


The article provides crucial insights into post-market surveillance for medical devices, underscoring its vital role in ensuring product safety and regulatory compliance. It asserts that effective post-market surveillance encompasses systematic monitoring, user feedback, and strict adherence to regulatory requirements. These elements collectively enhance patient safety and foster continuous improvement in medical technology. By emphasizing these key aspects, the article establishes the necessity for robust post-market surveillance in the Medtech landscape.
Post-market surveillance for medical devices stands as a critical pillar in safeguarding patient safety and ensuring the efficacy of medical technologies; it is not merely a regulatory requirement. As manufacturers navigate the complexities of compliance and strive for innovation, understanding the intricacies of post-market monitoring becomes paramount.
What challenges do they face in gathering accurate data? How can they leverage user feedback to enhance device performance?
This article delves into ten key insights that illuminate the evolving landscape of post-market surveillance, revealing strategies that can empower Medtech innovators to optimize their practices and ultimately protect patient welfare.
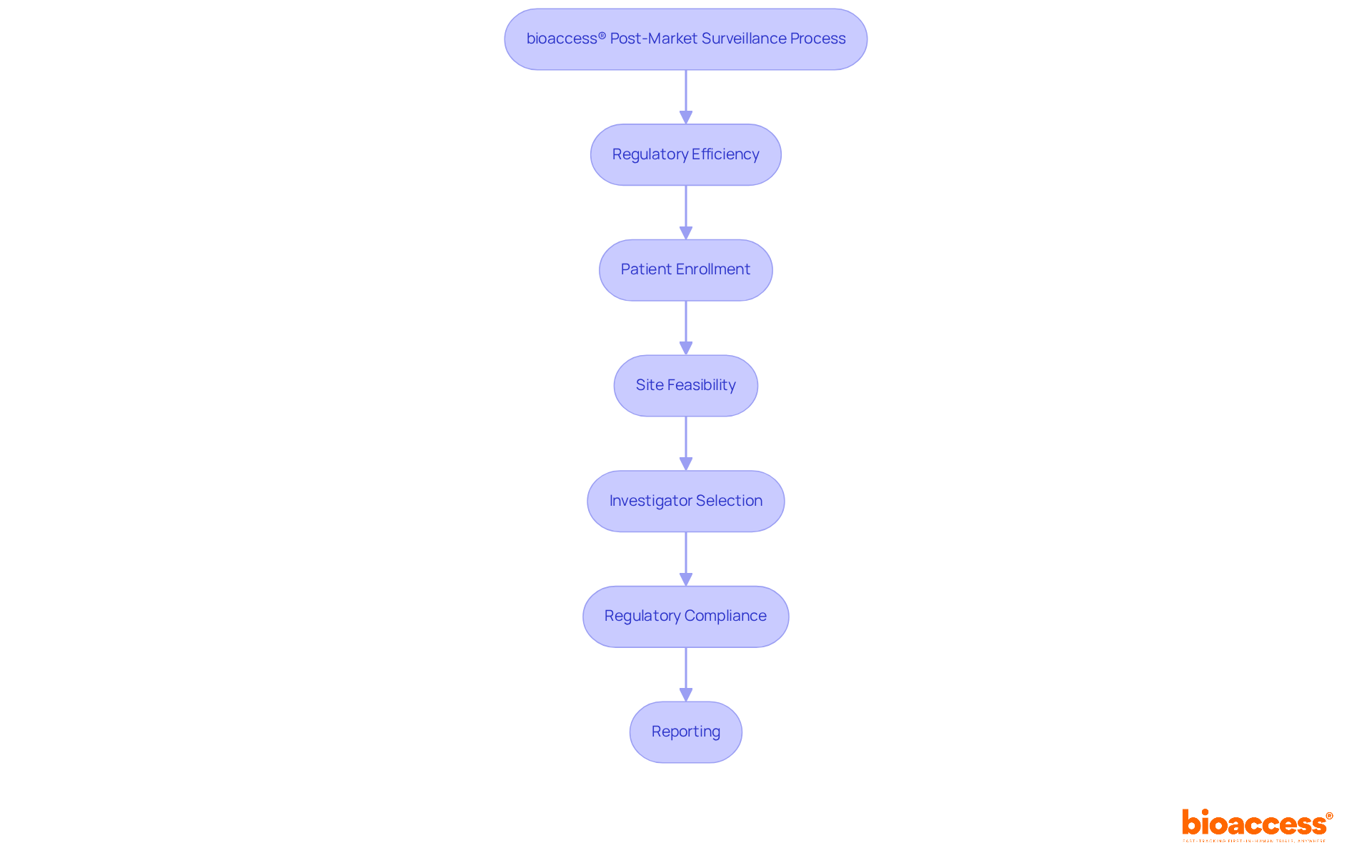
bioaccess® effectively integrates regulatory efficiency with diverse patient populations across Latin America, the Balkans, and Australia, significantly enhancing post-market monitoring for medical products. By securing ethical approvals in a mere 4-6 weeks and facilitating expedited enrollment, bioaccess® empowers Medtech innovators to enhance their post-market surveillance medical device performance and reliability monitoring with greater efficacy.
Focusing on comprehensive processes—including site feasibility, investigator selection, regulatory compliance, study project management, and reporting—bioaccess® not only enhances patient outcomes but also ensures robust regulatory compliance.
Furthermore, Colombia's competitive advantages, such as cost efficiency, rapid regulatory reviews, and a high-quality healthcare system, further bolster the acceleration of clinical trials, positioning it as an ideal locale for Medtech startups aspiring for swift market entry.
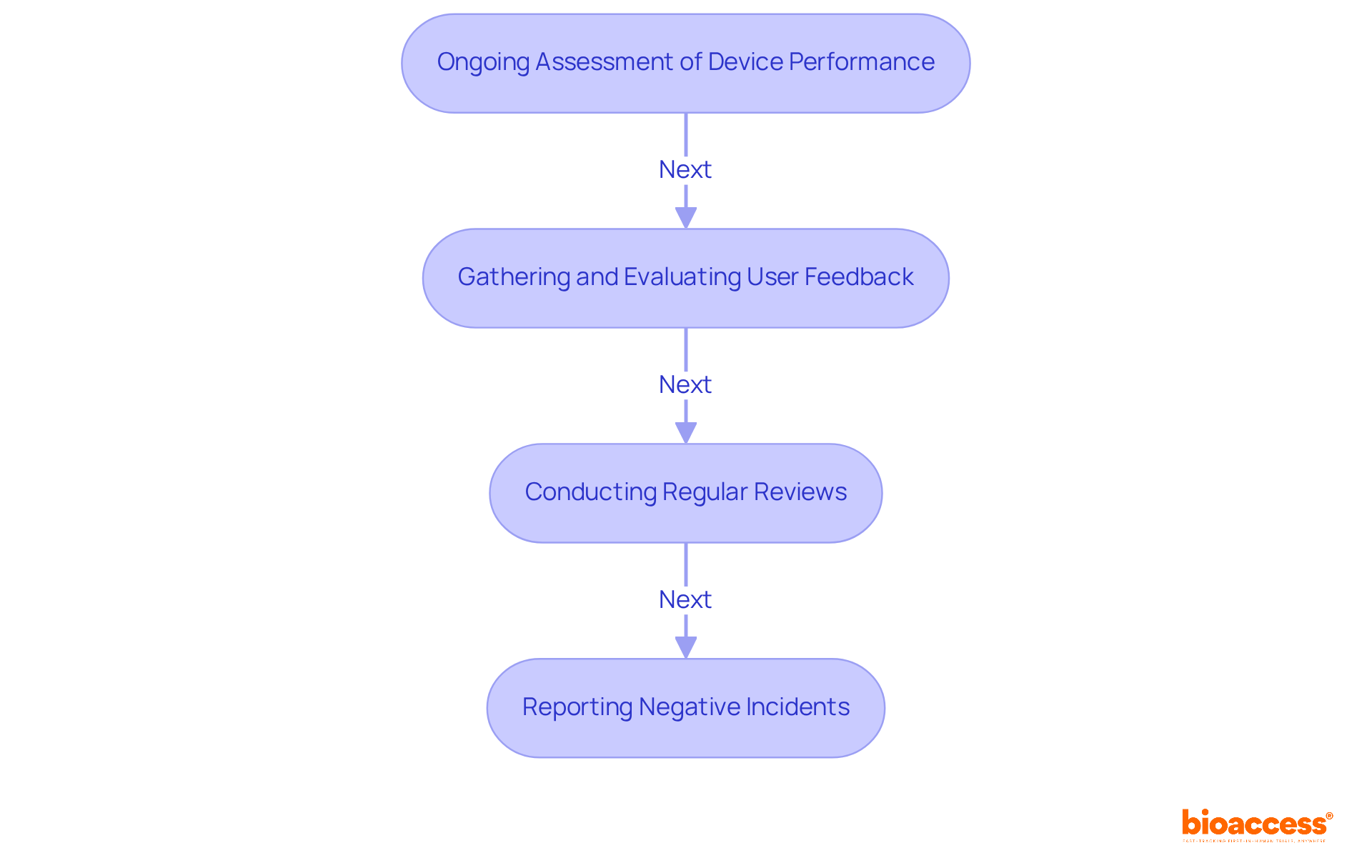
Post-market surveillance medical device serves as a systematic process that monitors medical products following their market approval. The primary objective is to ensure the ongoing protection and efficacy of these instruments, enabling producers to swiftly identify potential issues and implement corrective measures. This proactive approach is essential for safeguarding patient well-being and maintaining public trust in medical technologies.
At bioaccess®, we leverage over 20 years of expertise in managing clinical studies, including:
This ensures our clients not only fulfill regulatory requirements but also uphold the highest standards of innovation and safety in their medical products. Statistics reveal that serious incidents, such as those resulting in death or significant health deterioration, must be reported to regulatory authorities, highlighting the critical role of the post-market surveillance medical device in risk identification and mitigation.
For instance, the FDA mandates that manufacturers of certain Class II and III products submit comprehensive plans for post-market surveillance medical device within 15 months of approval. Continuous monitoring of equipment performance is vital, as it assists in adjusting the risk/benefit profile in accordance with regulatory standards, such as the EU Medical Device Regulation (MDR).
Recent studies have demonstrated the effectiveness of the post-market surveillance medical device in enhancing medical equipment security. A well-organized strategy for post-market surveillance medical device allows manufacturers to systematically assess equipment performance and implement necessary corrective actions, thereby improving overall product security. Practical examples illustrate how PMS has protected patient welfare; for example, investigations into adverse occurrences linked to medical instruments have led to crucial revisions in product labeling and safety communications.
In Latin America, regulatory bodies like INVIMA are instrumental in ensuring compliance and safety. In summary, effective post-market surveillance medical device practices transcend mere regulatory compliance; they are a vital element of quality management that promotes continuous improvement and innovation in the medical device industry.

Key regulatory requirements for post-market surveillance medical device encompass strict adherence to guidelines established by organizations such as the FDA and the European Medicines Agency (EMA). Manufacturers are mandated to develop a plan for post-market surveillance medical device that delineates:
Experts like Ana Criado, Director of Regulatory Affairs and a professor in biomedical engineering, underscore the critical nature of these regulations in maintaining market authorization and ensuring patient protection. With her extensive experience in regulatory roles at INVIMA and as a consultant for global companies, Ana's insights are essential for navigating the complexities of compliance within the medical equipment sector, particularly regarding Colombian regulations. Compliance with these regulations is imperative for sustaining market authorization and ensuring the effectiveness of post-market surveillance medical device practices to safeguard patient well-being.

Post-market surveillance medical device is essential for ensuring the reliability and effectiveness of medical products after their launch. Its core activities encompass:
These processes are vital for identifying potential hazards and ensuring that corrective actions are implemented swiftly to mitigate risks. For example, effective PMS systems empower manufacturers to derive insights from user experiences, leading to product enhancements and improved patient safety.
As we approach 2025, the emphasis on robust post-market surveillance medical device practices under regulations such as ANVISA will require manufacturers to adopt comprehensive monitoring strategies that adapt to emerging information and trends. This proactive stance not only guarantees compliance but also encourages innovation and builds trust within the medical technology sector.
Furthermore, techniques for information collection, including user surveys and post-market clinical follow-up studies, are crucial for obtaining in-depth feedback, enabling manufacturers to refine their products based on real-world usage. Engaging with users and healthcare professionals enriches the quality and quantity of PMS information, ultimately resulting in better health outcomes.
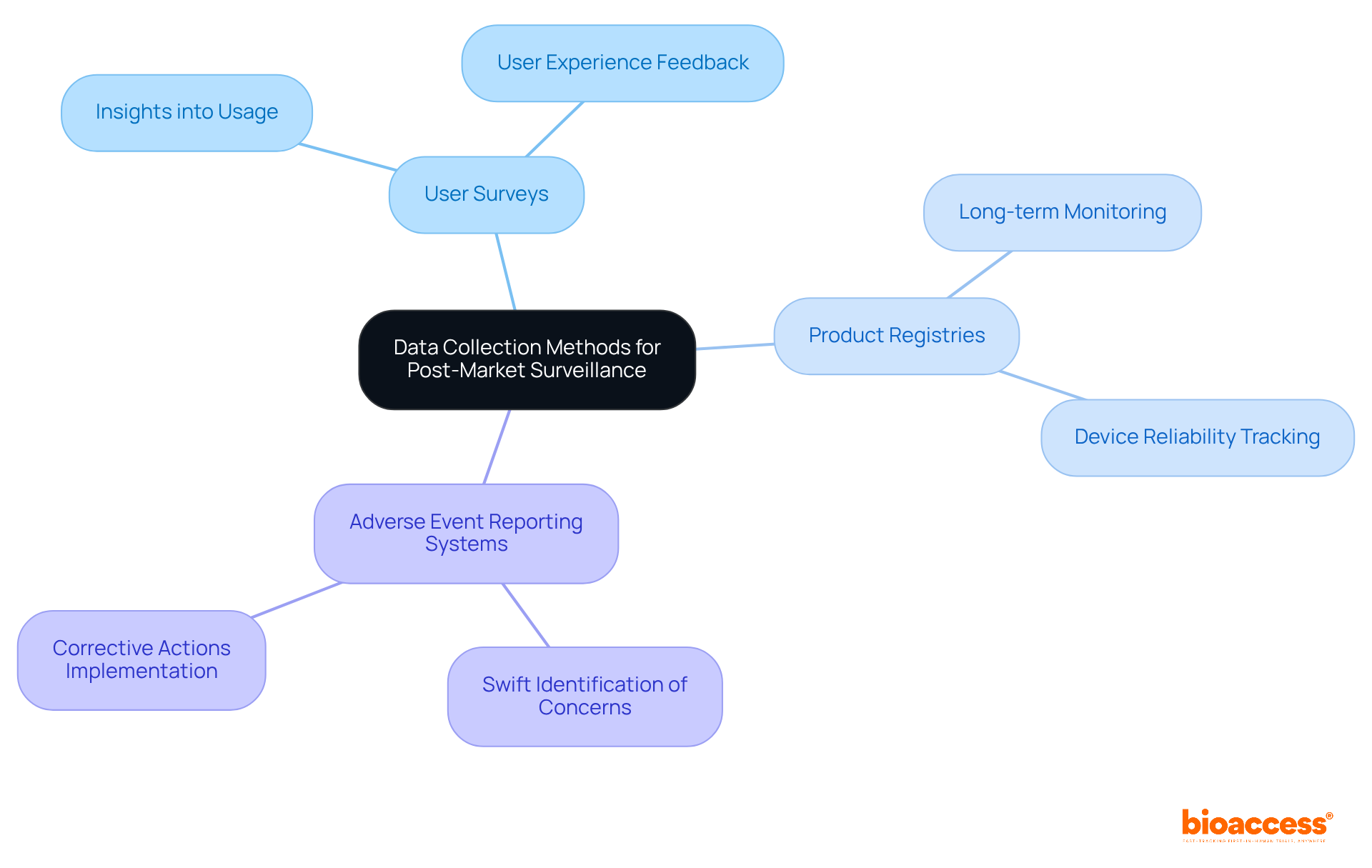
Effective post-market surveillance medical device is pivotal in the realm of clinical research, relying on robust information collection methods such as user surveys, product registries, and adverse event reporting systems. These essential tools empower manufacturers to acquire real-world data regarding device performance and user experiences.
For instance, user surveys provide critical insights into device utilization within clinical settings, while product registries facilitate long-term monitoring of device reliability and effectiveness across diverse populations. Furthermore, adverse event reporting systems are vital for the swift identification of concerns, allowing manufacturers to implement necessary corrective actions promptly.
Health Canada reports that approximately 200,000 adverse drug reactions (ADRs) are documented annually, underscoring the significance of effective adverse event reporting systems. Additionally, the European Union Medical Device Regulation (MDR) emphasizes that post-market surveillance medical device is crucial for safeguarding patient well-being.
By leveraging these information-gathering techniques, manufacturers can enhance patient protection, ensure compliance with regulatory standards, and maintain high levels of product quality throughout the product lifecycle.
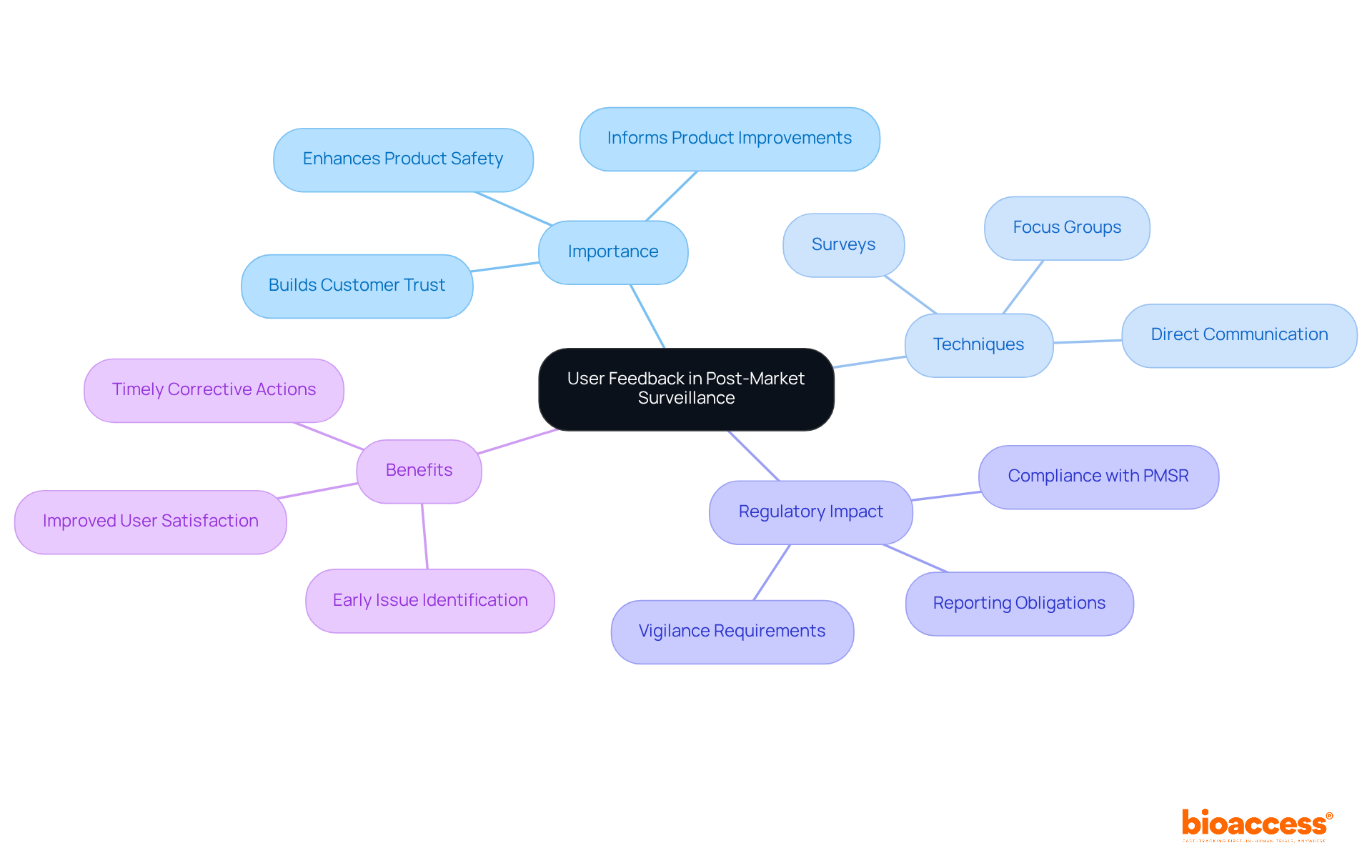
User feedback plays a vital role in post-market monitoring, providing manufacturers with crucial insights into product performance and potential risk issues. By involving users through various techniques such as surveys, focus groups, and direct communication avenues, manufacturers can gather essential data that informs product improvements and bolsters overall security.
As we move into 2025, the significance of user insights has become increasingly apparent, particularly as regulatory authorities stress the necessity for robust post-market surveillance systems. Proactive engagement strategies, including customer surveys and post-market clinical follow-up studies, have demonstrated effectiveness in early issue identification and timely corrective actions.
Real-world examples reveal that companies leveraging user feedback not only enhance device security but also cultivate trust and satisfaction within their customer base. As Medtech professionals continue to champion comprehensive user engagement, the integration of user insights into safety protocols is poised to redefine industry standards and practices.
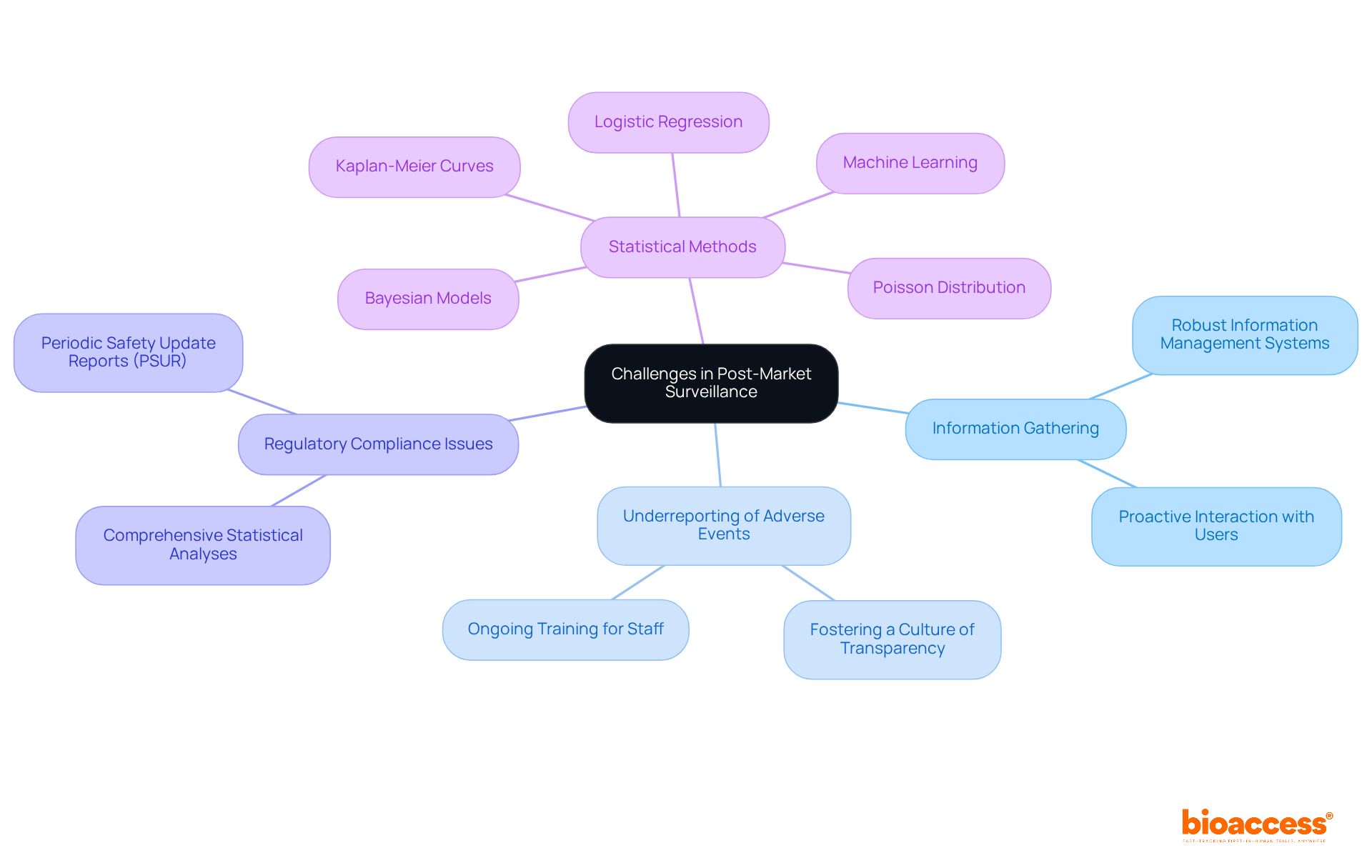
Obstacles in post-market surveillance medical devices present significant challenges, including difficulties in gathering information, underreporting of adverse events, and regulatory compliance issues. To effectively overcome these barriers, manufacturers of post-market surveillance medical devices must implement robust information management systems, foster a culture of transparency, and ensure ongoing training for staff involved in PMS activities. Additionally, proactive interaction with users and healthcare professionals can enhance reporting rates and improve the quality of information gathered.
Statistics clearly indicate that underreporting can lead to a substantial gap in safety data, undermining the effectiveness of post-market surveillance medical device efforts. Advanced statistical methods, such as logistic regression, machine learning, and the Poisson distribution, are crucial for recognizing patterns in adverse event reporting and improving the overall risk profile evaluation of medical equipment. Furthermore, Kaplan-Meier curves serve as valuable tools to assess the duration of medical equipment performance over time, pinpointing critical risk moments that require attention.
Incorporating case studies, such as the Post-Market Clinical Follow-up (PMCF), which involves ongoing collection and analysis of post-market clinical information, effectively demonstrates the application of these strategies. Moreover, the application of Bayesian models for wound disinfection tools illustrates how combining information from diverse sources can lead to a more thorough assessment of risk data across various populations.
As Felisiano Cipressi from Di Renzo Regulatory Affairs aptly states, 'Statistics plays a crucial role in the post-market surveillance medical device monitoring and in the assessment of the reliability of medical instruments.' This underscores the legal requirement of conducting comprehensive statistical analyses in safety reports, which is vital for ensuring patient welfare and the long-term success of medical instruments in the market.
Information from the post-market surveillance medical device is pivotal in shaping clinical assessments by providing essential real-world evidence regarding product performance and safety. This information is critical for:
By incorporating PMS information into clinical assessments, manufacturers can guarantee that their devices continually meet evolving standards of effectiveness and reliability throughout their lifecycle. Notably, approximately 70% of medical instruments in Brazil are subject to ongoing oversight, underscoring the necessity of robust post-market surveillance medical device systems to ensure compliance and enhance patient safety.
Furthermore, advanced analytics have demonstrated the capability to identify significant issues an average of 18 months earlier than traditional methods, emphasizing the importance of timely information in informing clinical decisions. As regulatory landscapes evolve, the integration of post-market surveillance medical device data will be indispensable for manufacturers aiming to tackle compliance challenges and improve the performance of their equipment.

Future trends in post-market surveillance medical devices are increasingly shaped by the integration of artificial intelligence (AI) and machine learning. These technologies enable the analysis of vast datasets, allowing for the identification of safety signals and emerging trends with greater efficacy. The ongoing observation of performance facilitated by AI systems permits prompt interventions when issues arise. Notably, AI systems can adapt over time, enhancing their accuracy and responsiveness to real-world conditions. However, this adaptability also introduces challenges related to output unpredictability.
Moreover, digital health technologies, including mobile applications and wearable gadgets, are revolutionizing information gathering and user feedback systems. These tools not only enhance real-time monitoring but also empower patients to report adverse events directly, thereby enriching the data pool available for PMS. The FDA's recent draft guidance underscores the importance of a total product life cycle approach, emphasizing the necessity for continuous performance assessments of AI-enabled technologies post-deployment.
Industry leaders advocate for a collaborative framework that encourages manufacturers and healthcare providers to actively engage in the post-market surveillance medical device process. This collaboration is critical to ensure that AI technology is monitored effectively, striking a balance between innovation and patient safety. As the landscape of medical equipment monitoring evolves, the role of AI and machine learning will be pivotal in enhancing the robustness and responsiveness of PMS systems.

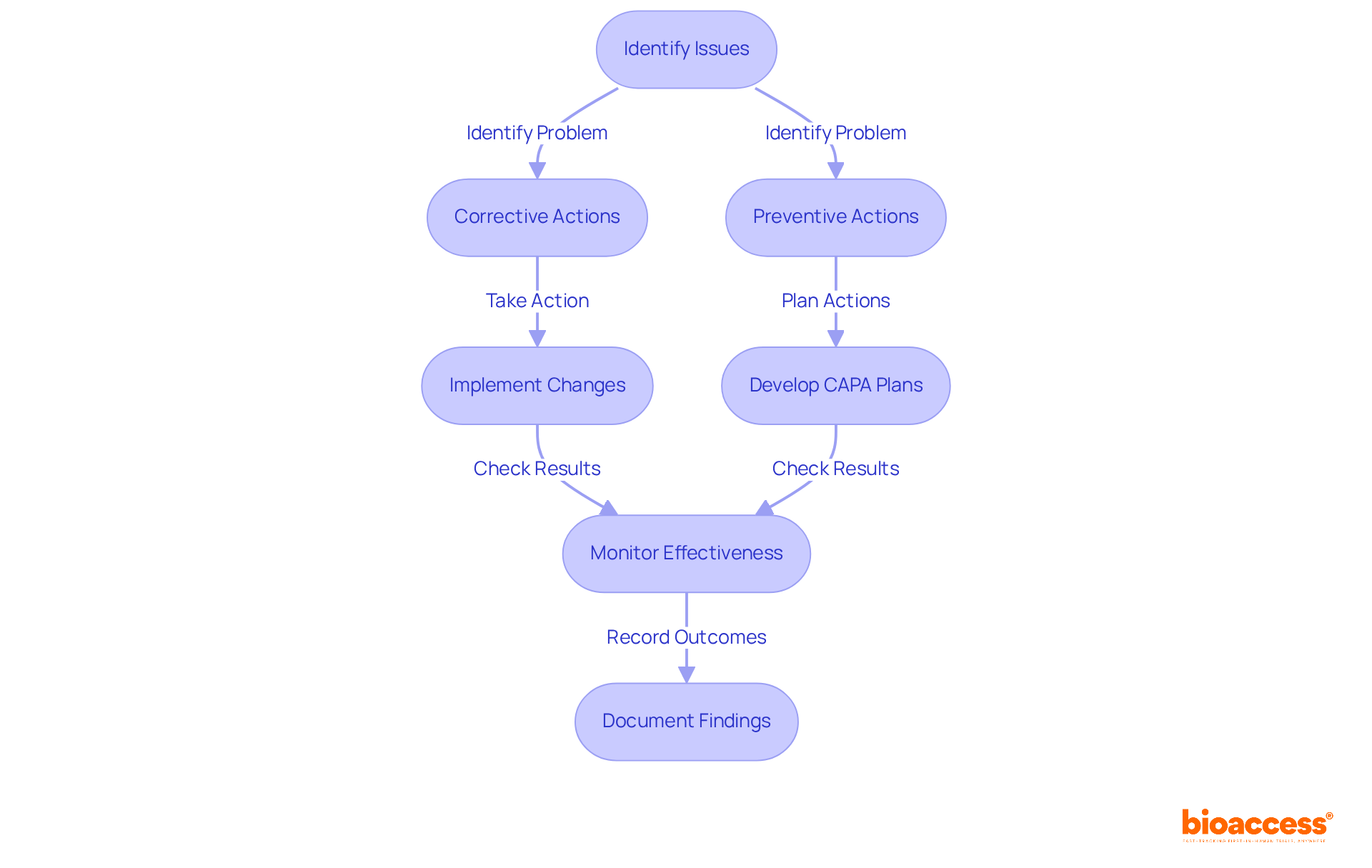
Corrective and preventive actions (CAPA) are indispensable elements of post-market surveillance, ensuring that identified issues are addressed with urgency. Manufacturers are required to develop and implement comprehensive CAPA plans that delineate specific actions to rectify problems and prevent their recurrence.
In Colombia, the INVIMA (Colombia National Food and Drug Surveillance Institute) occupies a crucial position in overseeing the marketing and production of health products, including medical instruments. As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA ensures that producers adhere to stringent standards for effectiveness and quality.
Regular reviews of post-market surveillance medical device data should inform CAPA strategies, fostering a culture of continuous improvement and enhancing overall safety of the device, in alignment with INVIMA's regulatory framework.
The significance of post-market surveillance in the medical device industry is paramount. It serves as a critical mechanism for ensuring the safety and efficacy of medical products after they have been approved for market use. By implementing comprehensive monitoring strategies, manufacturers can swiftly identify potential risks and take corrective actions, ultimately safeguarding patient well-being and maintaining public trust in medical technologies.
Key insights highlighted throughout the article include:
The integration of advanced technologies such as AI and machine learning is reshaping post-market surveillance, enabling more effective analysis of real-world data and timely interventions. Moreover, the emphasis on corrective and preventive actions underscores the ongoing commitment required from manufacturers to uphold the highest standards of safety and quality.
As the landscape of medical device monitoring evolves, it is imperative for stakeholders to remain vigilant and proactive. Embracing best practices in post-market surveillance not only fulfills regulatory obligations but also fosters innovation and improves patient outcomes. Engaging with healthcare professionals, leveraging user insights, and adopting new technologies will be crucial in navigating the challenges ahead. The collective efforts in enhancing post-market surveillance practices will ultimately contribute to a safer, more reliable healthcare environment for all.
What is bioaccess® and how does it enhance post-market surveillance for medical devices?
bioaccess® integrates regulatory efficiency with diverse patient populations across Latin America, the Balkans, and Australia, enhancing post-market monitoring for medical products. It secures ethical approvals in 4-6 weeks and facilitates expedited enrollment, allowing Medtech innovators to improve the performance and reliability monitoring of medical devices.
What processes does bioaccess® focus on to enhance post-market surveillance?
bioaccess® focuses on comprehensive processes including site feasibility, investigator selection, regulatory compliance, study project management, and reporting, which enhance patient outcomes and ensure robust regulatory compliance.
What advantages does Colombia offer for clinical trials in the Medtech sector?
Colombia offers competitive advantages such as cost efficiency, rapid regulatory reviews, and a high-quality healthcare system, making it an ideal location for Medtech startups seeking swift market entry.
What is post-market surveillance and why is it important?
Post-market surveillance is a systematic process that monitors medical products after market approval to ensure ongoing protection and efficacy. It allows producers to quickly identify potential issues and implement corrective measures, which is essential for safeguarding patient well-being and maintaining public trust in medical technologies.
What types of studies does bioaccess® manage in relation to post-market surveillance?
bioaccess® manages various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Clinical Follow-Up Studies (PMCF).
What are the regulatory requirements for post-market surveillance of medical devices?
Manufacturers must develop a plan for post-market surveillance that includes data collection methods, reporting procedures for adverse events, and mechanisms for implementing corrective actions, following guidelines set by organizations like the FDA and EMA.
Why is compliance with post-market surveillance regulations critical?
Compliance is critical for maintaining market authorization and ensuring patient protection. It helps manufacturers systematically assess equipment performance and implement necessary corrective actions, thereby improving overall product security.
How does post-market surveillance contribute to patient safety?
Effective post-market surveillance practices allow manufacturers to identify and mitigate risks associated with medical devices, leading to crucial revisions in product labeling and safety communications, ultimately protecting patient welfare.