


Post-marketing studies serve as a vital link between clinical trials and real-world application, ensuring that medications remain safe and effective long after their initial approval. For Clinical Research Directors, grasping the intricacies of these studies is essential as they navigate the complex landscape of regulatory requirements, patient safety, and market access.
With the rapid evolution of healthcare technologies and methodologies, how can these professionals effectively leverage post-marketing insights to enhance drug efficacy and streamline commercialization? This question not only highlights the challenges but also unveils transformative opportunities in the realm of clinical research.
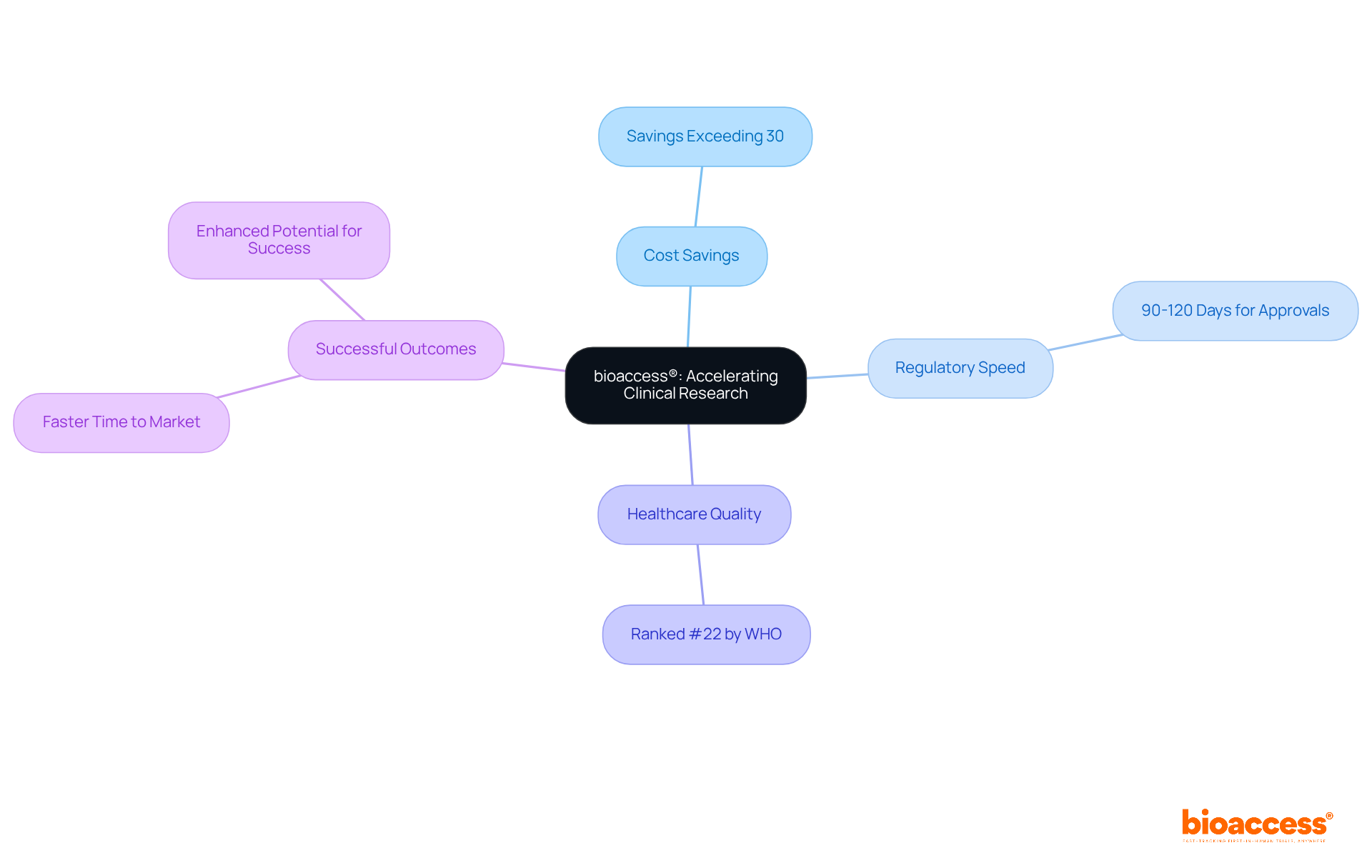
bioaccess® excels in delivering rapid clinical research services tailored for Medtech startups. By leveraging Colombia's competitive advantages - such as cost savings exceeding 30% compared to North America and Western Europe, regulatory speed that allows for ethical approvals in just 90-120 days, and a high-quality healthcare system ranked among the best globally - bioaccess® significantly accelerates clinical trials. For instance, the median time to regulatory authority (RA) approval in Colombia is notably efficient, with the World Health Organization ranking the country's healthcare system as #22 out of 191 countries. Furthermore, the median time from Ethics Committee/Institutional Review Board (EC/IRB) approval to the first patient randomized is streamlined, illustrating the effective processes available in Colombia.
This capability not only reduces time to market but also enhances the potential for successful outcomes in clinical research. The impact of such regulatory agility is profound; it empowers Medtech innovators to navigate the complexities of clinical trials effectively, fostering a more dynamic environment for medical advancements. Successful Medtech startups, like Welwaze Medical Inc. with its Celbrea® device, have harnessed this speed to bring their innovations to market faster, underscoring the importance of rapid clinical research services in today's competitive landscape.
Reducing bureaucratic burdens in clinical trials is essential for improving efficiency. This makes bioaccess® an invaluable partner for startups aiming to navigate the complexities of clinical research. Are you ready to explore how bioaccess® can help you overcome your clinical research challenges?
Post marketing studies are essential for ensuring the safety and effectiveness of medications after they receive public approval. This ongoing evaluation is crucial for identifying adverse effects that may not have surfaced during clinical trials. In Colombia, the National Food and Drug Surveillance Institute (INVIMA) plays a pivotal role in this process. Established in 1992 under the Ministry of Health and Social Protection, INVIMA is tasked with inspecting and supervising the marketing and manufacturing of health products, including medical devices.
The Directorate for Medical Devices and other Technologies within INVIMA specifically oversees the regulation of medical devices, ensuring compliance with standards of effectiveness and reliability. By systematically collecting and analyzing data from real-world use, regulatory bodies like INVIMA can promptly address potential risks, thereby safeguarding public health. This regulatory framework is vital for maintaining the integrity of post marketing studies and ensuring that products consistently meet the required safety and quality standards.
As we navigate the complexities of clinical research, the collaboration between regulatory bodies and healthcare providers becomes increasingly important. How can we leverage this partnership to enhance patient safety and product reliability? The next steps involve fostering stronger ties within the Medtech landscape to tackle key challenges effectively.

Post marketing studies play a crucial role in clinical research, and they can be categorized into two main types: passive and active surveillance.
Passive surveillance relies on voluntary reporting systems, where healthcare providers and patients report adverse events. While this method is cost-effective, it faces significant underreporting challenges, with estimates suggesting that only 10% of adverse events are reported in some systems. This gap can lead to missed security information, underscoring the necessity for improved data gathering and examination to enhance effectiveness.
In contrast, active surveillance involves proactive data gathering, often through structured studies or registries, including post marketing studies, allowing for more thorough evaluations of security. A prime example is the FDA’s Sentinel Initiative, which utilizes real-time data and advanced technologies like AI and machine learning to monitor drug security effectively. Researchers emphasize that active monitoring not only enhances the identification of adverse events but also provides a stronger framework for understanding drug security over time. As one expert noted, 'Active surveillance allows us to capture data that passive systems often miss, leading to better-informed regulatory decisions.'
Understanding these distinctions, along with the challenges faced by passive systems-such as operator variability and technological complexity-is essential for creating effective evaluations that prioritize individual well-being. This knowledge is vital for stakeholders in the Medtech landscape, as it informs strategies to address key challenges and improve patient outcomes.

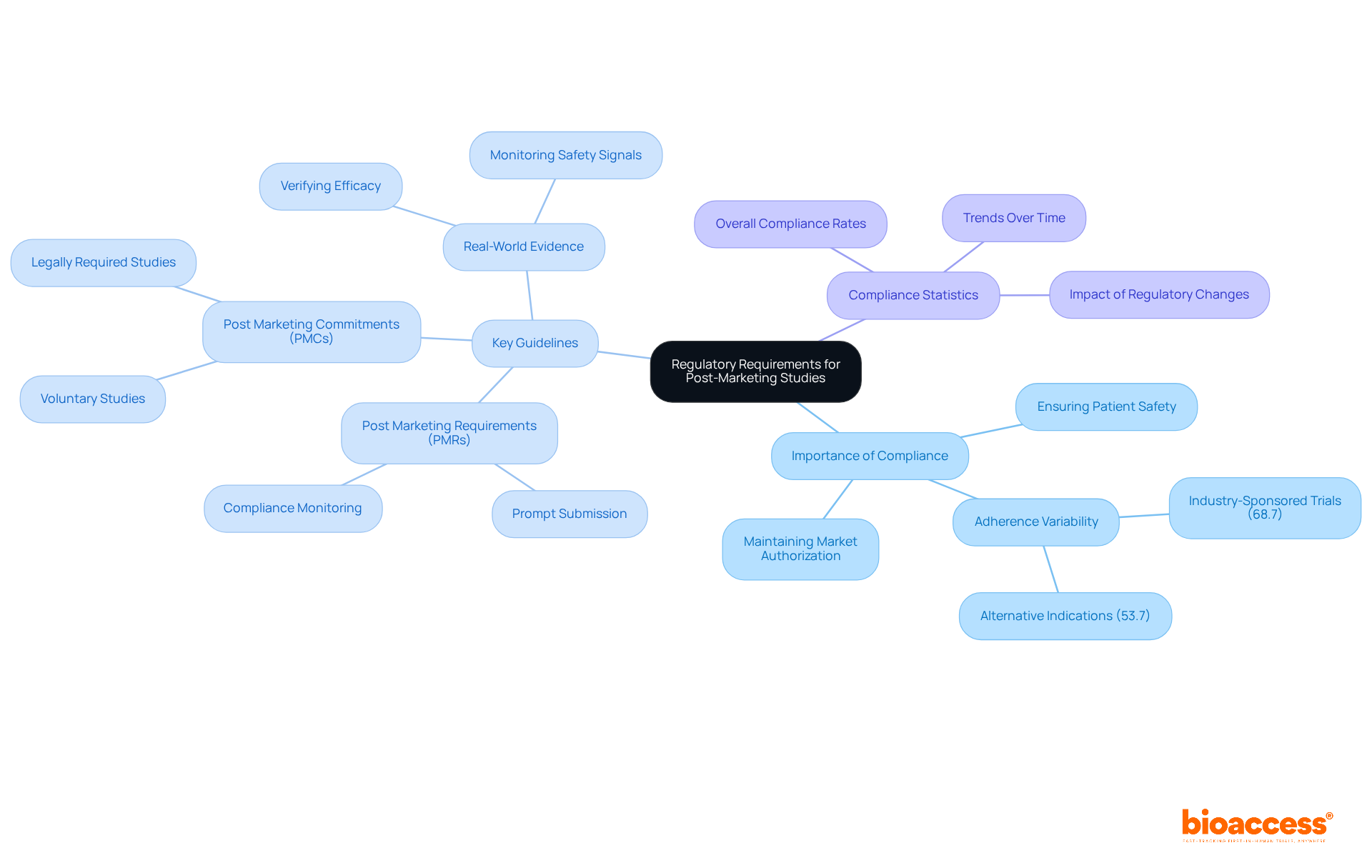
Navigating the regulatory environment for after-market research is crucial for clinical research professionals. A comprehensive grasp of the guidelines set by the FDA and EMA is essential. These regulations outline the types of data that must be collected, the timelines for reporting, and the ethical considerations necessary for compliance. Adhering to these regulations is vital for maintaining market authorization and ensuring patient safety.
In 2025, Clinical Research Directors must be particularly vigilant. Compliance rates with regulations for post marketing studies have shown variability, with industry-sponsored trials often leading in adherence. For instance, approximately 68.7% of research for initially authorized indications were funded by the industry, compared to 53.7% for alternative indications. Regulatory affairs specialists emphasize that staying updated on changes in these guidelines is essential for effectively managing studies.
Essential compliance guidelines include:
As the landscape evolves, understanding these requirements will be key for effectively navigating research related to post marketing studies. Are you prepared to meet these challenges head-on?
Real-world evidence (RWE) plays a crucial role in post marketing studies, offering essential insights into drug performance in everyday clinical settings. By leveraging data from electronic health records, registries, and insurance claims, researchers can uncover trends in medication effectiveness and safety that might remain hidden during clinical trials. This strategic use of RWE not only deepens our understanding of a drug's effects across diverse populations but also significantly enhances individual well-being and treatment outcomes.
Moreover, integrating electronic health records into drug risk monitoring allows for real-time tracking of adverse events and long-term effects, thereby improving the overall assessment of therapeutic interventions. Insights from researchers indicate that effectively utilizing RWE can lead to more informed decision-making regarding drug safety, ultimately benefiting both healthcare providers and patients. As RWE continues to gain traction, it is increasingly being applied in early development activities, streamlining regulatory and HTA approval processes while reducing time and budget constraints.
Early planning in real-world data (RWD) generation is vital for maximizing the impact of RWE in clinical research. By prioritizing RWD, stakeholders can address key challenges in the Medtech landscape, fostering collaboration and innovation. What steps can you take today to leverage RWE in your clinical research efforts?

Recruitment for post marketing studies often presents significant challenges, including limited patient access, strict eligibility criteria, and competition for participants. To effectively navigate these hurdles, Clinical Research Directors can adopt several targeted recruitment strategies:
Successful recruitment strategies hinge on transparent communication, cultural sensitivity, and sustained engagement. For instance, fostering participant feedback mechanisms and maintaining regular updates can strengthen the researcher-participant relationship, leading to improved retention rates. Engaged participants not only provide more detailed long-term follow-up data but also contribute to the overall integrity of the research, ensuring reliable outcomes and advancing therapeutic innovation. By applying these strategies, Clinical Research Directors can improve the success rates of their after-market evaluations, ultimately speeding up the market entry of new treatments. Notably, effective participant recruitment and retention strategies can lead to enrollment being six months ahead of schedule, demonstrating the potential impact of these approaches.

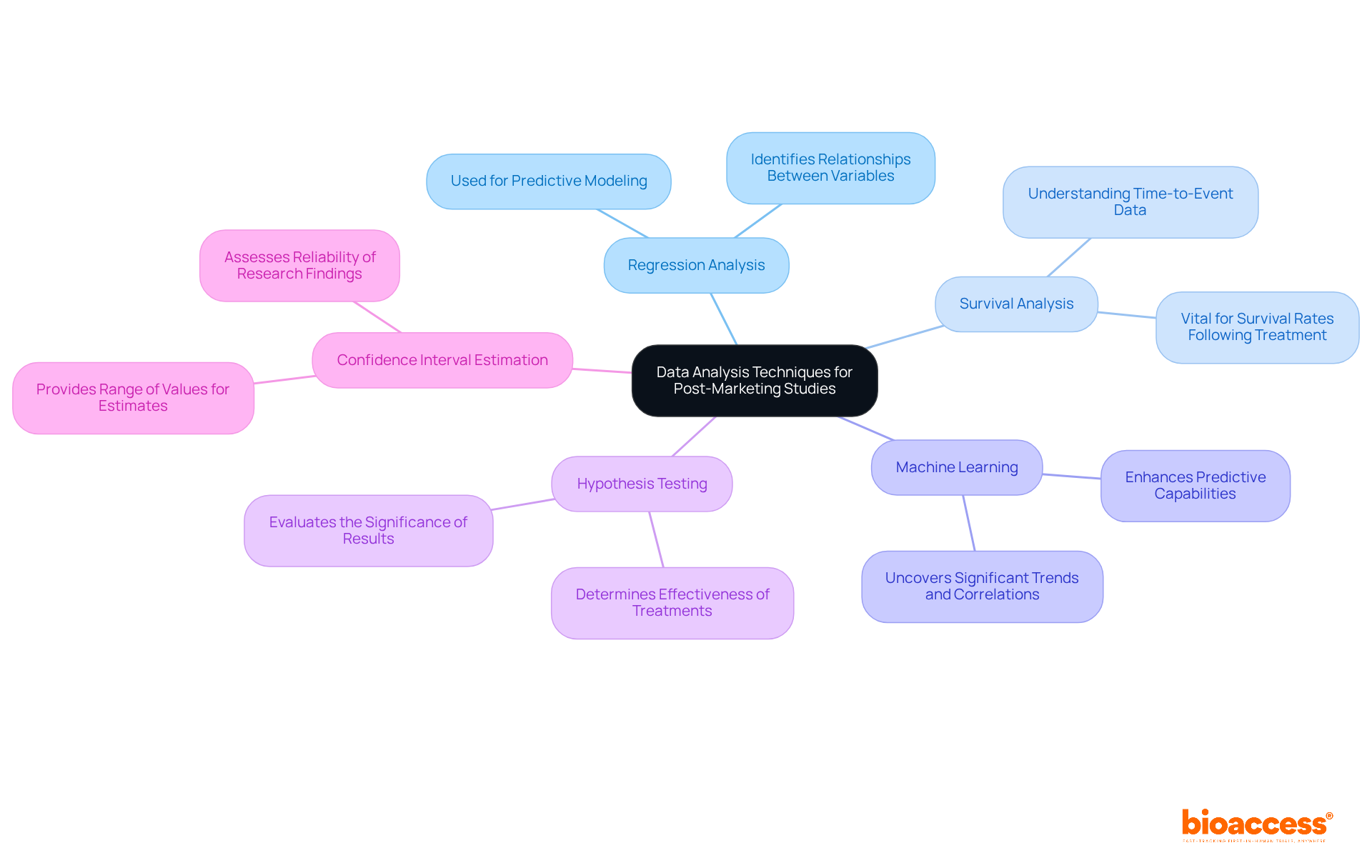
Data analysis in post marketing studies is crucial for deriving accurate interpretations of clinical results. By employing a variety of statistical techniques - such as regression analysis, survival analysis, and machine learning - researchers can uncover significant trends and correlations within the data. For example, regression analysis identifies relationships between variables, while survival analysis is vital for understanding time-to-event data, like survival rates following treatment. Additionally, machine learning algorithms enhance predictive capabilities, offering deeper insights into patient outcomes.
Biostatistical techniques are essential for evaluating the importance of results, ensuring that warning signals are identified swiftly. Techniques like hypothesis testing and confidence interval estimation are fundamental in assessing the reliability of research findings. Insights from biostatisticians underscore the necessity of robust methodologies for interpreting clinical data, particularly in post marketing studies, where the implications for patient safety and product efficacy are paramount. A thorough understanding of these statistical methods is critical for Clinical Research Directors to draw meaningful conclusions and make informed decisions based on their research.
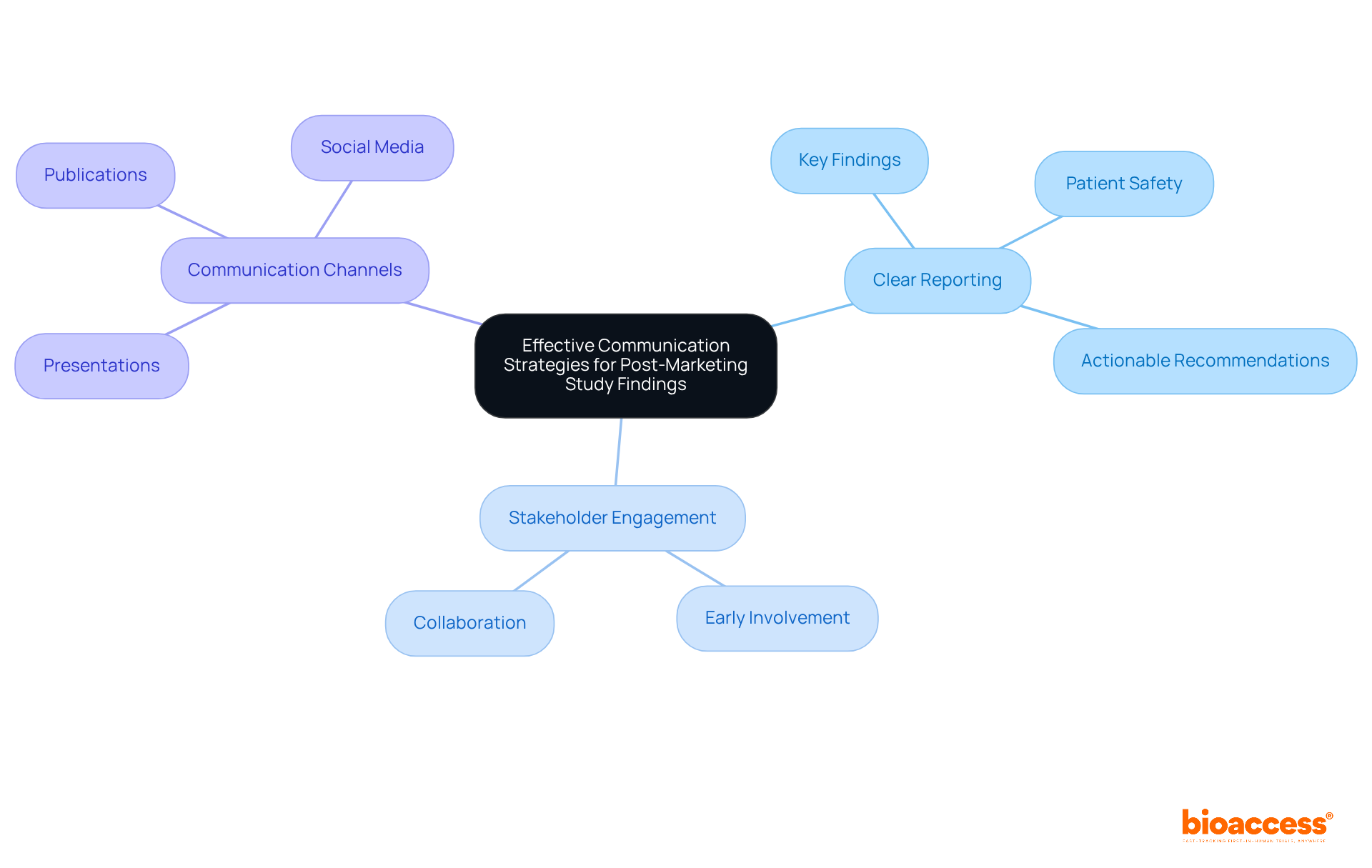
Effectively conveying the results of post marketing studies is crucial for influencing regulatory choices and improving healthcare practices. Clinical Research Directors must prioritize the development of clear and concise reports that emphasize key findings, implications for patient safety, and actionable recommendations. By employing various communication channels - such as presentations, publications, and social media - research findings can gain significant visibility and influence.
Involving stakeholders early in the process addresses their concerns and promotes collaboration. This collaboration enables the implementation of essential changes based on the research's findings. Successful examples of stakeholder engagement in clinical research demonstrate that open dialogue and transparency can lead to improved outcomes and trust in the research process.
As communication specialists emphasize, effective reporting is not merely about presenting data; it’s about ensuring that the message resonates with the audience. This approach ultimately drives informed decision-making and enhances the overall impact of the research.
Post marketing studies are crucial in shaping market access and the commercialization of medical products. By providing robust evidence of safety and effectiveness, these studies not only facilitate regulatory submissions but also ease market entry. Favorable outcomes from after-launch research can greatly enhance a product's reputation, fostering trust among healthcare professionals and patients, which in turn drives sales. Industry experts emphasize that manufacturers should regard evidence generation as an ongoing process throughout the drug lifecycle, extending beyond phase 3 trials. This perspective underscores the necessity for Clinical Research Directors to consider the commercial ramifications of their studies. Aligning after-launch research with broader business strategies is vital for maximizing benefits and ensuring successful product commercialization.
Current trends reveal that drugs introduced in areas with unmet medical needs tend to achieve greater success, especially when backed by real-world evidence (RWE). For instance, a recent analysis indicated that 25% of post-launch investigations could leverage RWE, particularly those focusing on underrepresented populations and safety. These insights highlight the importance of integrating post marketing studies into commercialization strategies, ultimately leading to improved health outcomes and market success.
At bioaccess®, we excel in comprehensive clinical trial management services, including:
Our expertise in these areas ensures that your follow-up evaluations are not only compliant but also strategically aligned with your commercialization goals.
The future of post-launch studies is on the brink of transformation, driven by several crucial trends. The increasing reliance on real-world evidence is reshaping how data is collected and analyzed, allowing for more accurate assessments of product performance across diverse populations. Moreover, advancements in data analytics are enhancing this process, enabling Clinical Research Directors to extract actionable insights from extensive datasets.
Digital health technologies are becoming indispensable in post marketing studies, as they facilitate real-time data collection and foster user engagement. Tools like mobile health applications and remote monitoring devices not only streamline data gathering but also empower patients to actively participate in their healthcare journeys. This shift towards patient-centered approaches is vital, aligning with the growing emphasis on stakeholder involvement in research design and execution.
As regulatory agencies increasingly prioritize post marketing studies, Clinical Research Directors must adjust their strategies to incorporate these innovations. The integration of digital health solutions will not only boost the efficiency of studies but also ensure they are responsive to the evolving needs of the healthcare landscape. With over 20 years of experience in Medtech, bioaccess® underscores the importance of embracing these technologies to maintain compliance and enhance patient outcomes. This represents a significant evolution in the approach to clinical research.
The significance of post-marketing studies cannot be overstated; they are a vital mechanism for ensuring the continued safety and efficacy of medications after approval for public use. These studies provide essential insights that help identify adverse effects and validate the benefits of treatments in real-world settings, ultimately safeguarding public health and enhancing patient outcomes.
Key insights presented throughout the article include:
These points emphasize the need for Clinical Research Directors to adapt to a rapidly evolving landscape.
As the future of post-marketing studies unfolds, embracing innovative technologies and methodologies will be crucial for enhancing the efficiency and effectiveness of clinical research. By prioritizing collaboration, transparency, and a patient-centered approach, stakeholders can improve the reliability of post-marketing evaluations and drive meaningful advancements in healthcare. Engaging with these insights and strategies empowers Clinical Research Directors to navigate the complexities of post-marketing studies, ultimately leading to improved market access and commercialization of vital medical products.
What services does bioaccess® provide for Medtech startups?
bioaccess® offers rapid clinical research services specifically tailored for Medtech startups, leveraging Colombia's advantages such as cost savings, regulatory speed, and a high-quality healthcare system.
How does bioaccess® help in accelerating clinical trials?
By utilizing Colombia's competitive advantages, bioaccess® can achieve ethical approvals in just 90-120 days and streamline the approval process, significantly reducing the time to market for Medtech innovations.
What are the cost advantages of conducting clinical trials in Colombia?
Clinical trials in Colombia can provide cost savings exceeding 30% compared to North America and Western Europe.
How does Colombia's healthcare system rank globally?
Colombia's healthcare system is ranked #22 out of 191 countries by the World Health Organization.
What role does INVIMA play in post-marketing surveillance in Colombia?
The National Food and Drug Surveillance Institute (INVIMA) is responsible for inspecting and supervising the marketing and manufacturing of health products, including medical devices, ensuring their safety and efficacy after public approval.
What are the two main types of post-marketing studies?
The two main types of post-marketing studies are passive surveillance, which relies on voluntary reporting, and active surveillance, which involves proactive data gathering through structured studies or registries.
What are the challenges associated with passive surveillance?
Passive surveillance faces significant underreporting challenges, with estimates suggesting that only 10% of adverse events are reported, which can lead to missed safety information.
How does active surveillance improve drug safety monitoring?
Active surveillance involves proactive data gathering, allowing for more thorough evaluations of drug safety, and utilizes advanced technologies like AI to monitor drug security effectively.
Why is collaboration between regulatory bodies and healthcare providers important?
Collaboration is crucial for enhancing patient safety and product reliability, helping to address key challenges in the Medtech landscape effectively.