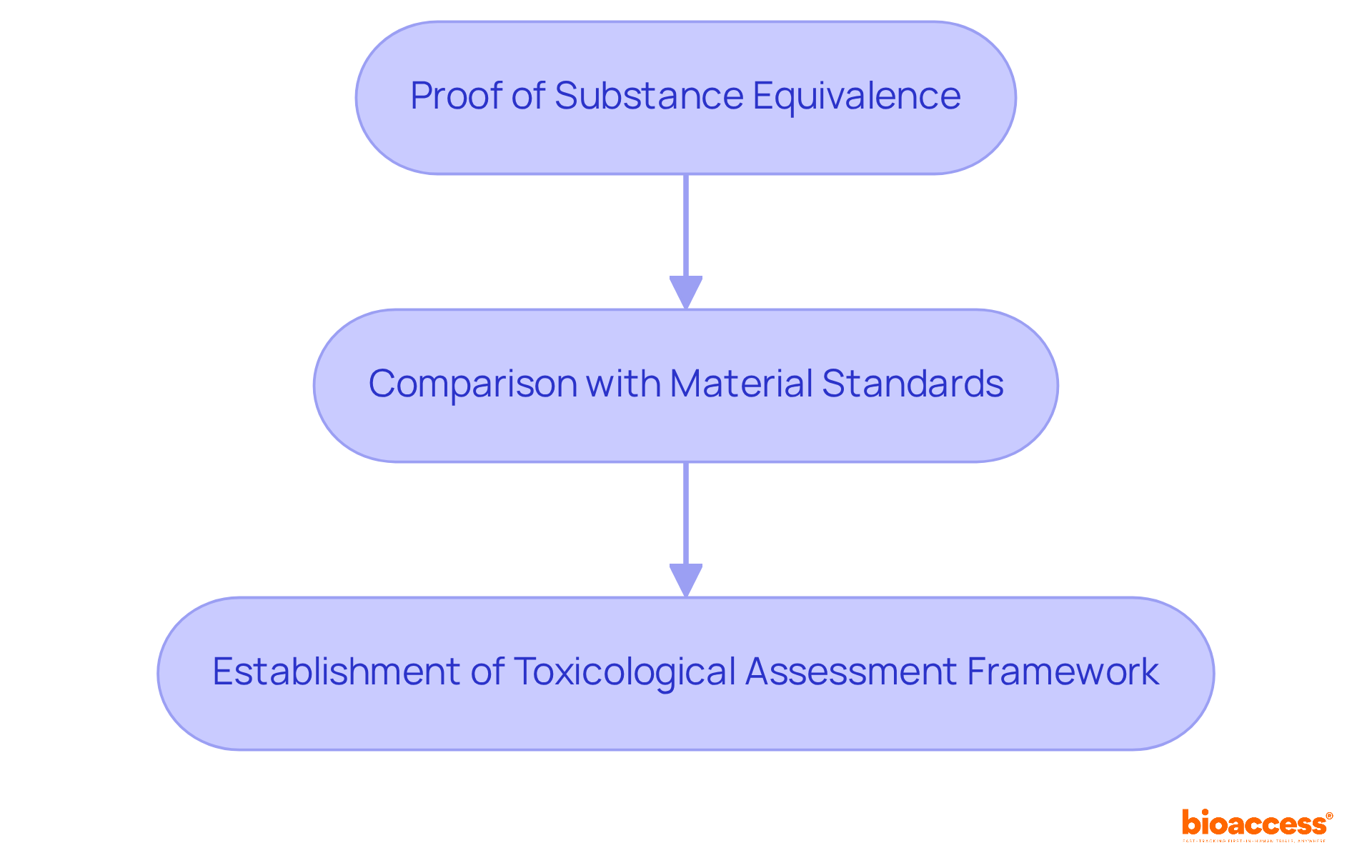
Substance characterization is a cornerstone of ISO 10993-18, focusing on the identification and quantification of material constituents in medical apparatus. This process is vital for assessing potential biological risks associated with the materials used in production. In 2025, the significance of thorough substance characterization is paramount, as it supports manufacturers in ensuring their products do not release harmful materials that could jeopardize patient health. Notably, research indicates that approximately 30% of medical instruments require further testing due to issues identified during substance characterization, underscoring the critical nature of this phase in the development process.
Successful examples of substance characterization illustrate its effectiveness in enhancing regulatory compliance and improving the safety profile of medical devices. Key stages in this process include:
Experts emphasize that a well-organized substance characterization strategy can mitigate hazards and streamline the path to market approval, ultimately benefiting both producers and patients. As Eric M. Sussman observes, "Chemical characterization can generate information for toxicological risk assessment and is an alternative approach for addressing some biocompatibility endpoints that can reduce the time and cost of testing and the need for animal testing." As the landscape of medical apparatus development evolves, integrating robust chemical characterization practices remains essential for safeguarding public health.
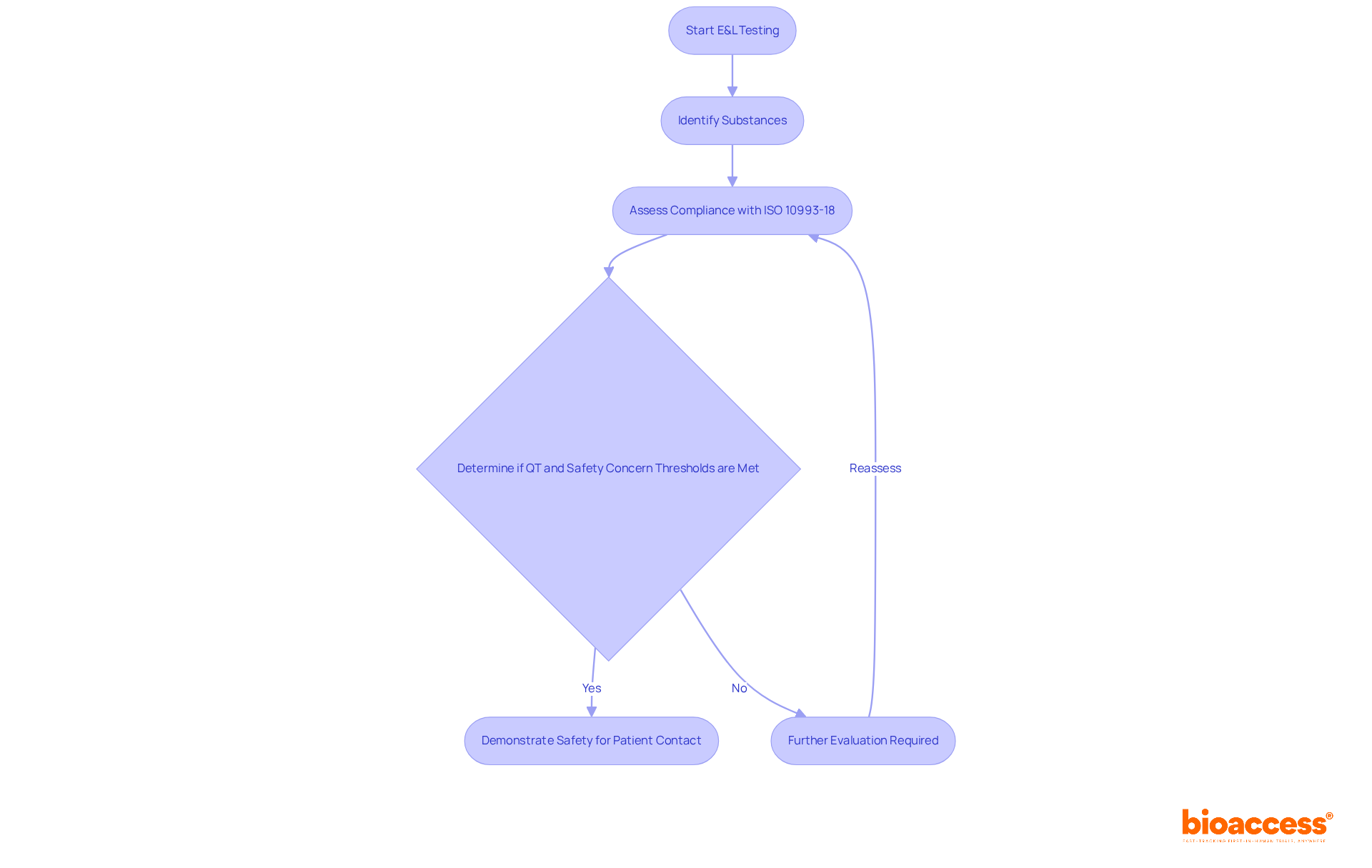
Extractables and leachables (E&L) testing is a critical component of ISO 10993 18, which is essential for assessing the safety of medical instruments. This testing identifies substances that may leach from devices into the body during use, potentially posing health risks. By conducting E&L testing, manufacturers can demonstrate compliance with biocompatibility standards, ensuring their products are safe for patient contact. This process not only satisfies regulatory requirements but also builds trust with healthcare providers and patients alike.
Recent updates indicate that by 2025, the percentage of medical devices passing biocompatibility tests following E&L testing has notably improved, reflecting advancements in testing methodologies and regulatory compliance. The integration of E&L testing into the development process has become increasingly vital, as manufacturers recognize its role in mitigating risks associated with chemical migration. The qualification threshold (QT) for E&L testing is established at 5 µg/day, while the safety concern threshold is set at 0.15 µg/day, emphasizing the stringent standards that must be adhered to.
As Chris Allen, CEO of Broughton, underscores, "Extractable and leachable studies are an essential part of product development to ensure safety and manage risk." This statement reinforces the fundamental significance of E&L testing within the medical equipment sector, particularly as regulatory bodies continue to enforce rigorous guidelines to safeguard patient health. Furthermore, the extractable and leachable testing market is anticipated to reach a valuation of $1 billion by 2028, underscoring the increasing importance of E&L testing in the industry.
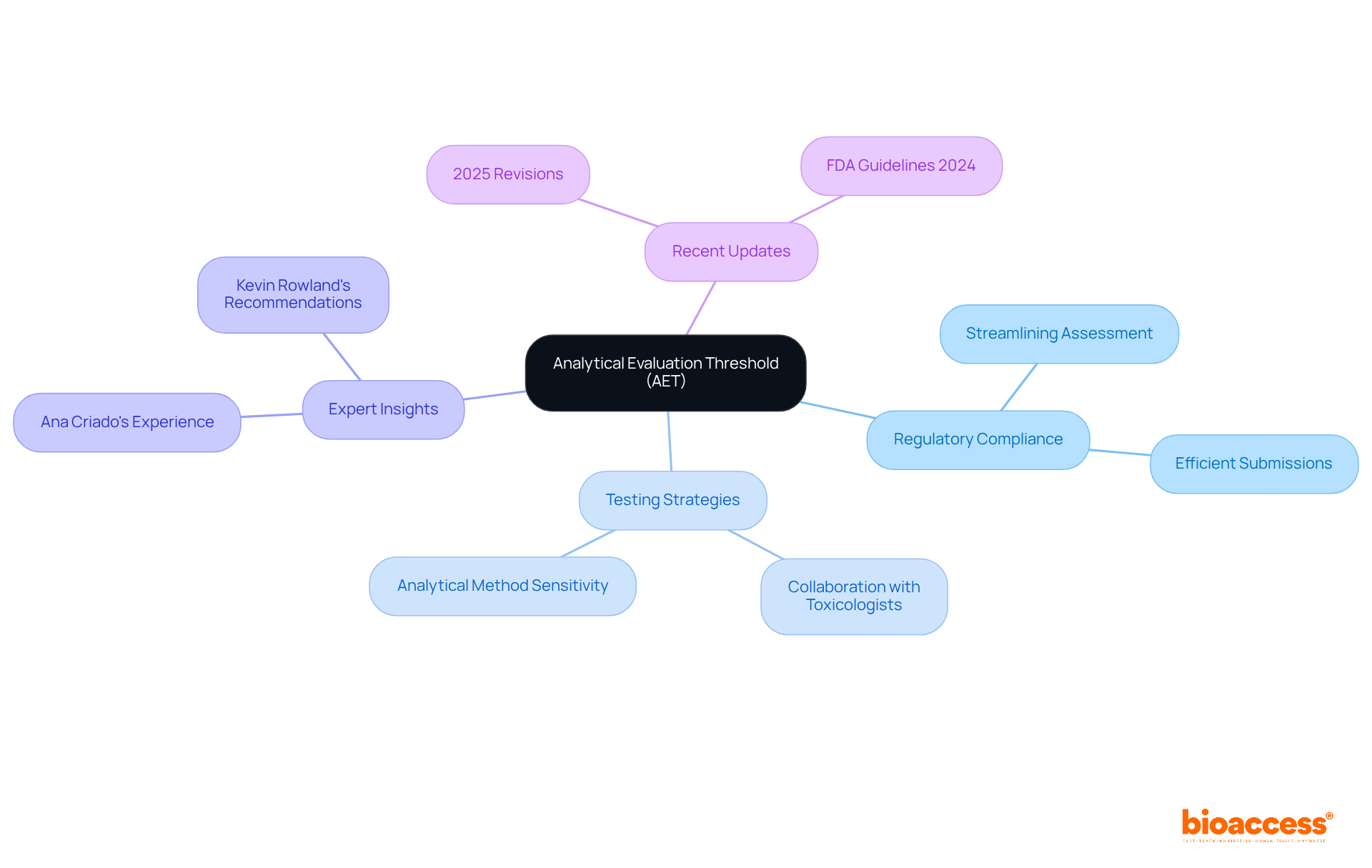
Understanding the Analytical Evaluation Threshold (AET) in ISO 10993-18
The Analytical Evaluation Threshold (AET) is a pivotal element in ISO 10993-18, establishing the minimum concentration of a substance that must be reliably quantified during chemical characterization. Substances detected below this threshold typically do not necessitate further toxicological evaluation, thereby streamlining the assessment process. By grasping the AET, manufacturers can strategically allocate resources to identify and assess potentially harmful substances, ensuring compliance while optimizing testing efforts. This focused strategy not only improves the effectiveness of the oversight process but also enables prompt market entry for medical devices.
Ana Criado, our Director of Regulatory Affairs and an expert in biomedical engineering and health economics, emphasizes the significance of AET in guiding manufacturers toward effective testing strategies that align with regulatory expectations. For instance, in her experience consulting for global companies, she has observed that a clear understanding of AET can lead to more efficient submissions and reduce the risk of costly delays. Recent updates in 2025 further emphasize the significance of the AET in substance characterization, promoting a cooperative method between toxicologists and chemists to guarantee precise application of the AET, ultimately aiding in successful submissions.
Safety Data Sheets (SDS) are essential documents that provide comprehensive information regarding the properties, hazards, and safe handling of substances utilized in medical devices. In alignment with ISO 10993-18, manufacturers must reference SDS to ensure thorough safety assessments of all chemical constituents. These documents not only assist in meeting legal standards but also play a pivotal role in managing uncertainties and safety evaluations throughout the product lifecycle. By keeping SDS current, manufacturers can showcase their dedication to safety and regulatory compliance.
The structured format of SDS, divided into 16 sections, ensures that critical information is readily accessible, facilitating effective hazard management. Expert insights indicate that proficient hazard management through SDS can considerably diminish potential risks, ensuring that organizations prioritize safety in their operations.

ISO 10993-18 and ISO 14971 are pivotal standards that collectively create a robust framework for addressing hazards associated with medical equipment. While ISO 14971 provides comprehensive management guidelines throughout the device lifecycle, ISO 10993-18 focuses on material characterization. By integrating ISO 10993-18 standards, manufacturers can systematically identify, assess, and mitigate risks related to chemical constituents, ensuring a thorough evaluation of potential hazards.
This comprehensive approach not only enhances product safety but also ensures regulatory compliance, ultimately leading to improved patient outcomes. Industry leaders emphasize that effective threat management is critical; as one expert stated, 'Medical equipment firms MUST have established threat management processes that adhere to ISO 14971.' Moreover, recent statistics reveal that over 70% of medical equipment manufacturers are now embracing both ISO standards, reflecting a growing acknowledgment of their significance in promoting safer medical innovations.
Examples of integrated safety management strategies include:
This ensures that every aspect of a device's safety profile is assessed, reinforcing the commitment to patient protection and compliance.

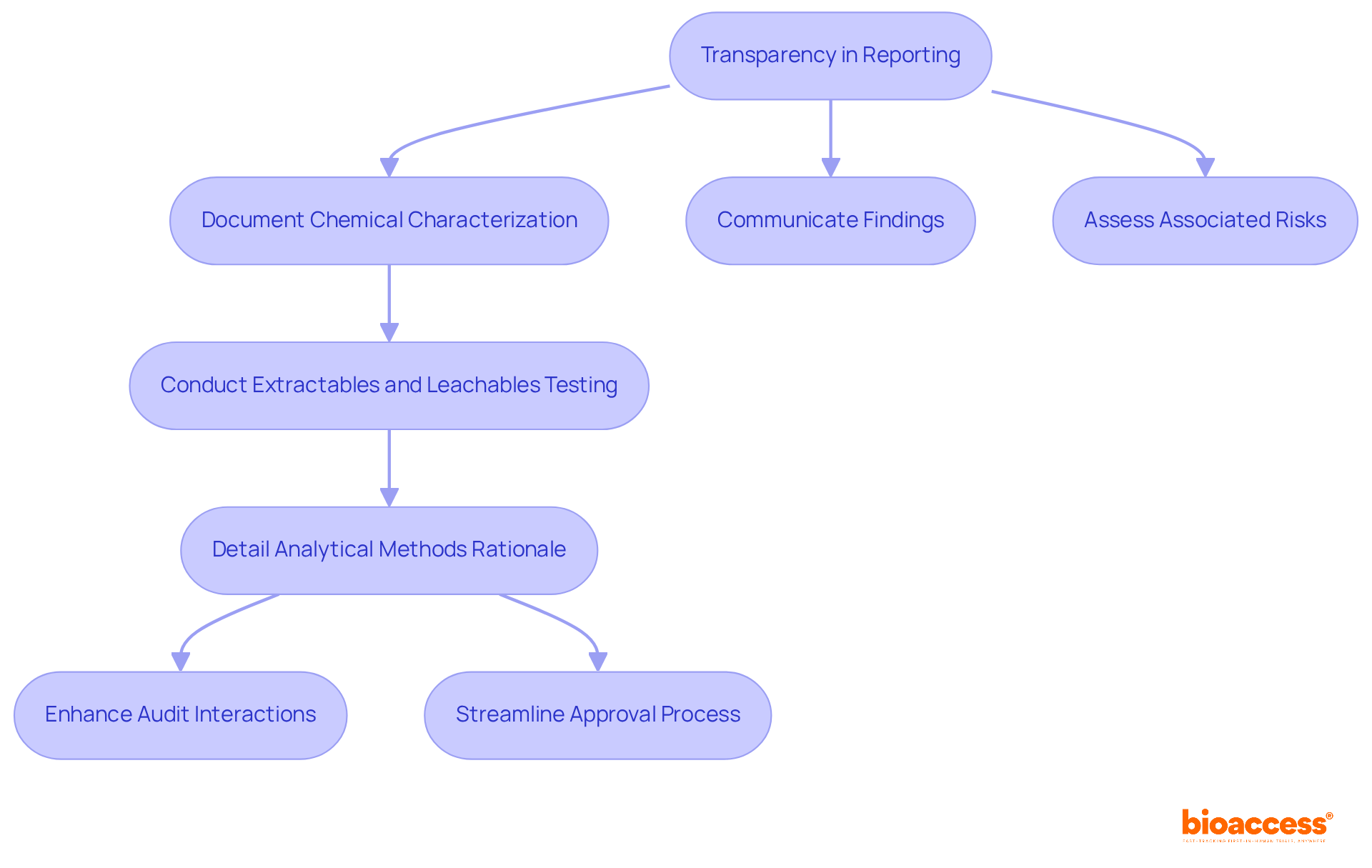
Transparency in reporting is vital for adherence to ISO 10993-18. Manufacturers must meticulously document and communicate their chemical characterization processes, findings, and associated risks. This requirement encompasses comprehensive reports on extractables and leachables testing, detailing the rationale behind the selected analytical methods. By adhering to strict documentation standards, including ISO 10993-18, manufacturers not only demonstrate their commitment to safety and compliance with regulations but also cultivate trust with oversight authorities and stakeholders. Effective reporting practices significantly enhance interactions during audits and reviews. Studies indicate that organizations with transparent documentation experience improved audit outcomes. Furthermore, insights from compliance specialists underscore that clear documentation can streamline the approval process, reducing the likelihood of delays and fostering a culture of accountability within the organization.
Demonstrating biological equivalence under ISO 10993-18 presents significant challenges for producers, particularly amid the evolving compliance landscape in Colombia. A major hurdle is the absence of harmonized guidance from regulatory bodies, which leads to discrepancies in the interpretation and application of the standard across various regions. Reports indicate that approximately 60% of producers face inconsistencies in guidance, complicating their compliance efforts.
Moreover, manufacturers must navigate complex testing requirements to ensure their materials comply with rigorous safety standards. Experts such as Ana Criado, Director of Compliance and a professor in biomedical engineering, and Katherine Ruiz, a specialist in compliance for medical tools and in vitro diagnostics, underscore the importance of a proactive approach. This includes:
As industry leaders emphasize, effectively navigating these regulatory challenges is vital for sustaining competitiveness in a rapidly changing environment.

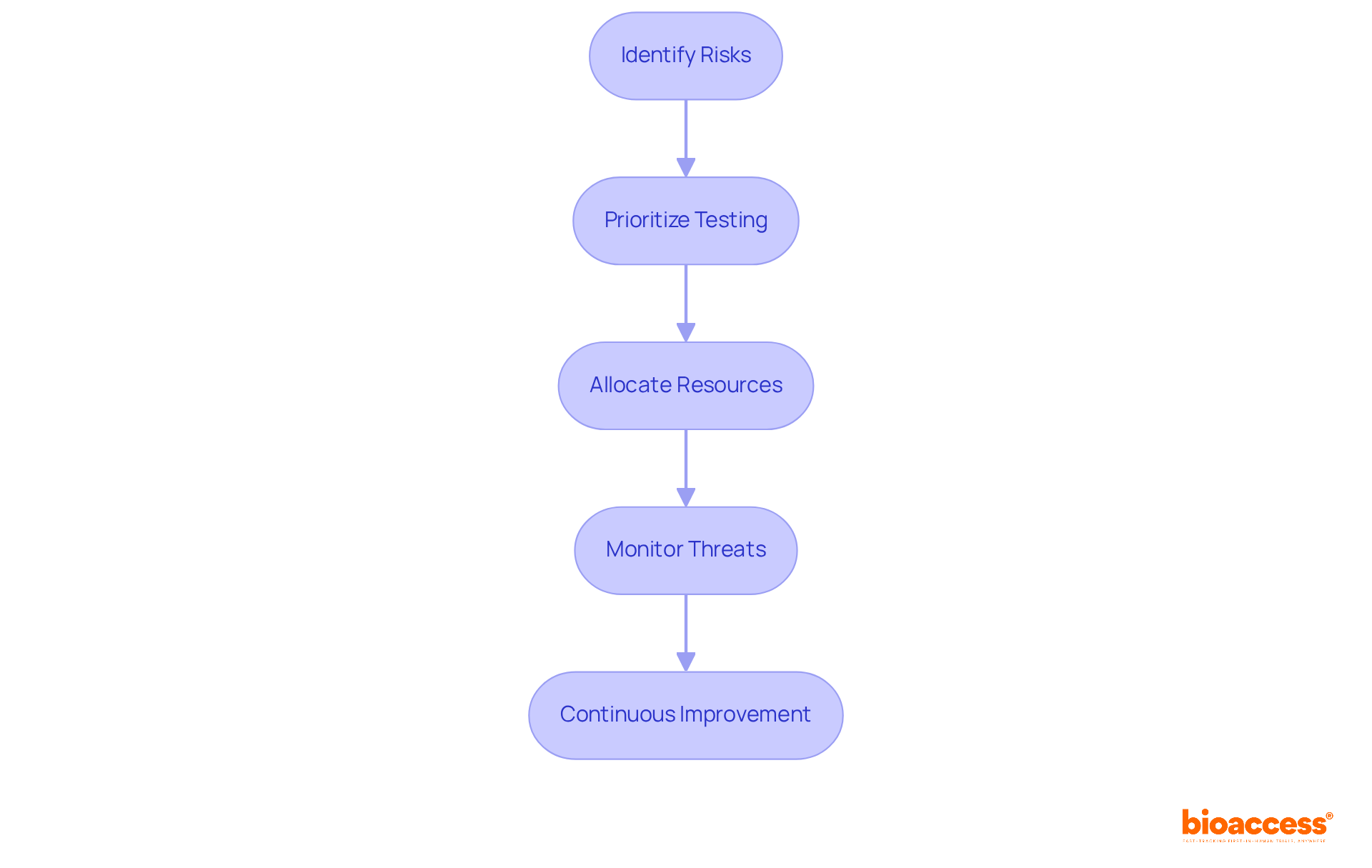
Adopting a risk-based strategy in substance characterization is essential for compliance with ISO 10993 18. This approach enables producers to prioritize testing activities based on the potential hazards associated with specific materials and their intended uses. By focusing on high-risk components, organizations can allocate resources more efficiently, streamlining the compliance process. This method not only enhances the efficiency of chemical characterization but also strengthens the overall safety and efficacy of medical devices, ensuring compliance with regulatory standards while minimizing unnecessary testing.
Industry leaders emphasize that focusing on the evaluation of challenges can lead to improved resource distribution. For instance, 31% of executives view third-party threats as the greatest challenge to company growth, underscoring the importance of prioritizing testing based on potential issues. Furthermore, early planning is crucial to avoid missing critical data, which can significantly impact compliance timelines.
Establishing a framework for continuous threat monitoring is also vital, as it allows organizations to adapt to evolving conditions and maintain compliance. By employing a formula like Likelihood x Impact, manufacturers can effectively prioritize their testing strategies, ensuring that they address the most significant hazards in their substance characterization processes.
The framework for the chemical characterization of materials established by ISO 10993-18 is pivotal in safeguarding the safety of medical instruments. This standard is instrumental in identifying potential biological risks linked to component elements, thereby protecting patient health.
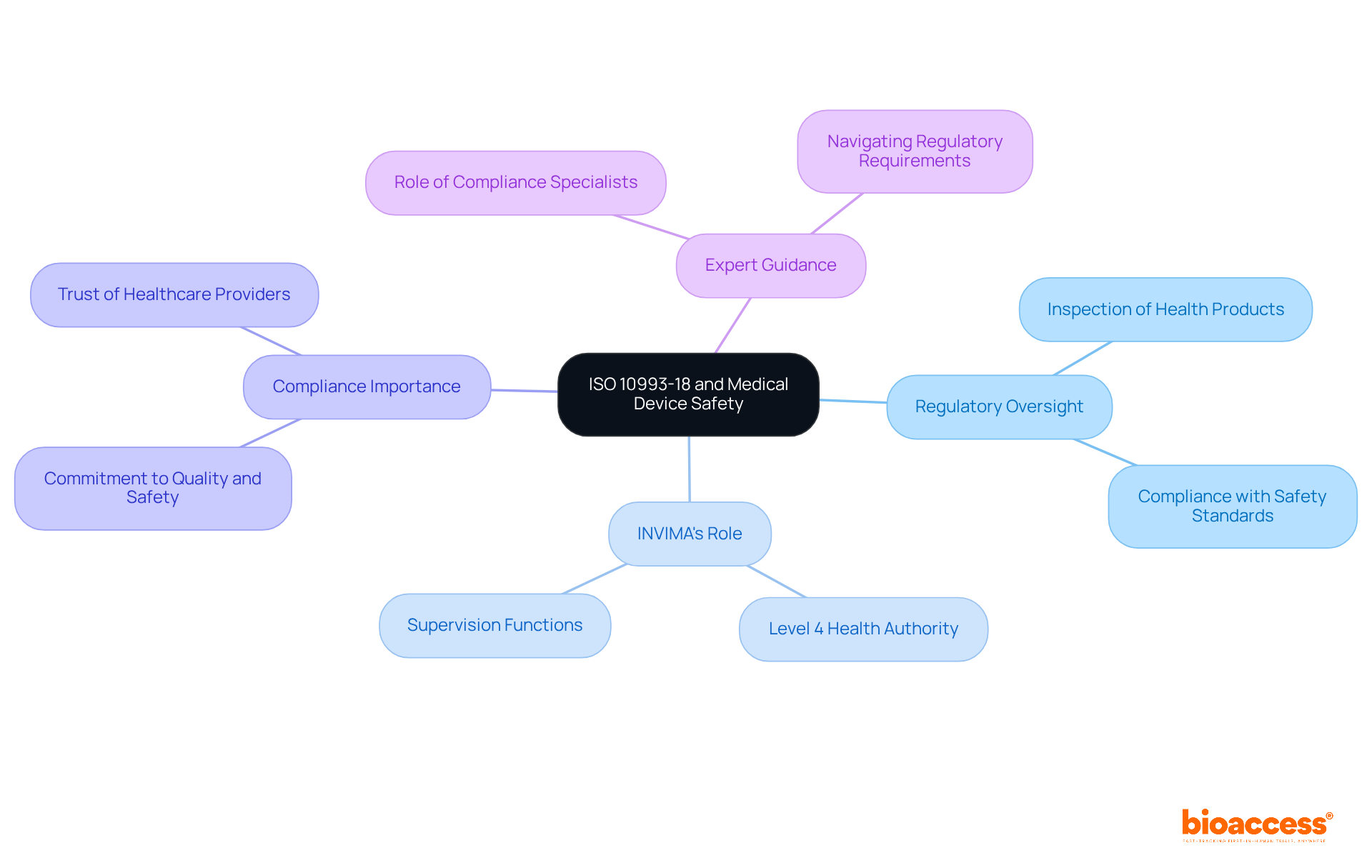
In Colombia, the oversight of medical devices is significantly shaped by INVIMA (Colombia National Food and Drug Surveillance Institute), recognized as a Level 4 health authority by PAHO/WHO. INVIMA's oversight functions include:
By adhering to the standards set by ISO 10993-18, manufacturers can affirm their commitment to quality and safety, ultimately bolstering the trust of healthcare providers and patients alike.
Specialists such as Ana Criado, Director of Compliance at Bioaccess, leverage their extensive experience in compliance and biomedical engineering to guide companies through the complexities of meeting regulatory requirements.
As the medical device landscape evolves, compliance with ISO 10993-18, supported by robust regulatory frameworks, remains a critical factor for the successful development and commercialization of safe and effective medical technologies.
The insights presented on ISO 10993-18 underscore its critical role in ensuring the safety and compliance of medical devices. By focusing on rigorous chemical characterization and biocompatibility testing, manufacturers can effectively navigate the complexities of regulatory requirements while prioritizing patient safety. Collaboration with bioaccess® further enhances the ability to meet these standards efficiently, showcasing the importance of strategic partnerships in clinical research.
Key arguments throughout the article highlight the significance of:
The integration of Safety Data Sheets (SDS) and a risk-based approach to chemical characterization also emerge as essential components for maintaining transparency and enhancing the safety profile of medical devices. As the landscape of regulatory compliance continues to evolve, the proactive adoption of these strategies is paramount for success.
Ultimately, embracing the principles outlined in ISO 10993-18 not only fosters regulatory compliance but also contributes to the advancement of safer medical technologies. Manufacturers are encouraged to prioritize these practices, as they safeguard patient health and build trust with healthcare providers and stakeholders. Engaging with experts and leveraging available resources can drive innovation and ensure that medical devices meet the highest standards of safety and efficacy.
What is bioaccess® and what role does it play in clinical research?
bioaccess® is a company that leverages its expertise in early-phase clinical research to ensure compliance with ISO 10993-18, focusing on the chemical characterization of materials used in medical devices. It helps innovators in Medtech, Biopharma, and Radiopharma navigate compliance effectively and supports successful clinical research projects.
How does bioaccess® ensure compliance with ISO 10993-18?
bioaccess® secures ethical approvals within 90-120 days by utilizing Colombia's competitive advantages such as swift regulations, cost efficiency, and a diverse patient population. This rapid turnaround is essential for meeting the rigorous standards set by ISO 10993-18.
What are the benefits of conducting clinical trials in Colombia?
Conducting clinical trials in Colombia offers cost savings exceeding 30% compared to North America, access to a high-quality healthcare system, and R&D tax incentives, making it an appealing location for clinical research.
What is the significance of substance characterization in ISO 10993-18?
Substance characterization is crucial for identifying and quantifying material constituents in medical devices, which helps assess potential biological risks. It is essential for ensuring that products do not release harmful materials that could jeopardize patient health.
What are the key stages in the substance characterization process?
Key stages include proof of substance equivalence, comparison with material standards, and the establishment of a toxicological assessment framework as outlined in ISO 10993-18.
Why is extractables and leachables (E&L) testing important?
E&L testing is critical for assessing the safety of medical instruments by identifying substances that may leach into the body during use. It ensures compliance with biocompatibility standards and helps build trust with healthcare providers and patients.
What are the established thresholds for E&L testing?
The qualification threshold (QT) for E&L testing is set at 5 µg/day, while the safety concern threshold is established at 0.15 µg/day, highlighting the stringent standards that must be adhered to.
What is the projected market value for extractable and leachable testing by 2028?
The extractable and leachable testing market is anticipated to reach a valuation of $1 billion by 2028, indicating its increasing importance in the medical equipment sector.