


This article presents key insights into the payment for participants in medical trials, underscoring the critical importance of equitable remuneration and the ethical considerations involved in recruitment. Competitive payment structures are highlighted as a significant factor that can enhance participant enrollment and retention. Moreover, the ethical implications of compensation are acknowledged, emphasizing the necessity of ensuring informed consent and fairness among diverse populations. These insights are crucial for advancing clinical research and addressing the challenges within the Medtech landscape.
The landscape of medical trials is rapidly evolving, with participant compensation emerging as a pivotal factor in enhancing recruitment and ensuring the success of clinical studies. Understanding the intricacies of payment structures allows individuals to navigate the complexities of participation while recognizing the ethical implications tied to compensation. Yet, the promise of faster enrollment and increased access to innovative treatments presents a challenge: how can stakeholders maintain ethical standards and transparency? Balancing the need for competitive remuneration with the imperative to protect participants' rights and welfare is essential in this dynamic environment.
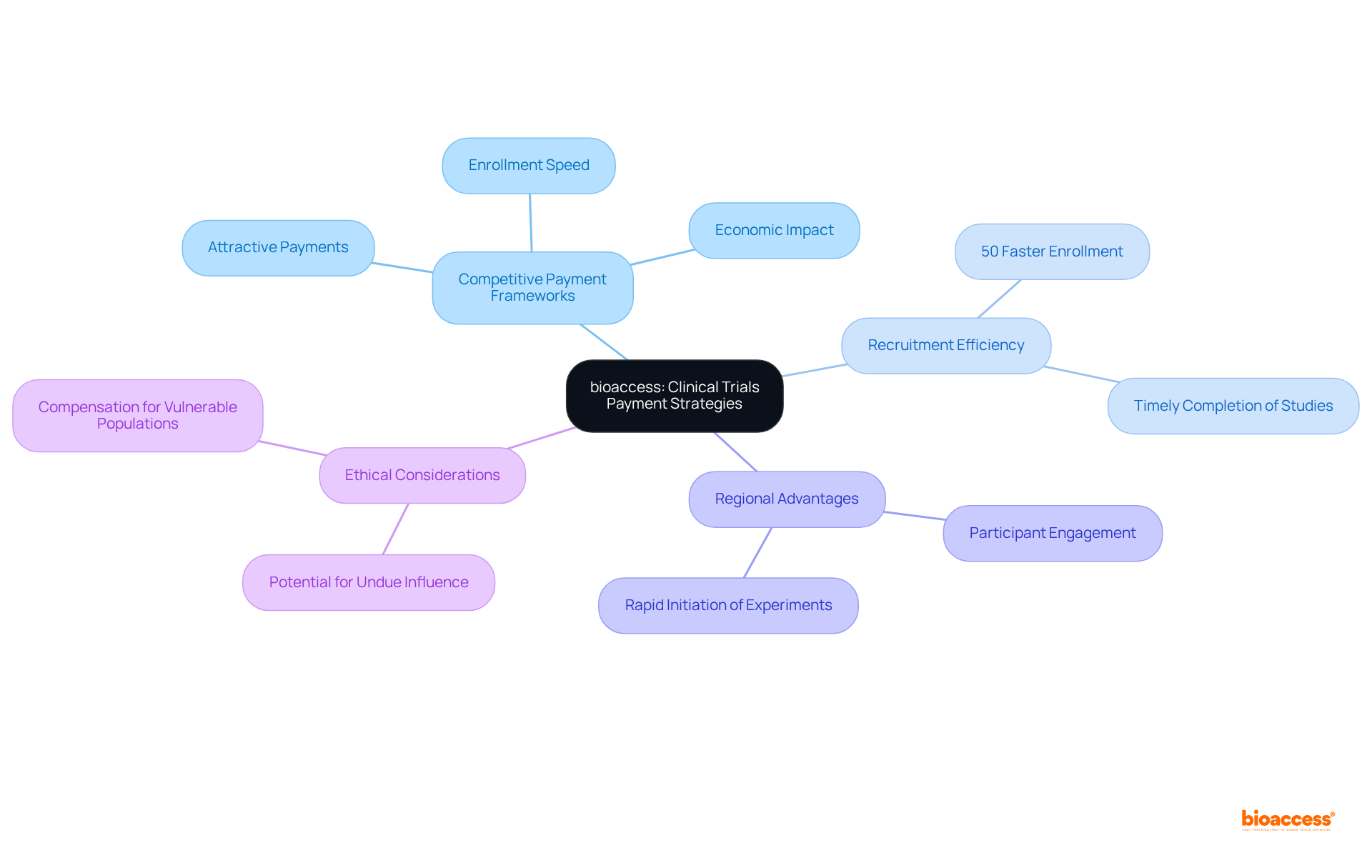
bioaccess® effectively employs competitive payment frameworks for medical trials payment to enhance the recruitment of individuals in clinical studies, resulting in a significant boost in efficiency. By providing attractive medical trials payment, the organization not only fosters participation but also facilitates the timely completion of studies. This strategy proves particularly successful in regions like Latin America, where the regulatory environment allows for the rapid initiation of experiments and participant engagement.
Research shows that medical trials payment can significantly reduce enrollment times; for instance, enrollment occurs 50% faster than in traditional markets. Furthermore, studies have highlighted that reduced medical trials payment of $3,200 may render recruitment impractical for certain demographics, underscoring the critical importance of competitive remuneration, especially among economically disadvantaged groups.
With ethical approvals secured in a mere 4-6 weeks, bioaccess® is strategically positioned to leverage these competitive advantages, ultimately accelerating access to innovative treatments and therapies. This approach not only enhances testing efficiency but also contributes to local economic growth through job creation and improved healthcare outcomes. However, it is vital to recognize potential ethical concerns associated with high compensation, as such incentives may unduly influence the decisions of participants.
Medical trials payment for individuals participating in clinical trials serves multiple essential purposes:
This practice is not a bribe; rather, it is an acknowledgment of the crucial role individuals play in the research process. Ethical standards regulate this practice, ensuring that individuals are treated fairly and with respect. Research indicates that average compensations for involvement in Phase I studies can vary from $2,000 to $5,000, reflecting the complexity and duration of the studies involved.
Moreover, ethical considerations emphasize that compensation should not excessively sway individuals' choices, thereby preserving the integrity of the research. By aligning payment frameworks with real-world standards, organizations like bioaccess® enhance trust and encourage participation, ultimately aiding the success of medical trials payment. This approach not only enhances the welfare of those involved but also contributes to the advancement of medical knowledge and innovation.
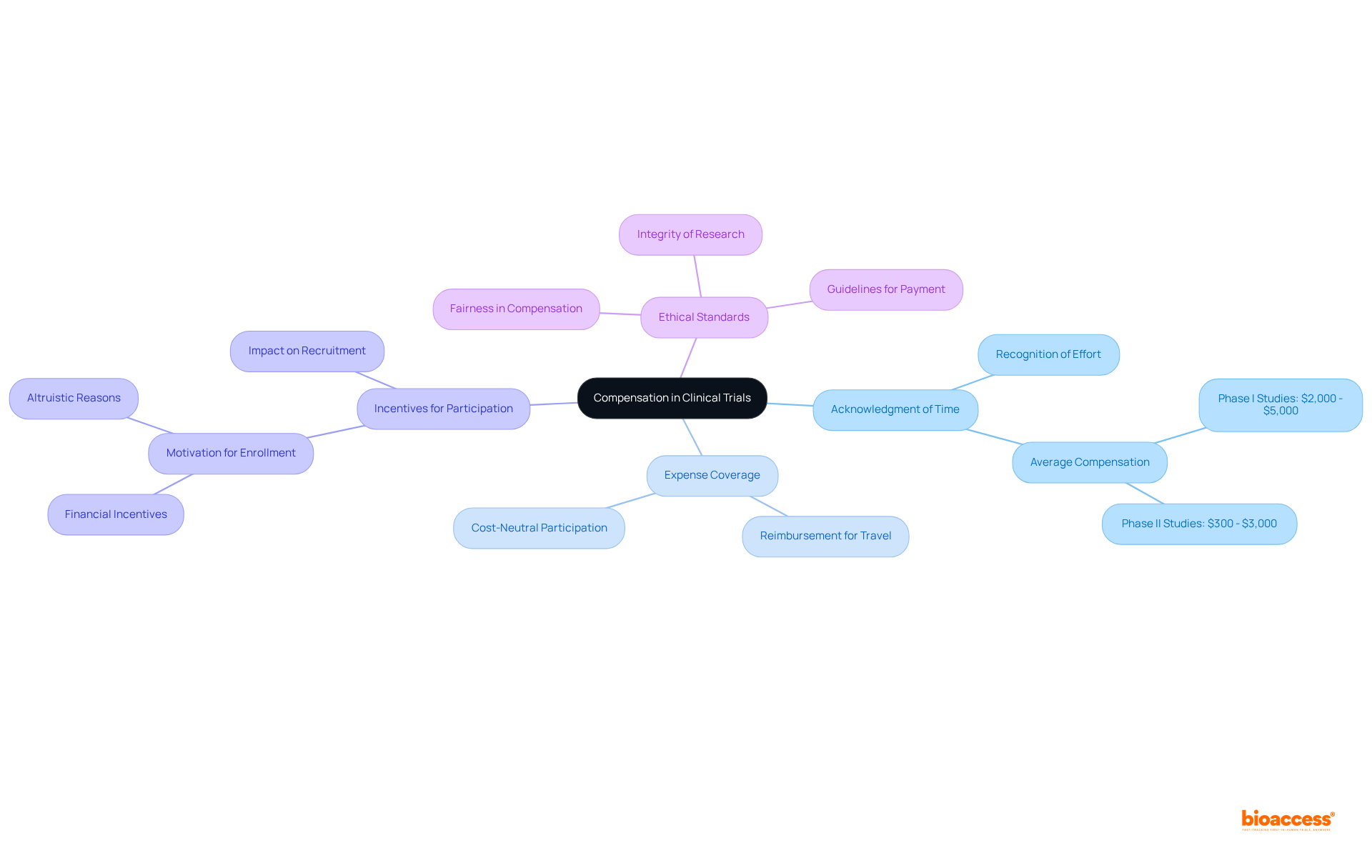
The medical trials payment provided to subjects in clinical studies is significantly influenced by various factors, particularly the complexity of the research. More intricate studies, which may require regular visits or invasive interventions, typically offer higher rewards to compensate for the increased demands on participants. For example, Phase III studies, involving larger patient groups and requiring stringent oversight, generally reimburse individuals between $2,000 and $7,000, depending on the specifics of the research. In contrast, Phase II studies offer reimbursements ranging from $1,000 to $5,000, highlighting the variability in payment across different study phases.
Moreover, the time commitment required from participants plays a crucial role in determining the levels of medical trials payment. Studies that demand longer durations or more extensive involvement often provide greater financial incentives. Local economic conditions also impact payment rates; studies conducted in metropolitan areas may offer higher remuneration due to the elevated cost of living.
Ethical considerations are paramount in establishing remuneration structures. While larger rewards can attract participants, they must not overshadow the potential risks associated with the study. Regulatory organizations ensure that participant safety and informed consent are prioritized, striking a balance between fair compensation and ethical research practices. The amount of medical trials payment offered to contributors often reflects the complexity, duration, and potential hazards associated with the study. Thus, understanding the intricacies of a study is essential for both participants and investigators in evaluating remuneration in clinical studies. Clear communication regarding compensation details is vital to ensure participants are fully informed about what to expect. Additionally, participants should recognize that engaging in multiple distinct studies can enhance their overall earnings, providing further motivation to explore various research opportunities.
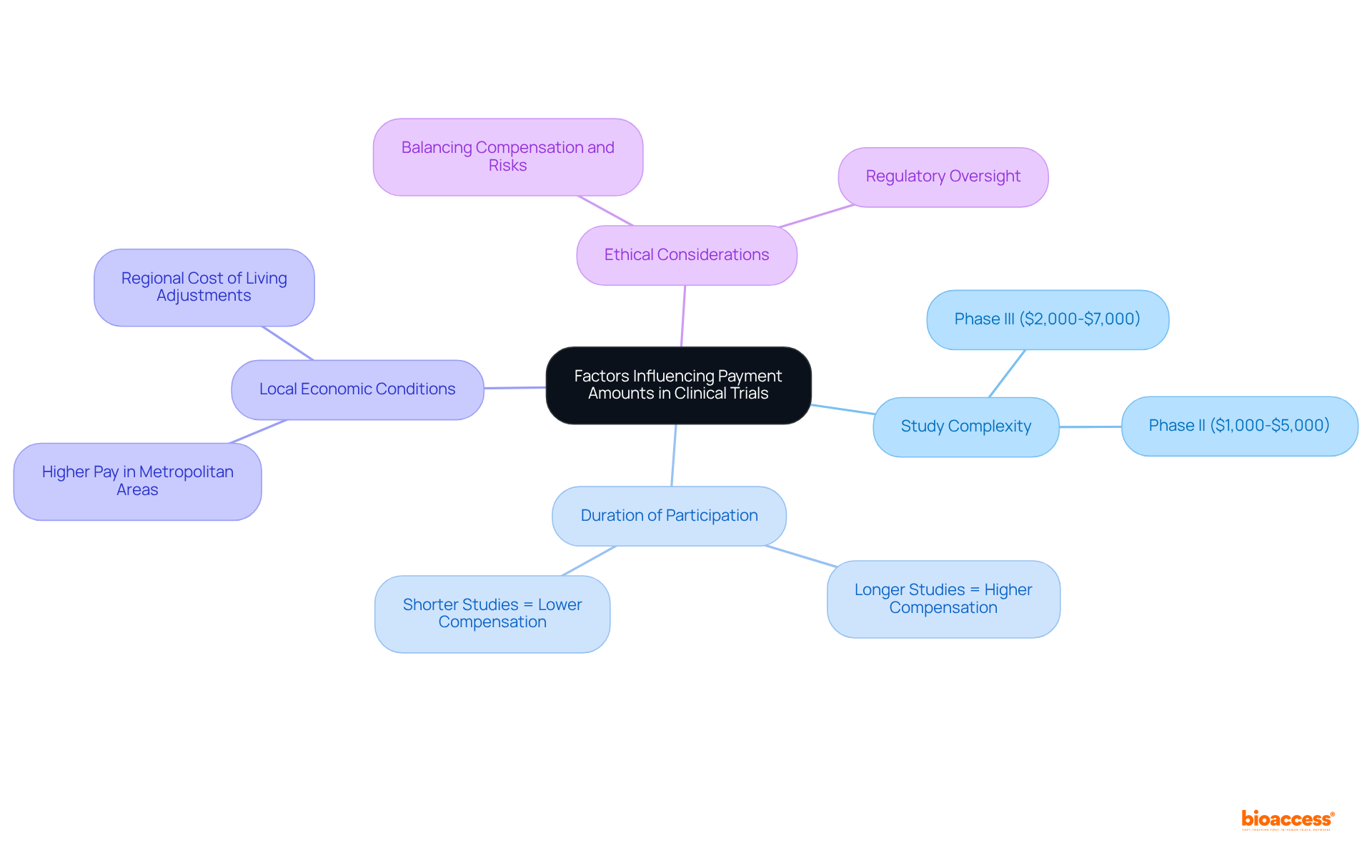
Medical trials payment for individuals is not only essential but also laden with substantial ethical considerations that require meticulous management. Medical trials payment must be equitable, accurately reflecting the time and effort involved, while avoiding coercive amounts that could unduly pressure individuals into participation against their better judgment.
Ethical guidelines underscore that medical trials payment should not exert undue influence, particularly over vulnerable populations. Researchers are tasked with the challenge of fostering engagement while upholding their ethical responsibilities, ensuring that medical trials payment practices are transparent, fair, and respectful of individuals' unique circumstances.
Engaging with community representatives can significantly enhance the relevance of payment processes, fostering trust and inclusivity. Clear communication regarding medical trials payment processes is paramount to cultivate understanding and trust among all parties involved.
Furthermore, researchers bear an ethical obligation to ensure that the medical trials payment processes do not inadvertently exclude participants based on their circumstances, addressing potential barriers to participation. Balancing these considerations is vital for maintaining the integrity of research and promoting inclusivity.
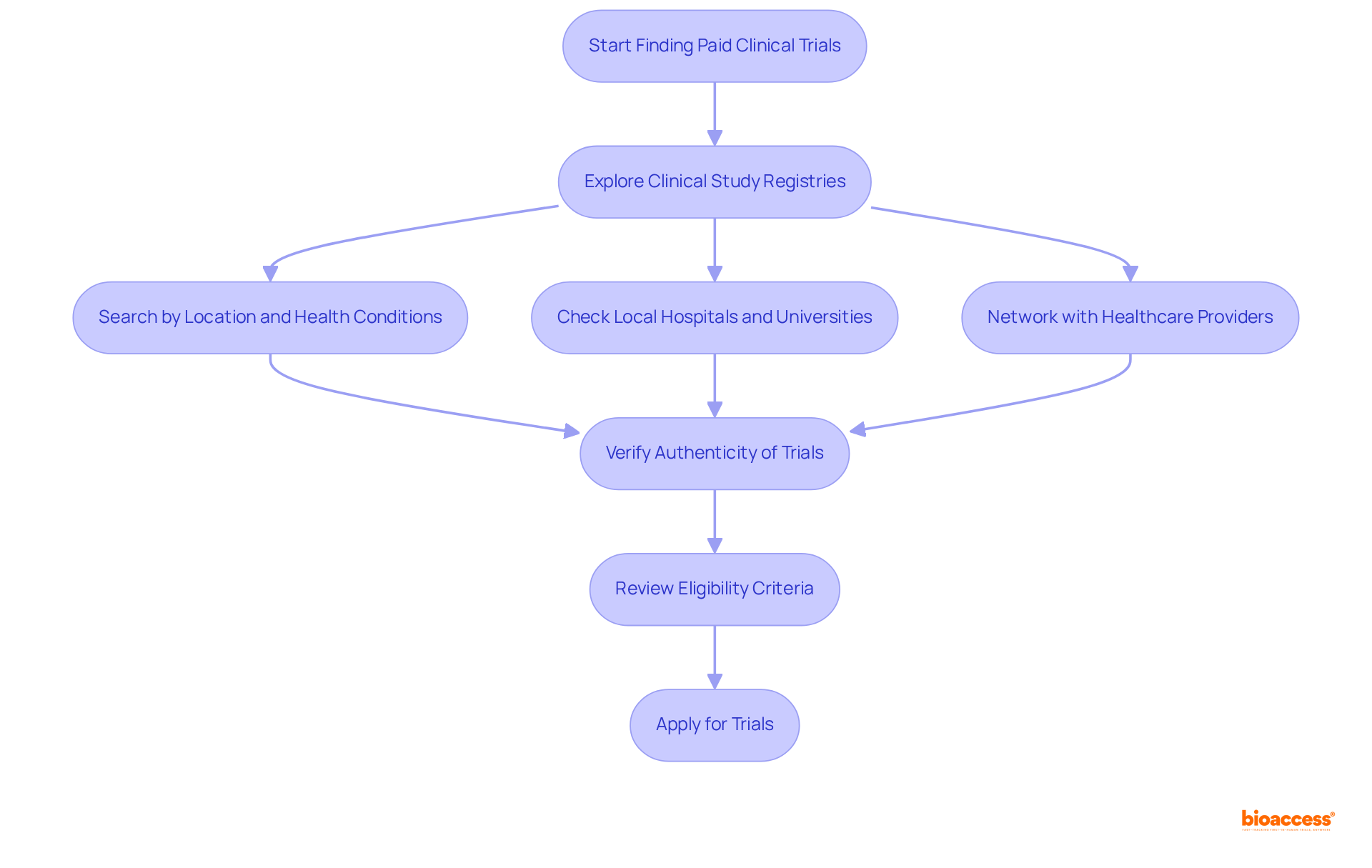
Finding compensated research studies can be a straightforward process when employing the right approach, particularly through comprehensive services that facilitate medical trials payment. Begin by exploring clinical study registries such as ClinicalTrials.gov, which allows you to search for research based on your location and specific health conditions. These registries offer valuable insights into medical trials payment, helping you understand what to expect. The medical trials payment typically increases with the duration and complexity of the process, ranging from below $100 to several thousand dollars. Additionally, local hospitals, universities, and research institutions often maintain catalogs of ongoing studies, providing another avenue for discovery.
At bioaccess, we facilitate the assessment and selection of research sites and lead investigators, ensuring that studies are organized efficiently and adhere to ethical standards. Networking with healthcare providers can also uncover information about upcoming studies that may not be widely advertised. It is crucial to verify the authenticity of the trials and thoroughly comprehend the prerequisites before applying. Ethical committees oversee these studies to prioritize participant safety and compliance with ethical standards, emphasizing participant welfare throughout the process.
Participants possess the fundamental right to withdraw from research studies at any time without repercussions on their future medical treatment, ensuring informed involvement. It is essential to review the eligibility criteria before applying to a study, as each research project has specific requirements that determine who can participate. Engaging in medical trials payment not only contributes to advancing health research but also provides individuals with the opportunity to receive compensation for their involvement, ultimately fostering job creation and economic development within local communities.
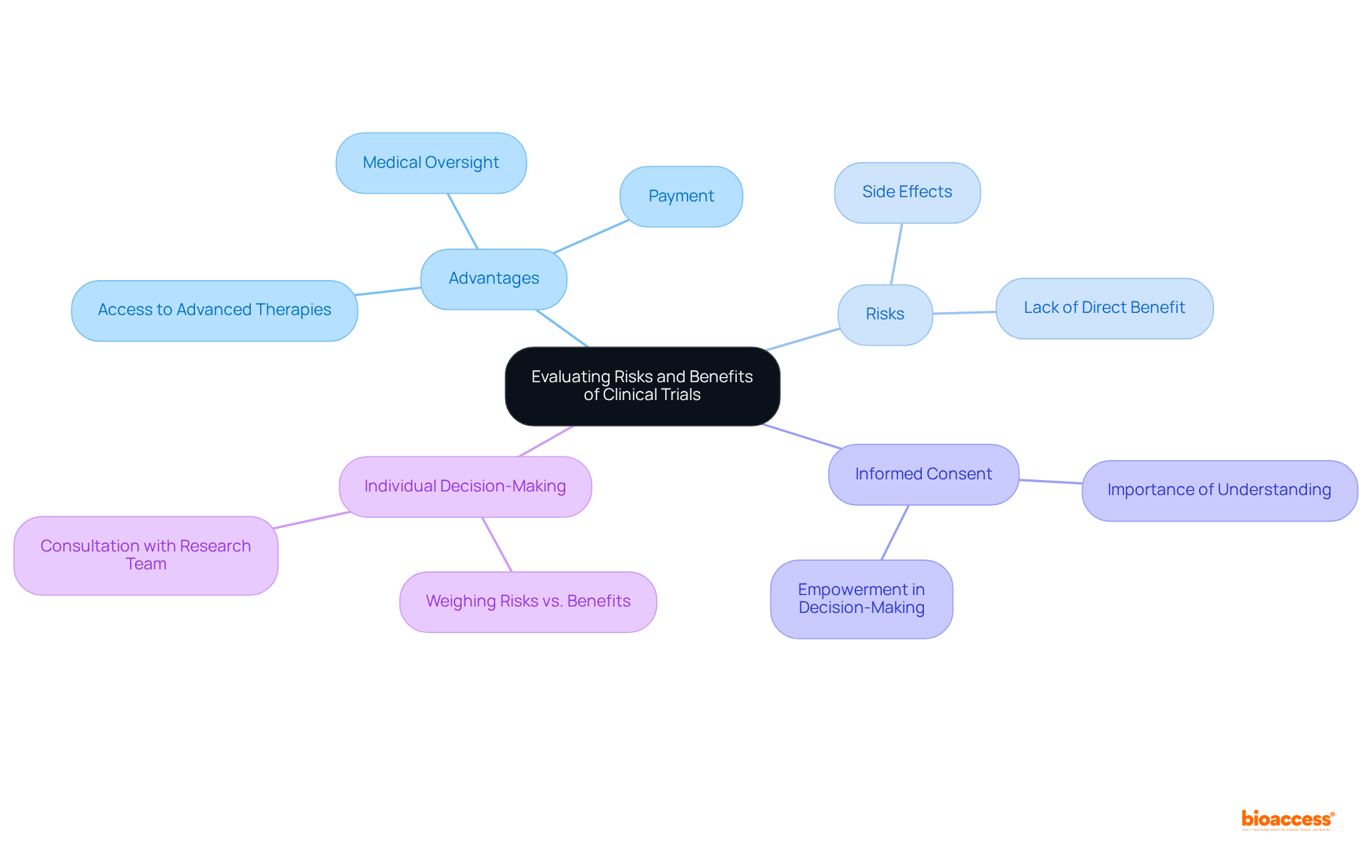
Engaging in a research study necessitates a thoughtful evaluation of both risks and advantages. On the positive side, individuals may gain access to advanced therapies, receive comprehensive medical oversight, and often receive medical trials payment for their time and participation. For instance, average remuneration for research subjects can vary significantly in terms of medical trials payment, reflecting the complexity and requirements of the study. However, potential risks must also be acknowledged, including side effects from experimental treatments and the possibility of not receiving any direct medical benefit from participation.
Before enrolling, individuals should meticulously review informed consent documents, which outline the study's purpose, procedures, risks, and benefits. Engaging in discussions with the research team is essential to address any concerns and ensure a clear understanding of what participation entails. Studies indicate that informed consent is not merely a formality; it plays a vital role in empowering individuals to make informed choices regarding their involvement in medical studies.
Furthermore, research on individual decision-making emphasizes that people weigh the risks of involvement against the potential advantages of managing their health issues. This evaluation process is crucial, as it influences their willingness to engage in research that may contribute to scientific advancements and the development of new therapies. Ultimately, a knowledgeable individual is better prepared to navigate the intricacies of research studies, ensuring that their decisions align with their health objectives and personal principles.
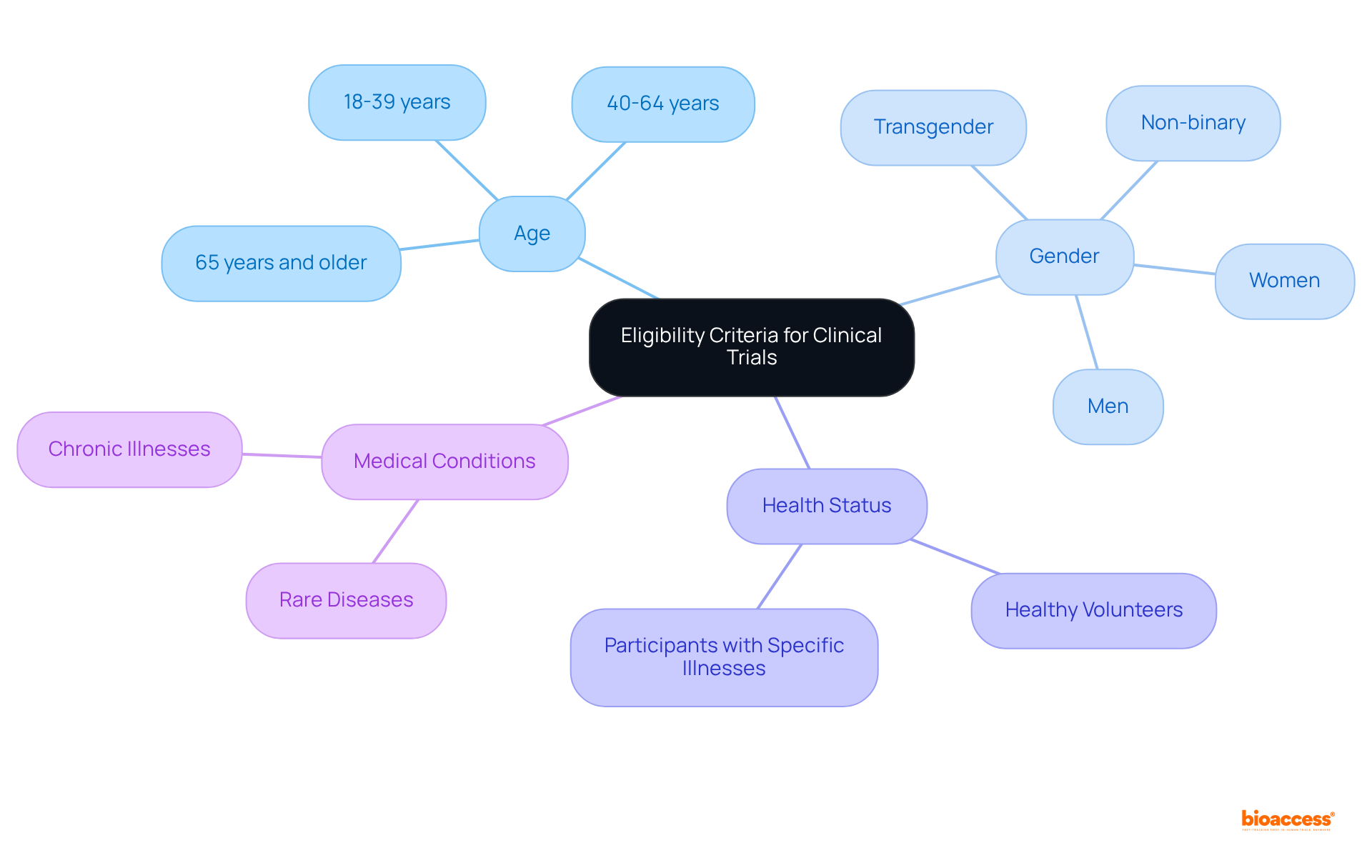
Eligibility requirements for clinical studies are crucial and vary significantly based on the research objectives. Common factors encompass age, gender, health status, and specific medical conditions. Some studies may necessitate that individuals be healthy volunteers, while others may specifically seek participants with certain illnesses. It is essential for potential candidates to carefully review these criteria and consult with the research team to ascertain their eligibility before applying.
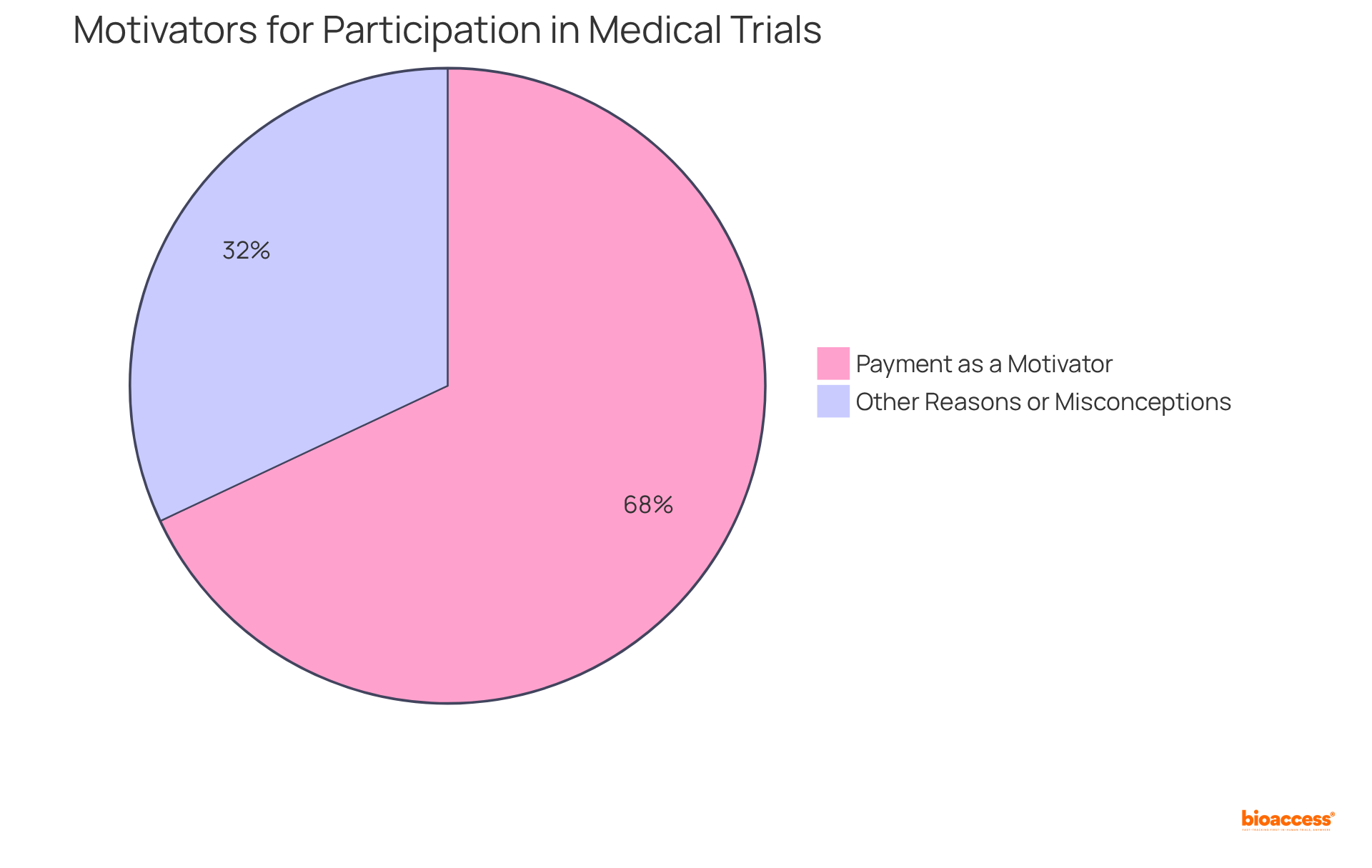
Numerous myths persist regarding medical trials payment for involvement in clinical research, often leading to misconceptions about the nature of these studies. A common misconception is that participants receive substantial financial rewards, which fosters the belief that trials exploit individuals. In reality, the medical trials payment is generally modest, intended to reflect the time and effort required for participation.
A survey indicates that 68% of respondents listed medical trials payment as a motivator for participation, underscoring its significance in decision-making. Another prevalent misconception suggests that individuals serve as test subjects for unproven medications; however, all research studies operate under strict regulatory supervision, prioritizing safety and ethical principles.
Furthermore, many erroneously believe that research studies are exclusively for those with life-threatening diseases, whereas they are designed to include a diverse range of participants, encompassing healthy individuals and those with various health issues. This diversity is essential for understanding how new treatments perform across different demographics.
The complexity of medical trials payment arises from varying income levels among contributors, making equitable compensation a challenge. By dispelling these myths, potential participants can attain a clearer perspective, alleviating fears and encouraging informed consideration of involvement in medical research.
Enhanced public awareness regarding the realities of medical studies, including the medical trials payment system and the stringent ethical guidelines established, can significantly elevate participation rates and foster trust in the research process.
Engaging in multiple research studies can be intricate and demands careful evaluation. At bioaccess, we understand the complexities inherent in research management, encompassing:
It is imperative for participants to disclose their involvement in other studies to the research teams, as this can significantly influence eligibility and safety. Research indicates that only 5% of eligible patients participate in clinical studies, underscoring the challenges faced by participants in navigating various studies. Furthermore, overlapping study schedules may lead to conflicts in appointments or medication interactions, which are critical safety considerations. Transparent communication with all research groups is essential to ensure that participation in multiple studies is safe and compliant with study protocols. Additionally, 73% of respondents assert that it is crucial for clinical study volunteers to represent diverse communities, highlighting the need for inclusivity in study participation.
To effectively manage involvement, individuals should:
By leveraging our extensive services, bioaccess aims to streamline the testing process, ultimately fostering job creation, economic growth, and enhancing healthcare within local communities.
The landscape of research study payments, including medical trials payment, is evolving, marked by an increasing recognition of the importance of equitable remuneration for participants. Collaborations, such as that between bioaccess™ and Caribbean Health Group, are vital in positioning Barranquilla as a leading hub for clinical studies in Latin America, with the support of Colombia's Minister of Health.
Key insights include:
Participants must be informed of their rights, the risks and benefits associated with their involvement, and the eligibility criteria for studies. By clarifying the payment process and highlighting the extensive clinical trial management services—including:
bioaccess™ and similar organizations can enhance participation in clinical research. This not only propels medical knowledge and improves patient outcomes but also bolsters local economies through job creation and healthcare advancements, ultimately driving global health innovation through international collaboration.
The evolving landscape of medical trials payment underscores the critical role that compensation plays in fostering participant engagement and advancing clinical research. By implementing competitive payment structures, organizations like bioaccess® not only attract individuals to participate in studies but also enhance the overall efficiency and speed of clinical trials. This strategic approach is vital in ensuring that innovative treatments reach the market more swiftly, thus benefiting both participants and the wider community.
Key insights from the article reveal the multifaceted nature of participant compensation, including its ethical implications, the factors that influence payment amounts, and the importance of transparency in communication. It is essential for potential participants to understand their rights, the risks and benefits associated with involvement, and the eligibility criteria for various studies. By demystifying the payment process and addressing misconceptions, stakeholders can encourage informed participation, ultimately driving advancements in medical knowledge and improving healthcare outcomes.
In conclusion, recognizing the significance of equitable compensation in clinical trials is crucial for enhancing participation rates and fostering trust in the research process. As the industry continues to evolve, it is imperative for researchers, participants, and organizations to collaborate in creating a transparent and ethical framework that prioritizes participant welfare while advancing global health innovation. Engaging in clinical trials not only offers individuals the opportunity to contribute to medical advancements but also supports local economies through job creation and improved healthcare services.
What is bioaccess® and how does it enhance clinical trials?
bioaccess® employs competitive payment frameworks to improve recruitment for medical trials, resulting in faster enrollment and timely study completion, especially in regions like Latin America.
How does competitive payment influence enrollment in clinical trials?
Competitive payment can reduce enrollment times by 50% compared to traditional markets, making participation more attractive, particularly for economically disadvantaged groups.
What are the ethical considerations regarding compensation in clinical trials?
While compensation acknowledges participants' contributions, it should not unduly influence their decisions. Ethical standards ensure fair treatment and respect for individuals involved in research.
What purposes does compensation serve for participants in clinical trials?
Compensation acknowledges participants' time and effort, covers expenses incurred during the trial, and incentivizes involvement in research that can lead to medical advancements.
How much can participants expect to be compensated for their involvement in clinical trials?
Average compensation for Phase I studies ranges from $2,000 to $5,000, while Phase II studies offer between $1,000 and $5,000, and Phase III studies can reimburse participants from $2,000 to $7,000 depending on the study's complexity.
What factors influence the amount of payment in clinical trials?
Payment amounts are influenced by the complexity of the research, the time commitment required from participants, and local economic conditions, such as the cost of living in metropolitan areas.
How do ethical considerations affect payment structures in clinical trials?
Ethical considerations prioritize participant safety and informed consent, ensuring that compensation does not overshadow potential risks associated with the study while balancing fair compensation.
Can participants earn more by engaging in multiple studies?
Yes, participants can enhance their overall earnings by engaging in multiple distinct studies, providing further motivation to explore various research opportunities.