


This article presents essential insights regarding ISO 14155:2020 that Clinical Research Directors must grasp to ensure compliance and enhance clinical study outcomes. It underscores the significance of:
These elements are critical for upholding the integrity and safety of medical research, as mandated by ISO 14155:2020. Understanding these aspects is not merely a regulatory requirement but a pathway to achieving excellence in clinical research.
Understanding the intricacies of ISO 14155:2020 is essential for Clinical Research Directors navigating the complex landscape of clinical trials. This standard establishes the framework for regulatory compliance, enhancing both patient safety and data integrity. As the medical research field evolves, the challenge lies in effectively implementing these updated guidelines while ensuring efficient trial execution.
What strategies can Directors adopt to seamlessly integrate ISO 14155:2020 into their operations and optimize their research outcomes?
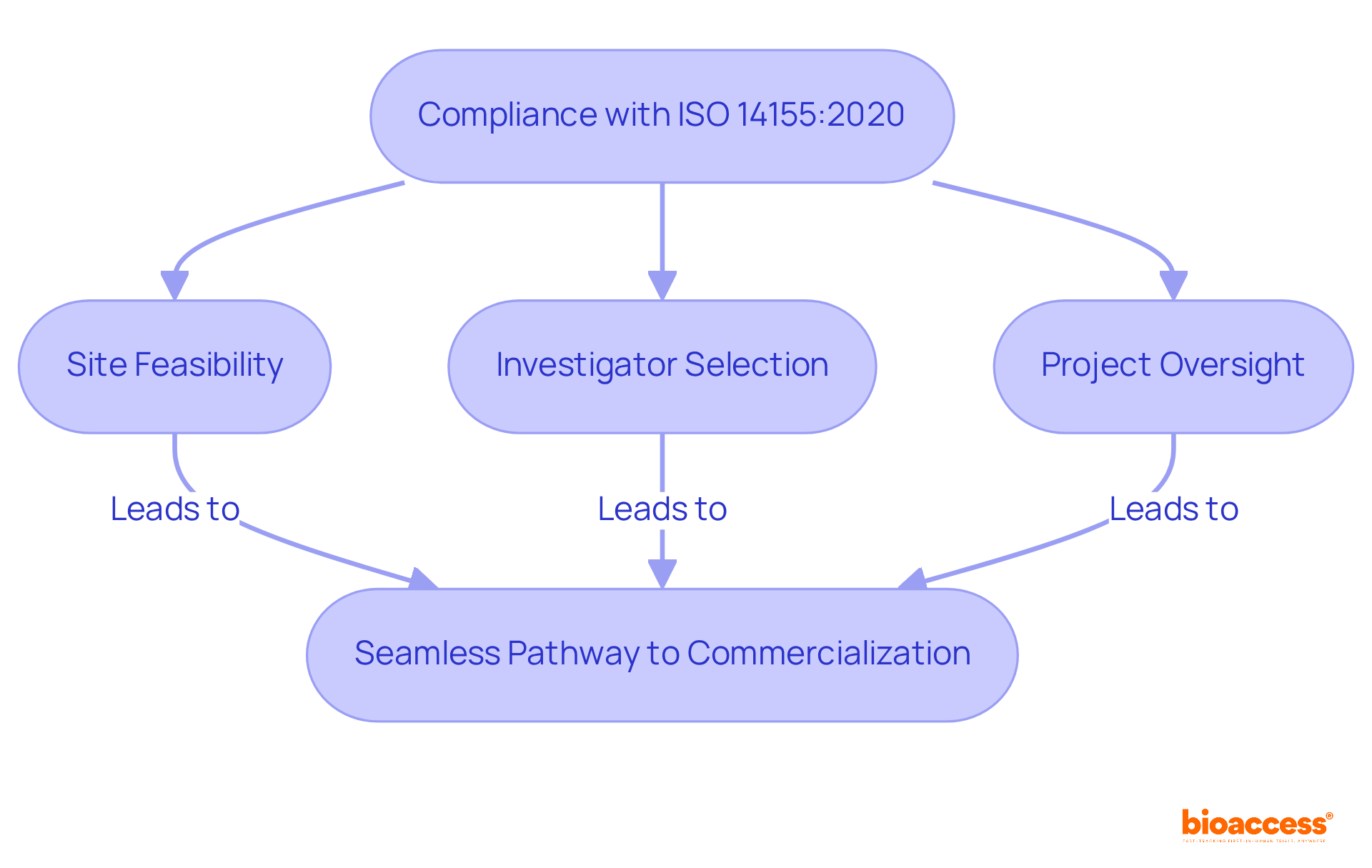
Bioaccess® leverages its extensive knowledge of regulatory frameworks across Latin America, the Balkans, and Australia to ensure compliance with ISO 14155:2020. By integrating local regulatory speed with international standards, bioaccess® guarantees that clinical studies are not only compliant but also efficient, significantly reducing the time to market for innovative medical devices.
In Colombia, where the total IRB/EC and MoH (INVIMA) review takes only 90-120 days, bioaccess® capitalizes on the country's cost efficiency—offering savings exceeding 30% compared to trials in North America or Western Europe.
This strategic approach empowers clients to focus on their core innovations while bioaccess® adeptly manages the complexities of regulatory requirements, including:
Ensuring a seamless pathway to commercialization.
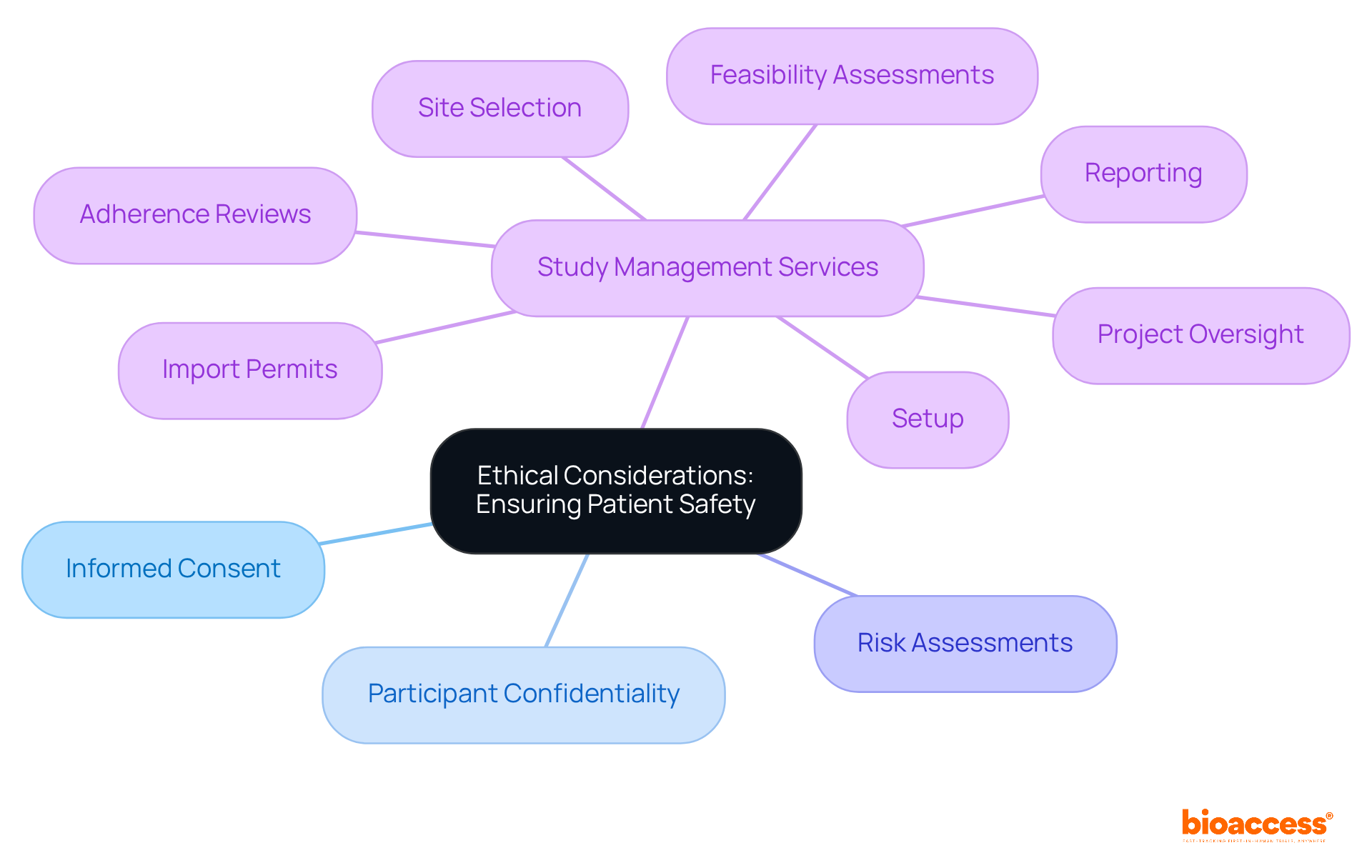
Ensuring patient safety is a cornerstone of medical investigations that adhere to ISO 14155:2020. Ethical considerations encompass obtaining informed consent, safeguarding participant confidentiality, and conducting risk assessments to minimize potential harm.
To uphold these ethical standards, bioaccess offers comprehensive study management services, including:
A critical aspect of these services involves the meticulous review and feedback on study documents to ensure compliance with country requirements. These services are meticulously designed to uphold the integrity of studies while protecting the rights and well-being of participants.
Furthermore, ongoing training and updates on ethical practices are essential for all team members engaged in research studies, ensuring they are thoroughly prepared to navigate the complexities of medical investigations.
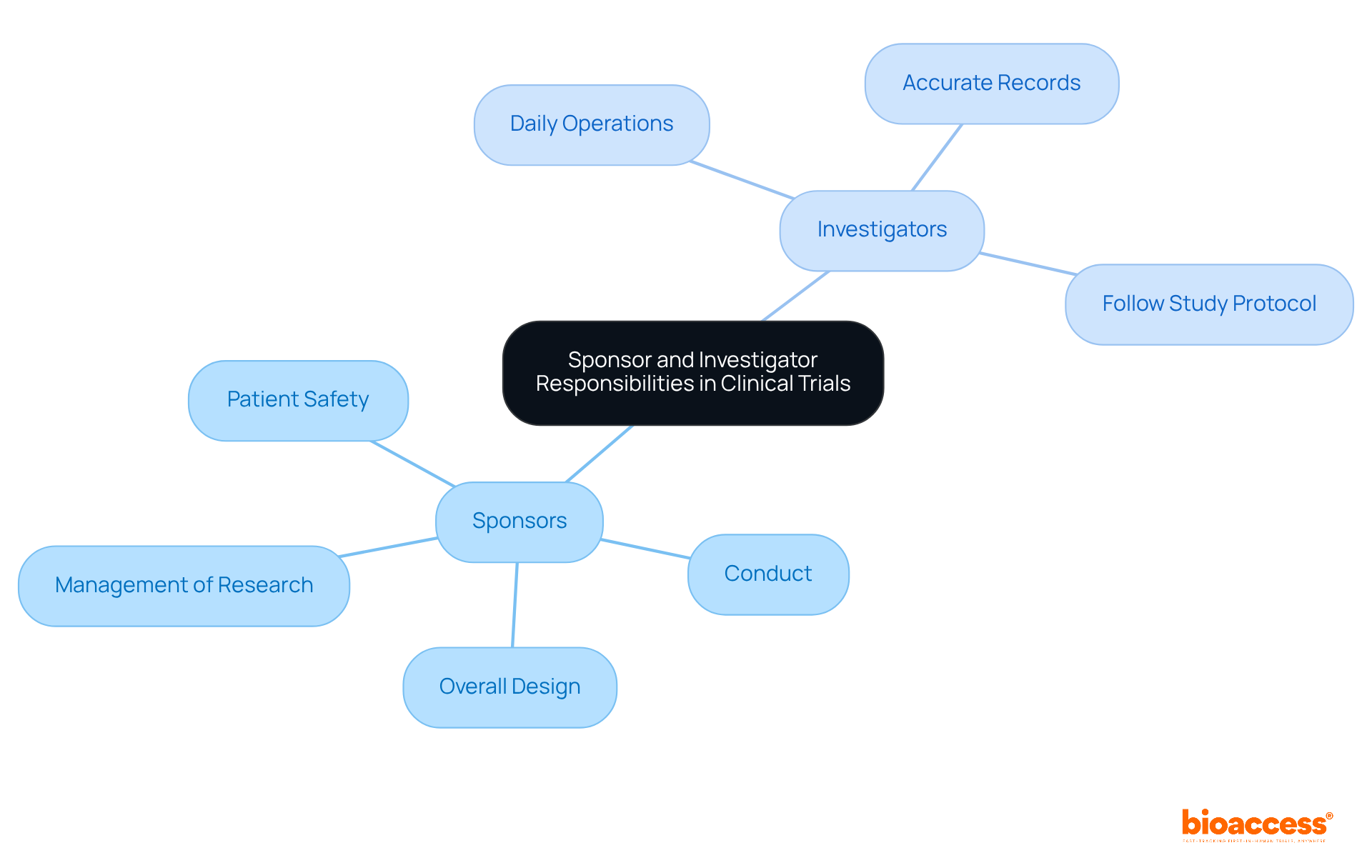
In clinical studies, sponsors hold the primary responsibility for the overall design, conduct, and management of research, whereas investigators manage the daily operations at the study site. This collaboration is crucial to ensure strict adherence to ISO 14155:2020, especially in addressing the patient recruitment challenges that Medtech and Biopharma startups frequently encounter.
It is vital to maintain accurate records, ensure participant safety, and strictly follow the study protocol. Clear communication and well-defined roles are essential for the successful execution of trials and compliance with regulations, especially as these organizations aim to recruit patients both effectively and efficiently.
Furthermore, leveraging innovative solutions for data management and adherence can significantly enhance the recruitment process.
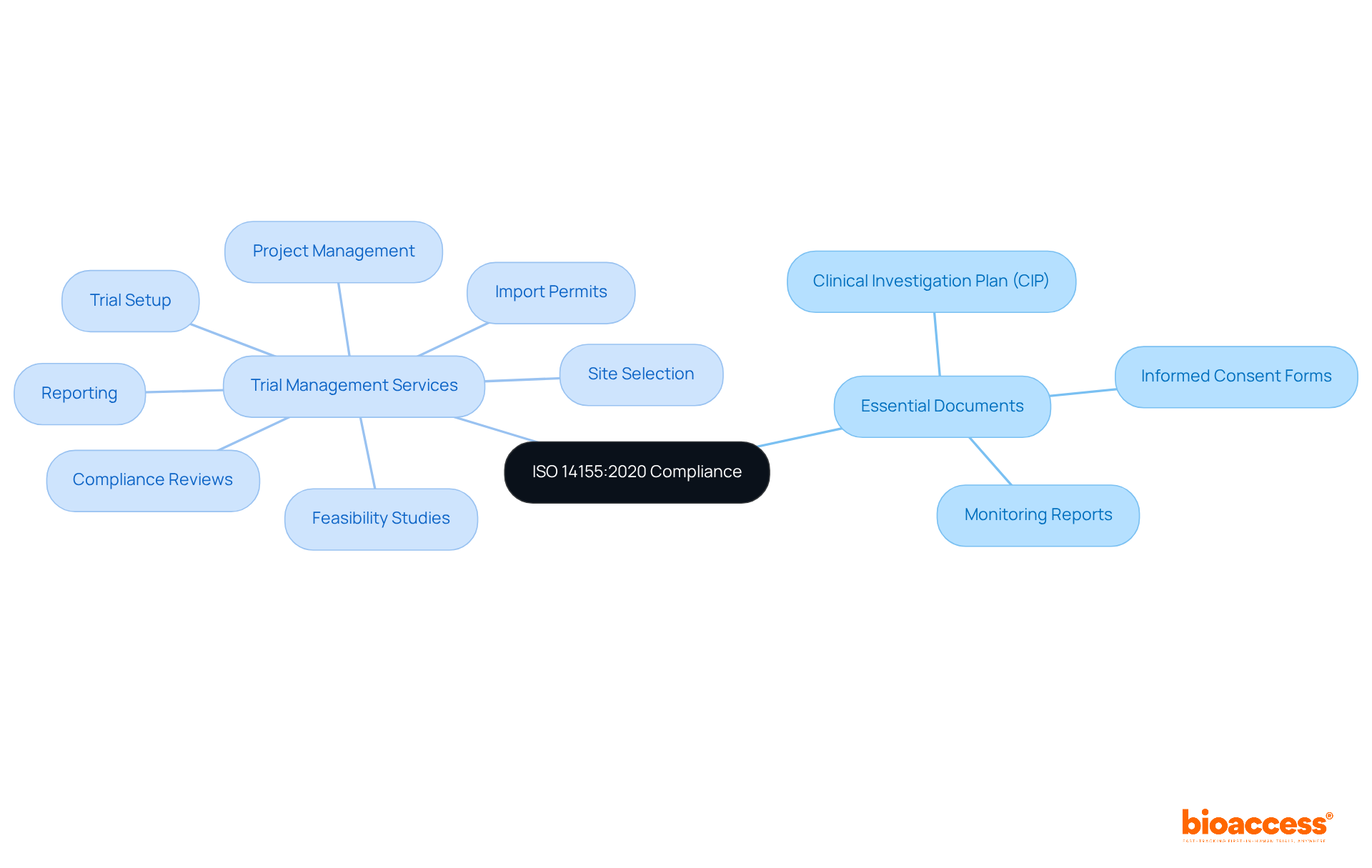
The critical importance of comprehensive documentation throughout the research investigation process is underscored by ISO 14155:2020. This encompasses the maintenance of essential documents, including:
Adequate documentation, as outlined in ISO 14155:2020, not only ensures adherence to regulations but also fosters clarity and accountability in research studies. At Bioaccess, we offer comprehensive trial management services, which include:
These services guarantee meticulous adherence to all documentation practices as outlined in ISO 14155:2020, thereby enhancing the overall integrity of the research investigation. Routine audits and assessments of documentation practices are instrumental in identifying areas for improvement and ensuring compliance with regulatory standards such as ISO 14155:2020. This ultimately contributes to the success of medtech research studies and their positive impact on local economies.
Clinical Research Directors must be aware of the new requirements introduced by ISO 14155:2020, which include:
These modifications are designed to enhance the safety and effectiveness of research studies. To navigate these updates efficiently, Directors should leverage comprehensive research study oversight services, such as those offered by bioaccess. These services encompass:
It is crucial for Directors to ensure that their teams are trained on these updates and that their trial protocols reflect the standards of ISO 14155:2020 to maintain compliance and protect participant safety. Collaborating with experts like Katherine Ruiz, who specializes in regulatory affairs for medical devices and in vitro diagnostics in Colombia, can significantly enhance Directors' understanding and implementation of these requirements.
Risk oversight is a cornerstone of ISO 14155:2020, which mandates that Research Directors establish systematic processes for identifying, assessing, and mitigating risks throughout research investigations. Regular risk assessments are not merely beneficial; they are essential, complemented by the development of robust contingency plans.
Training team members to recognize and respond to potential issues is crucial, fostering a proactive risk management culture. This approach enhances the safety and effectiveness of medical studies while aligning with contemporary trends in risk evaluation techniques that emphasize the integration of innovative technologies and data analysis.
Effective risk evaluation necessitates ongoing oversight and adjustment, ensuring that organizations adeptly navigate the complexities of medical studies. By embracing these practices, Clinical Research Directors can significantly elevate study outcomes and ensure compliance with regulatory standards.
Leveraging comprehensive research study oversight services—including:
can enhance the overall efficiency of medical investigations. These services not only streamline processes but also bolster local economies through job creation, economic growth, and healthcare enhancement, thereby promoting international collaboration within the Medtech sector.
Electronic Data Capture (EDC) systems play a pivotal role in enhancing adherence to the standards of ISO 14155:2020 by providing a systematic and effective method for collecting and managing research data. These systems enable real-time data entry, significantly reduce the risk of errors, and guarantee that data is securely stored and readily accessible for audits.
In addition to comprehensive clinical trial oversight services—such as:
Clinical Research Directors must prioritize the selection of validated EDC systems that comply with regulatory standards and align with the overarching goals of the clinical investigation.

Planning for clinical investigation is an essential aspect of compliance with ISO 14155:2020. It necessitates the development of a comprehensive Clinical Investigation Plan (CIP) that delineates the study's objectives, methodology, and regulatory requirements. The CIP must encompass:
By aligning the CIP with ISO 14155:2020 standards, Clinical Research Directors can ensure that their trials are meticulously structured and compliant from the very beginning.
Post-market follow-up (PMCF) is an essential element that ISO 14155:2020 outlines, requiring continuous monitoring of medical devices after their authorization for market use. This process involves the collection of data regarding the device's performance and safety in real-world environments, enabling the identification of any potential issues that may arise. Clinical Research Directors are tasked with developing robust PMCF plans that adhere to ISO 14155:2020 standards, ensuring the ongoing collection of valuable data that informs future product enhancements and regulatory compliance.
At bioaccess®, we leverage over 20 years of expertise in managing a diverse range of research studies, including:
Our team, which includes experts such as Katherine Ruiz, specializes in regulatory affairs for medical devices and in vitro diagnostics in Colombia. We provide tailored support to address the unique challenges present in the Latin American market, reinforcing our commitment to advancing clinical research in the region.

The role of ISO 14155:2020 is pivotal in shaping research practices by emphasizing ethical considerations, comprehensive documentation, and robust risk management. Adhering to ISO 14155:2020 empowers Clinical Research Directors to enhance the quality and integrity of their studies, which is essential for achieving better patient outcomes and ensuring compliance with regulatory requirements.
The incorporation of electronic data capture systems, coupled with meticulous planning processes, is integral to the successful execution of research investigations, aligning them with both ethical and regulatory standards. Recent trends highlight a growing emphasis on ethical factors within medical studies, reflecting a shift towards more patient-centered methodologies.
The effective integration of these ethical frameworks not only enhances the quality of trials but also cultivates trust among stakeholders, ultimately benefiting the entire clinical research ecosystem.
The insights provided on ISO 14155:2020 underscore its vital role in enhancing clinical research practices. By emphasizing ethical considerations, comprehensive documentation, and robust risk management, this standard empowers Clinical Research Directors to elevate the quality and integrity of their studies. Adhering to ISO 14155:2020 is not merely a regulatory obligation; it is a pathway to achieving better patient outcomes and fostering trust within the clinical research ecosystem.
Key arguments presented highlight the importance of:
Moreover, the introduction of electronic data capture systems and the focus on effective clinical investigation planning further align research practices with ethical and regulatory standards. These elements collectively contribute to a more patient-centered approach in clinical trials, reflecting a significant shift in the industry.
Ultimately, the implications of ISO 14155:2020 extend beyond regulatory compliance; they represent a commitment to advancing clinical research quality and patient safety. Embracing these insights and implementing the recommended practices will not only enhance the efficiency of clinical investigations but also support the growth of the Medtech sector, fostering international collaboration and economic development. It is imperative for Clinical Research Directors to prioritize these standards, ensuring their teams are equipped to navigate the complexities of modern clinical trials effectively.
What is bioaccess® and how does it ensure compliance with ISO 14155:2020 for clinical research?
Bioaccess® leverages its extensive knowledge of regulatory frameworks across various regions to ensure compliance with ISO 14155:2020. It integrates local regulatory speed with international standards, ensuring clinical studies are compliant and efficient, which reduces the time to market for innovative medical devices.
How does bioaccess® benefit clinical trials in Colombia?
In Colombia, bioaccess® capitalizes on a quick IRB/EC and MoH (INVIMA) review process that takes only 90-120 days, offering cost savings exceeding 30% compared to trials in North America or Western Europe.
What complexities of regulatory requirements does bioaccess® manage for its clients?
Bioaccess® manages various complexities, including site feasibility, investigator selection, and project oversight, allowing clients to focus on their core innovations while ensuring a seamless pathway to commercialization.
What ethical considerations are involved in clinical investigations adhering to ISO 14155:2020?
Ethical considerations include obtaining informed consent, safeguarding participant confidentiality, and conducting risk assessments to minimize potential harm.
What study management services does bioaccess® provide to uphold ethical standards?
Bioaccess® offers comprehensive study management services such as feasibility assessments, site selection, adherence reviews, setup, import permits, project oversight, and reporting.
How does bioaccess® ensure compliance with country requirements during clinical studies?
Bioaccess® meticulously reviews and provides feedback on study documents to ensure they comply with country requirements, upholding the integrity of studies and protecting participant rights and well-being.
What are the key responsibilities of sponsors and investigators in clinical trials?
Sponsors are primarily responsible for the overall design, conduct, and management of research, while investigators manage daily operations at the study site. Collaboration between both roles is crucial for adherence to ISO 14155:2020 and effective patient recruitment.
Why is clear communication important in clinical trials?
Clear communication and well-defined roles are essential for the successful execution of trials, ensuring accurate record-keeping, participant safety, and strict adherence to study protocols.
How can innovative solutions enhance the recruitment process in clinical trials?
Leveraging innovative solutions for data management and adherence can significantly improve the efficiency and effectiveness of patient recruitment in clinical studies.