


This article presents key insights into microbiology pharmaceutical practices that are vital for clinical research. It underscores the significance of regulatory compliance, innovative technologies, and strategic recruitment in enhancing the efficiency and effectiveness of clinical studies. These elements are crucial as they ultimately lead to improved patient outcomes and the advancement of new therapies within the field. By focusing on these aspects, the article establishes a foundation for understanding the current Medtech landscape and the essential role of bioaccess in addressing critical challenges faced in clinical research.
The landscape of microbiology pharmaceuticals is rapidly evolving, propelled by innovative practices that enhance clinical research outcomes. Advancements in technology and regulatory efficiencies empower organizations to conduct studies that not only accelerate the development of new therapies but also prioritize patient safety and compliance.
However, as researchers navigate this complex terrain, they confront critical questions:
bioaccess® leverages its expertise in early-phase research to accelerate the advancement of microbiology pharmaceutical products. By capitalizing on Colombia's competitive advantages—such as:
bioaccess® ensures that clinical studies are conducted effectively. Furthermore, investments in R&D benefit from substantial tax incentives, enhancing the appeal of conducting experiments in Colombia. The rigorous ICH/GCP certification process for hospitals in Colombia guarantees adherence to high-quality healthcare standards. This efficient approach, bolstered by these advantages, not only speeds up the investigation process but also facilitates the rapid introduction of innovative therapies in the microbiology pharmaceutical field to the market.
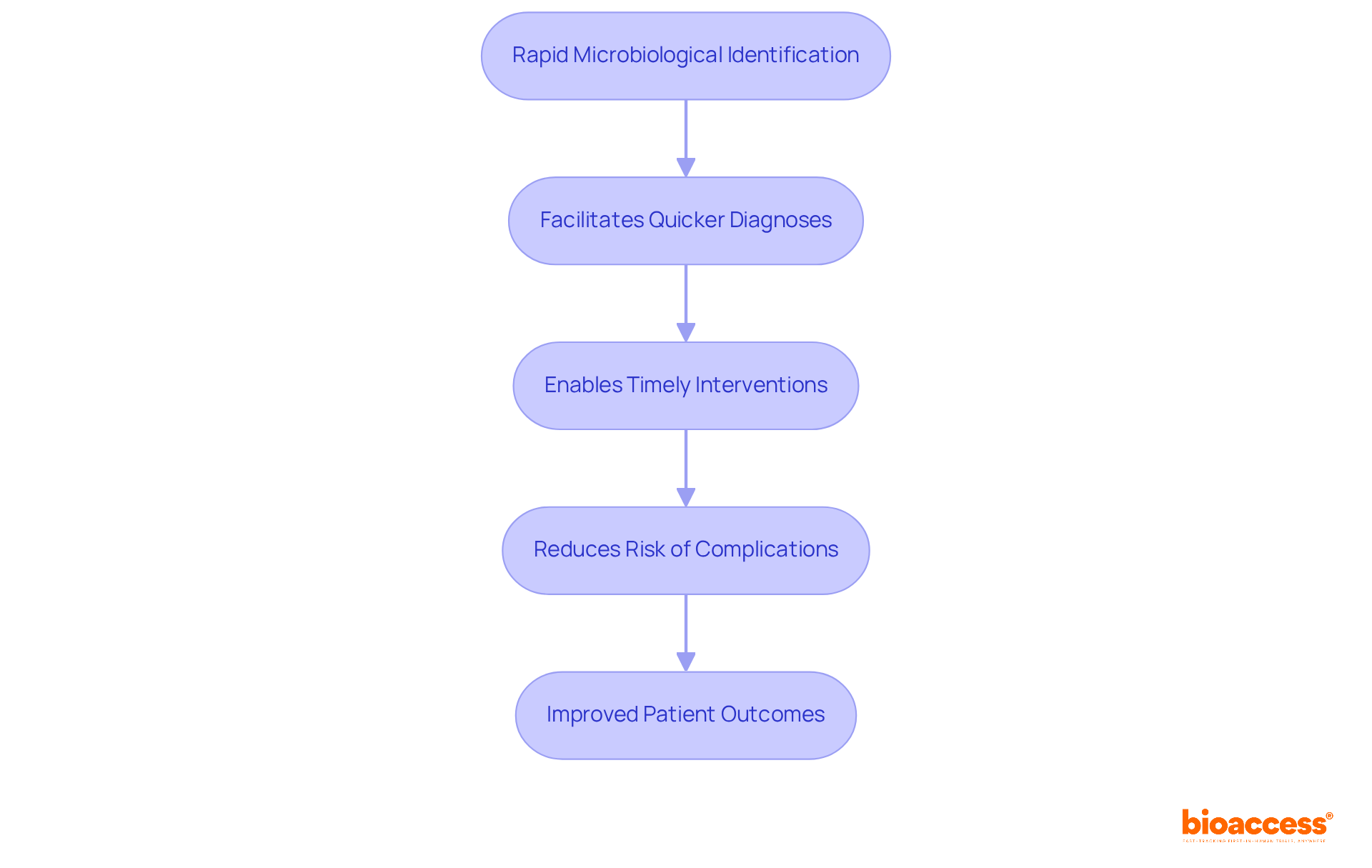
Rapid methods for microbiology pharmaceutical identification, including PCR and mass spectrometry, are pivotal in enhancing safety and efficiency within medical studies. By facilitating quicker diagnoses of infections, these methods enable timely interventions that significantly reduce the risk of complications and improve patient outcomes. The integration of these technologies into medical research not only accelerates the testing process but also ensures that safety protocols are rigorously followed. This advancement is crucial for addressing the challenges faced in the Medtech landscape, reinforcing the importance of innovation in clinical research.
Monitoring microbial origins is essential for ensuring adherence in medical studies. Implementing robust tracking systems enables researchers to identify contamination sources and mitigate risks associated with microbial infections. This proactive approach not only guarantees compliance with regulatory standards but also enhances the reliability of results.
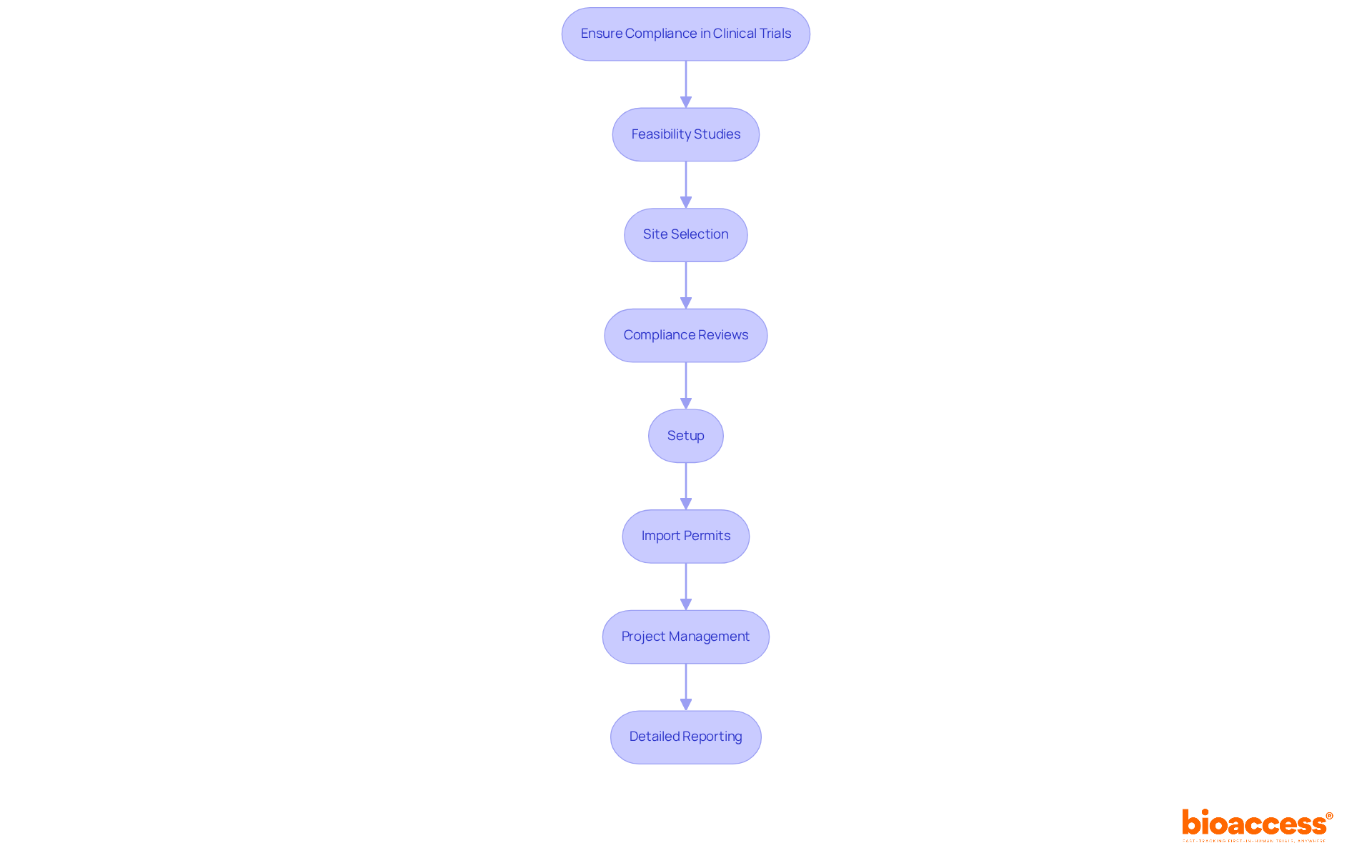
At bioaccess, our comprehensive research management services bolster this process through:
These services are pivotal for the successful advancement of microbiology pharmaceuticals, ensuring that all aspects of the study are meticulously managed and conform to industry standards.
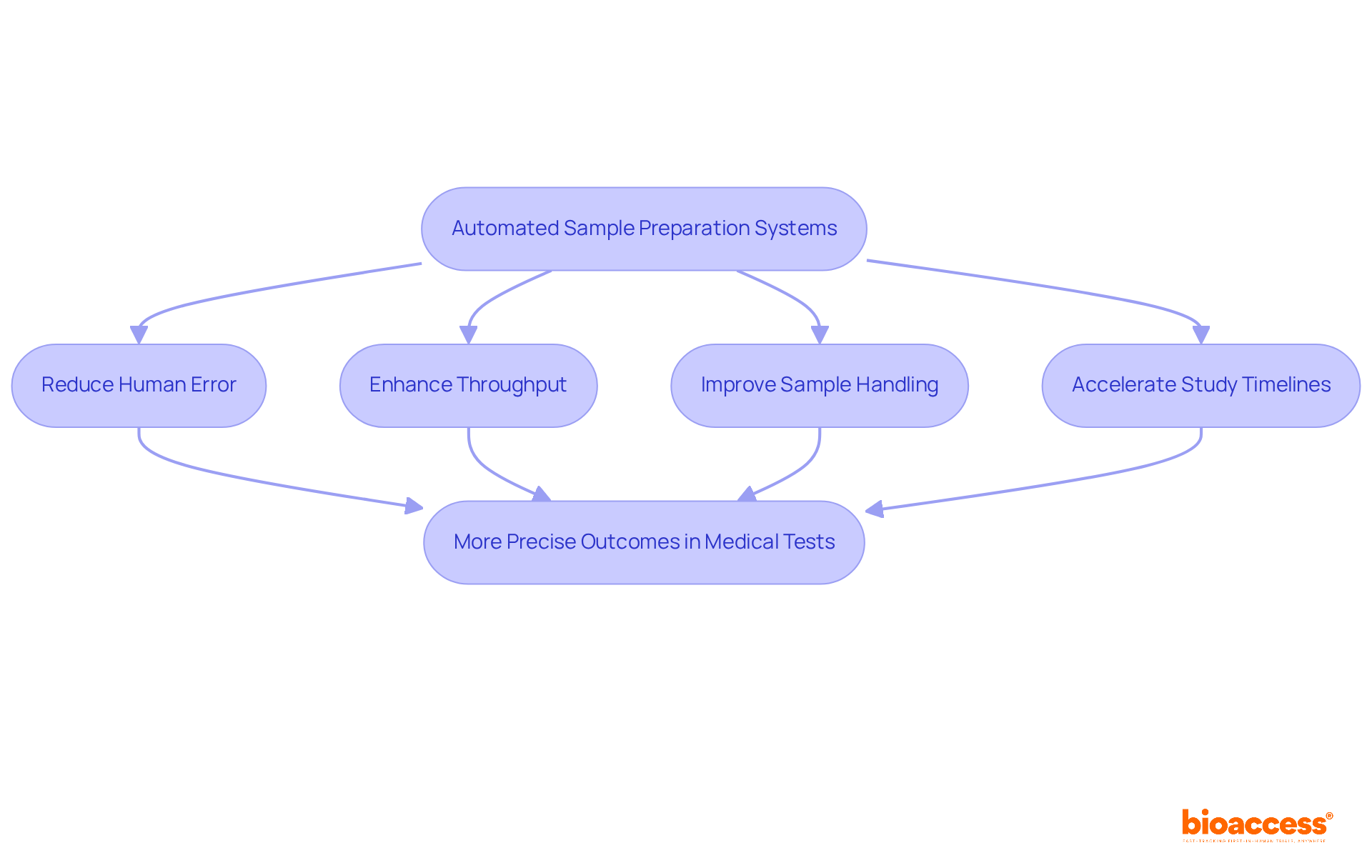
Automated sample preparation systems are revolutionizing procedures in microbiology pharmaceutical research within medical studies. By significantly reducing human error and enhancing throughput, these systems facilitate more consistent and reliable sample handling. This heightened efficiency accelerates study timelines and ensures that high-quality specimens are readily available for examination. Consequently, this advancement leads to more precise outcomes in medical tests, underscoring the critical role of technology in clinical research.
A comprehensive grasp of regulatory demands is essential for successful studies in microbiology pharmaceutical. In Colombia, researchers must navigate a multi-step process to obtain clinical study approval. This includes:
Staying informed about these local and international guidelines not only facilitates smoother approvals but also enhances the credibility of the research, fostering trust among stakeholders and participants. Moreover, understanding the difficulties encountered by medical device startups, including regulatory obstacles and hiring problems, is crucial for efficient management of studies. This knowledge empowers researchers to implement strategies that ensure adherence throughout the study process.
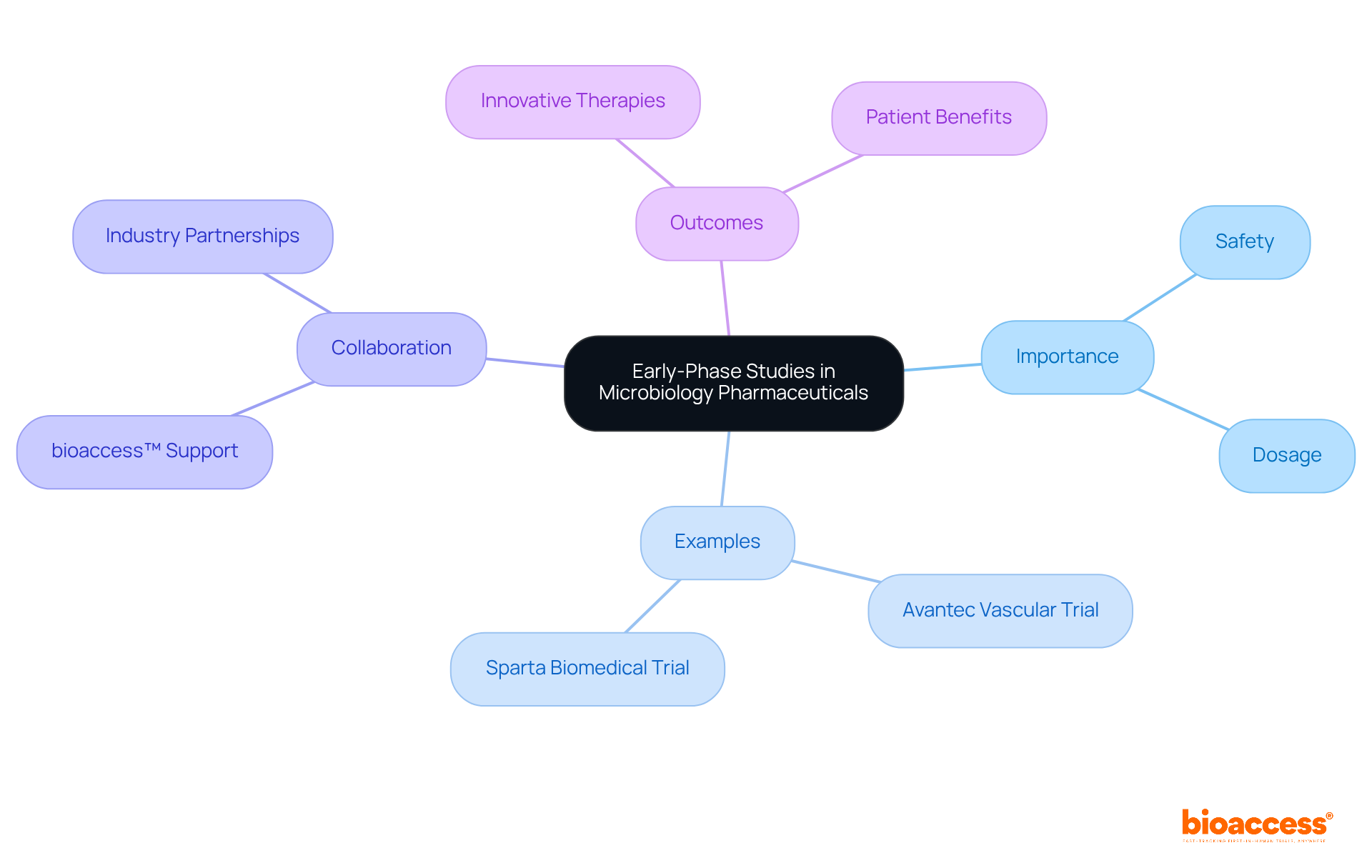
Early-phase studies are crucial for paving the way for innovative microbiology pharmaceutical products. These preliminary tests provide vital information on safety and dosage, enabling researchers to make informed decisions regarding further development.
For instance, bioaccess™ has been instrumental in supporting Avantec Vascular's first-in-human trial of a groundbreaking vascular device in Latin America, underscoring its commitment to advancing healthcare solutions.
Furthermore, Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its initial human trial in Colombia, which further emphasizes the importance of collaboration in clinical studies.
By focusing on early-stage studies, bioaccess® aids innovators in navigating the complexities of introducing new therapies in the microbiology pharmaceutical market, ultimately benefiting patients in need.
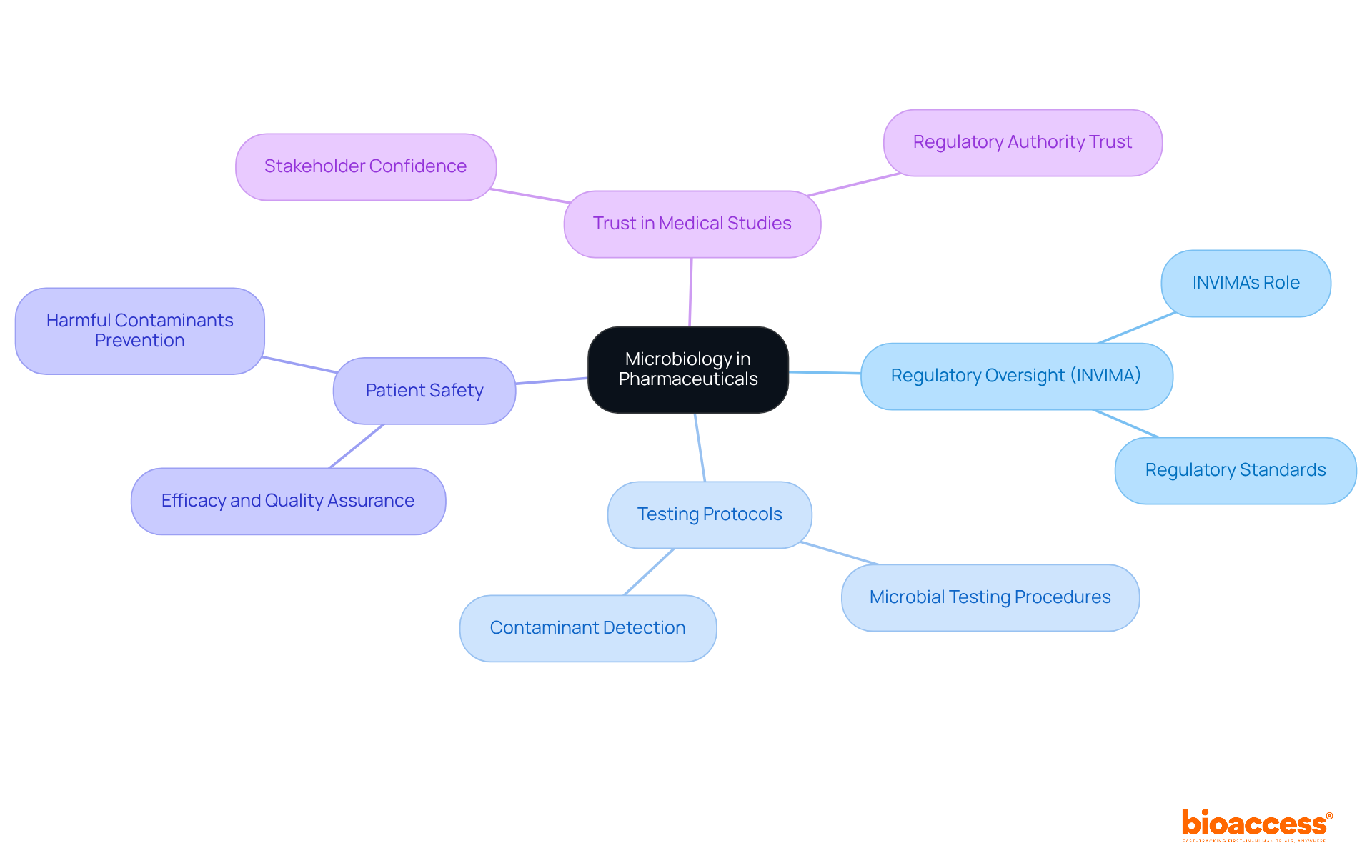
Microbiology pharmaceutical plays a crucial role in quality assurance within the pharmaceutical sector, particularly under the rigorous oversight of regulatory bodies such as INVIMA (Colombia National Food and Drug Surveillance Institute). Established in 1992, INVIMA is tasked with inspecting and supervising the marketing and manufacturing of health products, ensuring adherence to health standards.
By implementing stringent testing protocols concerning microorganisms, researchers in the microbiology pharmaceutical field can ascertain that products are devoid of harmful contaminants, aligning with INVIMA's mandate to ensure the safety, efficacy, and quality of medicines. This unwavering commitment to quality not only protects patient safety but also enhances the overall integrity of medical studies, fostering trust among stakeholders and regulatory authorities.
Recognized as a Level 4 health authority by PAHO/WHO, INVIMA's standards underscore the importance of regulatory excellence in microbial practices, which is essential for advancing Medtech studies in Latin America.
The significance of microbiology pharmaceutical practices in medical research is paramount, as they directly influence patient outcomes. Efficient microbiological testing and oversight in the microbiology pharmaceutical field, supported by comprehensive research management services—such as:
lead to timely interventions. This proactive approach mitigates the risk of adverse effects and enhances overall treatment efficacy. By prioritizing these practices and ensuring strict adherence to regulatory standards, researchers can elevate the quality of care provided to patients involved in research studies, ultimately resulting in improved health outcomes.

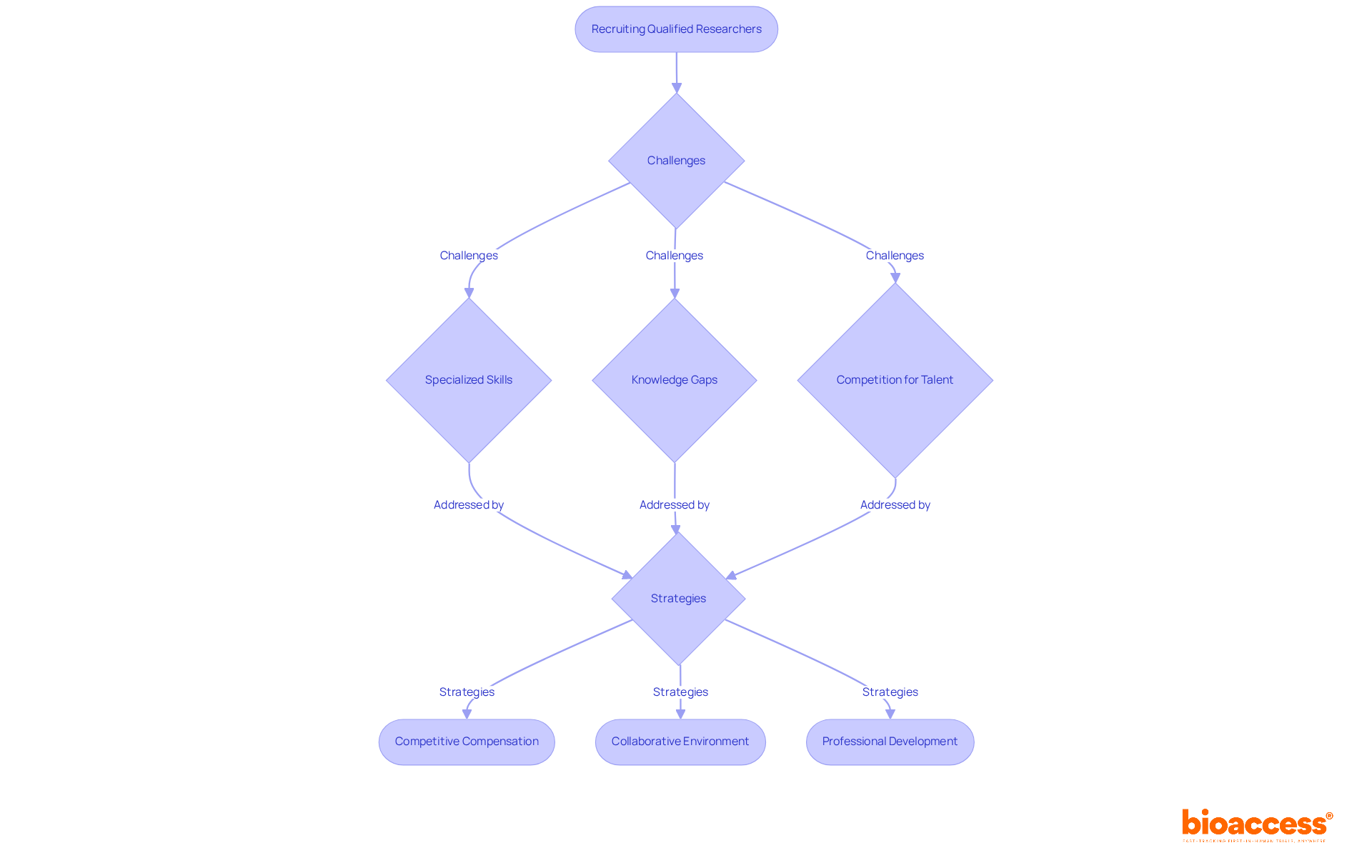
Recruiting qualified researchers in microbiology pharmaceutical presents distinct challenges, particularly the necessity for specialized skills and knowledge. To effectively support comprehensive research study management services—such as:
Organizations must implement targeted recruitment strategies. Such strategies may include:
By overcoming these challenges, research organizations can construct robust teams capable of advancing innovative studies in microbiology pharmaceutical. This effective recruitment not only enhances the quality of medical trials but also contributes to local economies through job creation and healthcare improvement, ultimately driving global health innovation through international collaboration.
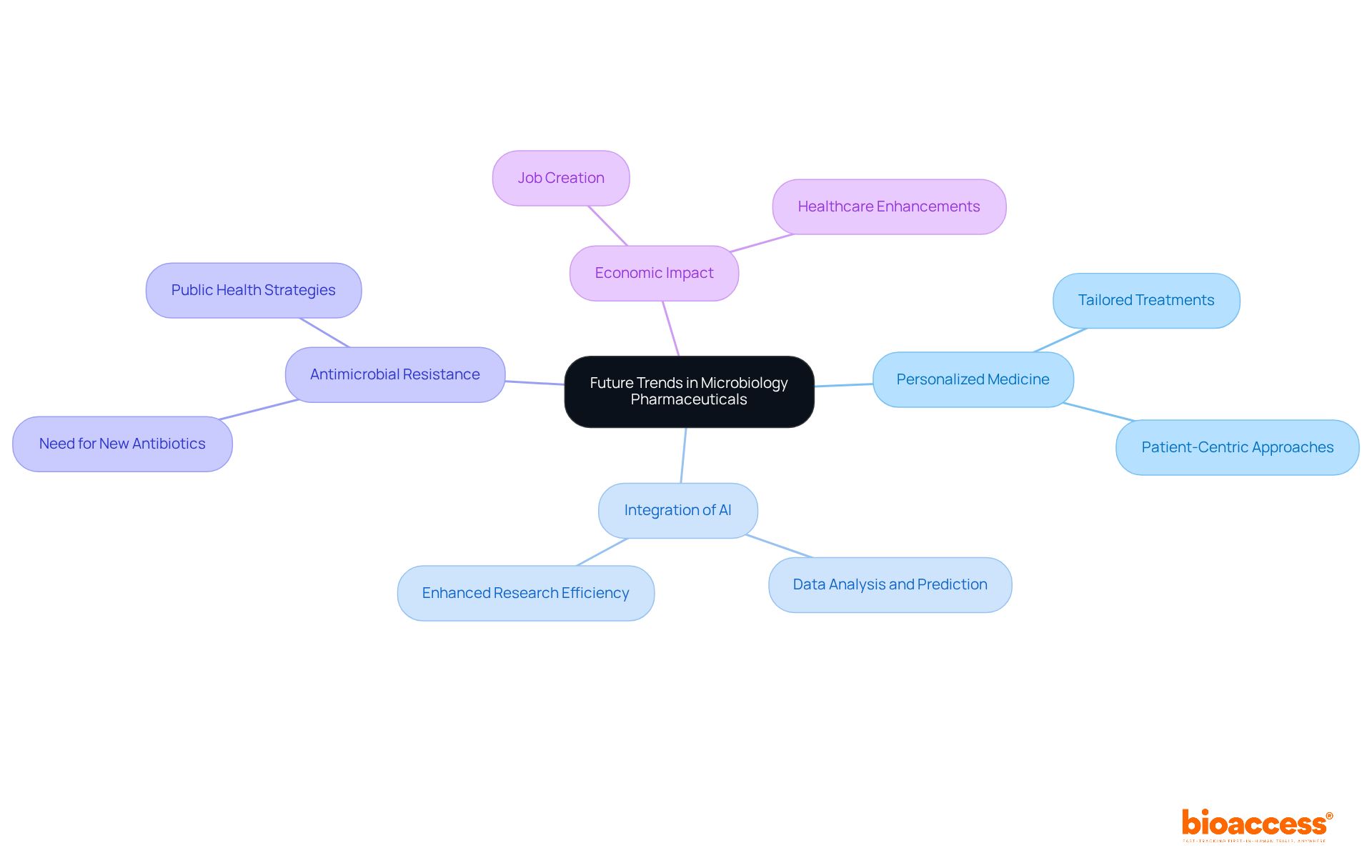
As the field of microbiology pharmaceutical evolves, several trends warrant the attention of medical researchers. These trends include:
Importantly, the influence of Medtech clinical studies on local economies is significant; these studies not only create jobs but also foster economic growth and enhance healthcare systems. Leading experts, such as Dr. Sergio Alvarado, who specializes in innovative medical research and artificial intelligence in Latin America, are driving these advancements. Furthermore, professionals like Ana Criado, with her extensive expertise in regulatory affairs and biomedical engineering, are essential in navigating the complexities of this dynamic landscape. By remaining vigilant regarding these trends, researchers can strategically position themselves to capitalize on new opportunities and propel innovation in microbiology pharmaceutical therapies.
The exploration of microbiology pharmaceutical practices unveils a landscape rich with opportunities for enhancing clinical research. By leveraging the competitive advantages of regions such as Colombia—characterized by cost efficiency and regulatory agility—organizations like bioaccess® are strategically positioned to expedite the development of innovative therapies. This approach not only accelerates research timelines but also highlights the essential role of microbiological practices in improving patient outcomes.
Key insights from this examination underscore the significance of:
A comprehensive understanding of regulatory requirements and an investment in early-phase studies further enhance the efficacy and safety of new microbiology pharmaceuticals. Moreover, a steadfast commitment to quality assurance ensures that these products adhere to the highest standards, fostering trust among stakeholders and bolstering the credibility of research efforts.
As the field evolves, embracing emerging trends such as personalized medicine and artificial intelligence becomes imperative. Researchers must remain proactive in adapting to these developments, which not only promise to enhance clinical practices but also significantly impact global health innovation. By prioritizing these insights and strategies, the microbiology pharmaceutical sector can pave the way for groundbreaking advancements that ultimately benefit patients and society at large.
What is bioaccess® and what does it specialize in?
bioaccess® specializes in accelerating clinical research in microbiology pharmaceuticals by leveraging its expertise in early-phase research.
What competitive advantages does bioaccess® have in Colombia?
bioaccess® benefits from cost reductions exceeding 30% compared to North America and Western Europe, regulatory efficiency with ethical approvals secured in 90-120 days, and access to a diverse patient population of over 50 million.
How does bioaccess® ensure effective clinical studies?
bioaccess® ensures effective clinical studies through substantial investments in R&D, which benefit from tax incentives, and by adhering to high-quality healthcare standards through the rigorous ICH/GCP certification process for hospitals in Colombia.
What role do rapid microbiological identification methods play in clinical research?
Rapid methods like PCR and mass spectrometry enhance safety and efficiency by facilitating quicker diagnoses of infections, enabling timely interventions, and ensuring rigorous adherence to safety protocols.
Why is tracking microbial sources important in clinical trials?
Tracking microbial sources is crucial for ensuring compliance with regulatory standards, identifying contamination sources, and mitigating risks associated with microbial infections.
What research management services does bioaccess® offer?
bioaccess® offers comprehensive research management services including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and detailed reporting to ensure meticulous management of microbiology pharmaceutical studies.