


The article emphasizes the critical aspects of study feasibility in clinical trials, underscoring the significance of operational, regulatory, and recruitment viability for achieving successful research outcomes.
Comprehensive feasibility assessments are highlighted as essential tools that can significantly reduce risks and enhance the likelihood of success by effectively addressing challenges such as participant enrollment and budget constraints.
This proactive approach ultimately facilitates the efficient introduction of new therapies to the market, reinforcing the necessity of thorough planning and collaboration in the clinical research landscape.
Understanding the feasibility of clinical trials has become increasingly critical in a landscape where the success of new therapies hinges on efficient execution. This article delves into ten key insights that illuminate the multifaceted nature of study feasibility, exploring how operational, regulatory, and recruitment factors intertwine to influence trial outcomes. As the stakes rise, the challenge remains: how can researchers navigate the complexities of feasibility assessments to ensure timely and effective trials? The answers lie in strategic planning, innovative technology, and a thorough understanding of the dynamics at play.
bioaccess® leverages the regulatory speed of Latin America, the diverse patient populations in the Balkans, and Australia's efficient pathways to secure ethical approvals in just 4 to 6 weeks. This global-first agility empowers Medtech, Biopharma, and Radiopharma innovators to significantly accelerate studies, achieving enrollment rates that are 50% quicker than conventional markets.
The typical length of research studies has been reported at around 574 days; however, with bioaccess®, companies can anticipate a streamlined process that enhances their competitive advantage. By integrating these strategic components, bioaccess® not only expedites the clinical evaluation process but also enables its clients to introduce innovative solutions to market more rapidly.
This approach addresses the urgent demands of the healthcare environment while ensuring FDA-ready data that saves $25K per patient without rework or delays.

The viability of clinical studies includes various critical aspects, which are encompassed by study feasibility clinical trials, such as operational, regulatory, and patient recruitment viability.
The study feasibility clinical trials evaluates the capacity to recruit a sufficient number of participants, which is a crucial factor in the success of clinical studies. Research indicates that approximately 80% of studies fail to meet their initial enrollment targets, leading to substantial financial repercussions, estimated at $8 million daily for pharmaceutical development companies. This highlights the urgent need for effective recruitment strategies and underscores the importance of understanding the demographics and health equity of the target population.
Every phase of study feasibility clinical trials is integral to the overall success of a clinical study. By conducting comprehensive assessments, researchers and sponsors can make informed decisions that enhance the likelihood of success, streamline processes, and ultimately facilitate the more efficient introduction of innovative therapies to the market. For instance, a meticulously prepared Clinical Research Assessment (CRFA) not only verifies the viability of a study but also aligns stakeholder expectations early in the planning process, ensuring that all logistical and regulatory factors are thoroughly considered.
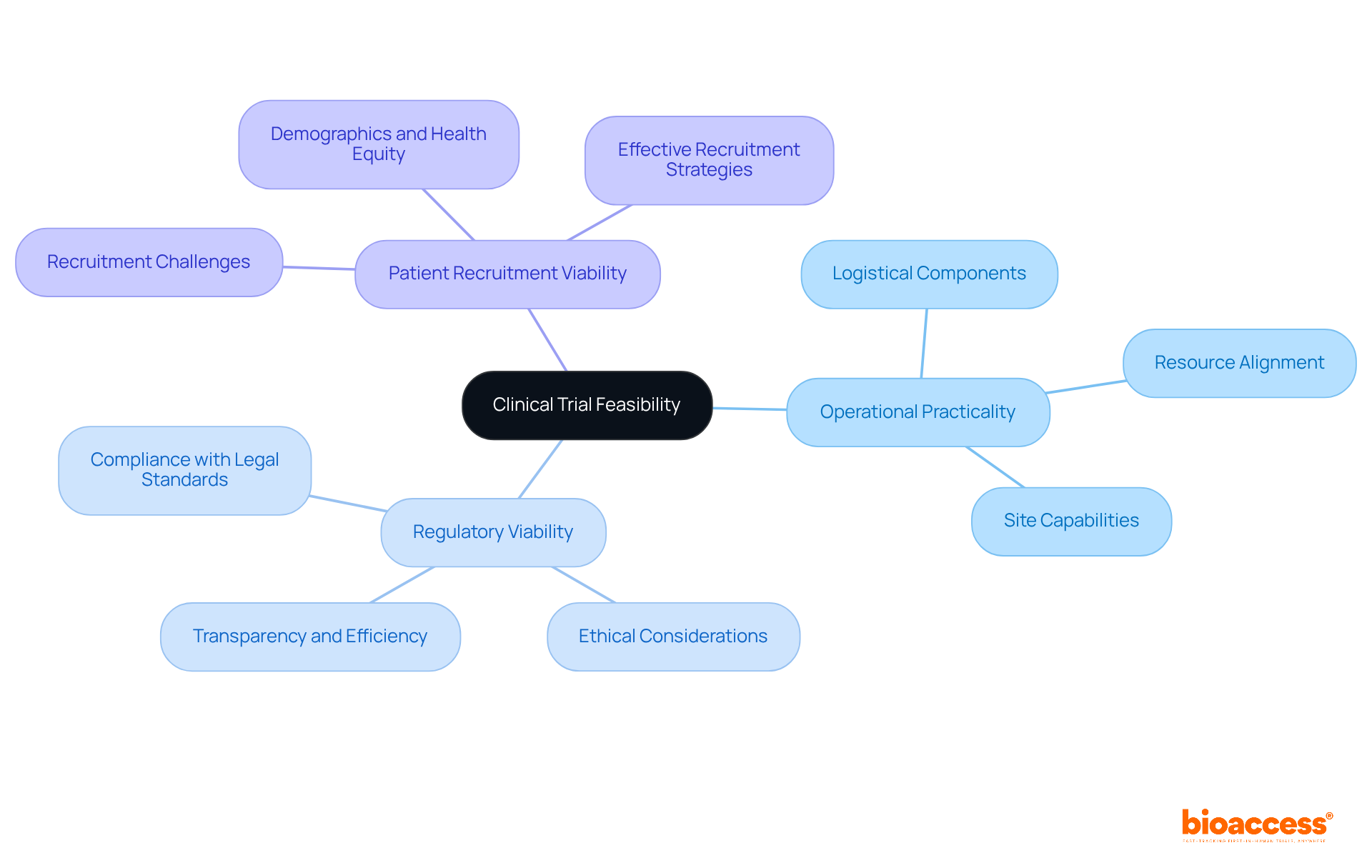
Evaluations of study feasibility clinical trials hinge on several critical components:
Each of these elements demands a thorough evaluation to ensure effective execution of the tests. For instance, selecting locations with robust patient groups can significantly expedite recruitment timelines. A dermatology study exemplifies this, achieving its enrollment targets six weeks ahead of schedule and saving approximately $500,000 in additional expenses.
Furthermore, understanding financial constraints is vital; research expenditures can soar into the millions, with key studies averaging a median cost of $41,117 per patient. A well-structured budget evaluation not only safeguards financial sustainability but also coordinates resources efficiently, ultimately contributing to the project's success.
By prioritizing meticulous site selection and comprehensive budget evaluations, sponsors can mitigate risks and enhance the study feasibility clinical trials to achieve their research objectives. The collaboration between bioaccess™ and Caribbean Health Group to position Barranquilla as a leading site for research studies in Latin America, supported by Colombia's Minister of Health, exemplifies the strategic approach necessary for evaluating study feasibility clinical trials.
As articulated, "By starting with a CRFA, sponsors give their clinical programs the structure, foresight, and clarity they need to succeed.
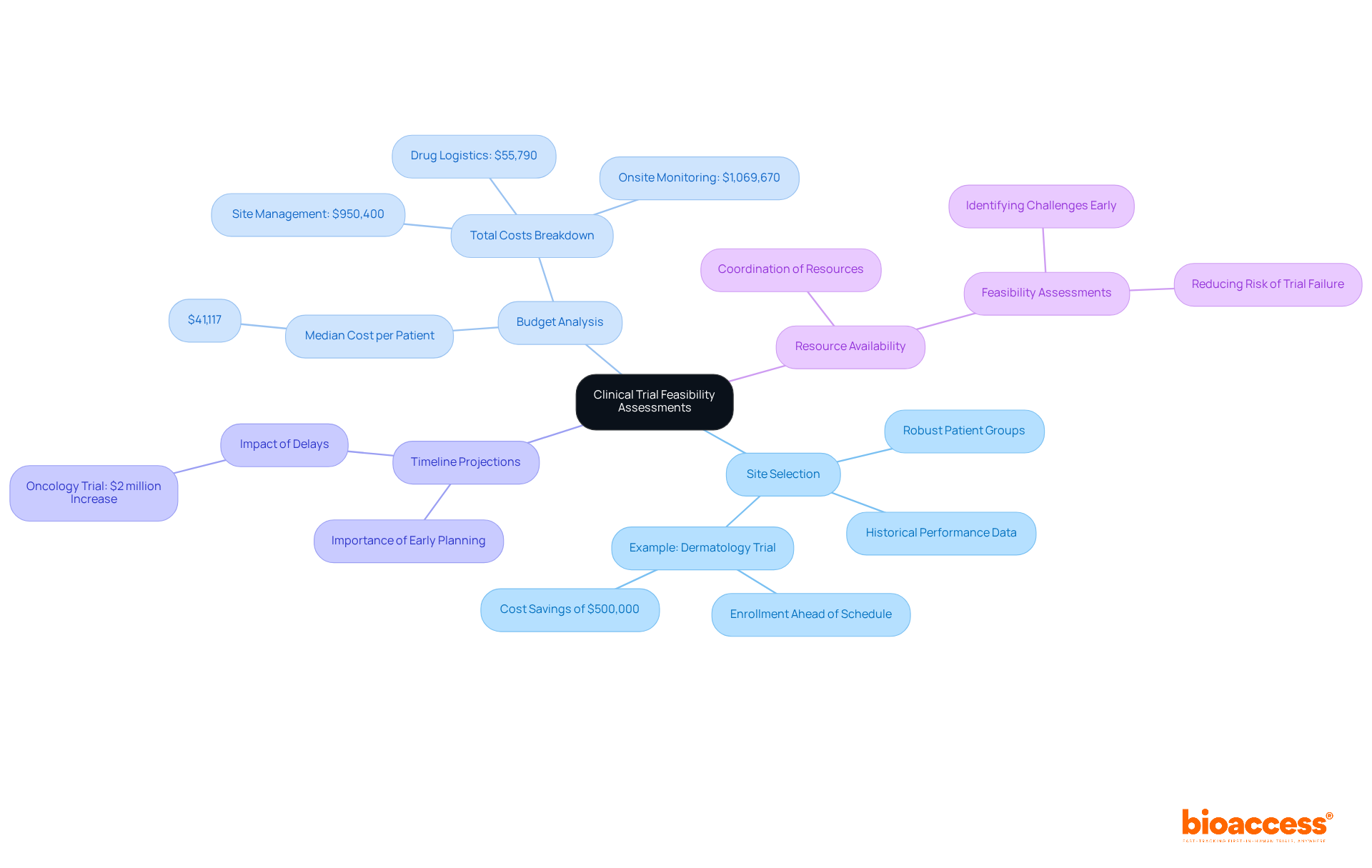
The study feasibility clinical trials frequently encounters significant challenges, including participant enrollment issues, regulatory hurdles, and budget constraints. The complexity of regulatory requirements can lead to extended timelines, with nearly 39% of sponsors attributing rising costs to intricate protocols. To navigate these challenges effectively, proactive planning is essential. Collaborating with local patient advocacy organizations can enhance enrollment efforts, while comprehensive training ensures adherence to regulatory standards. Furthermore, conducting detailed budget analyses helps identify potential financial pitfalls early in the process.
Best practices for overcoming recruitment difficulties involve leveraging diverse strategies, such as digital outreach and community engagement, which have proven effective in reaching broader audiences. Regular communication with stakeholders is crucial for promptly addressing concerns and adapting strategies as needed. For instance, a recent dermatology study that implemented patient-friendly protocols achieved its enrollment goals six weeks ahead of schedule, avoiding an estimated $500,000 in additional costs.
Moreover, employing advanced viability methods and instruments can significantly reduce risks and enhance the cost-effectiveness of experiments. By incorporating real-world data and predictive analytics, sponsors can optimize study designs and boost patient involvement, ultimately leading to more successful outcomes. As the landscape of medical research evolves in 2025, these solutions and best practices will be essential for addressing the persistent challenges in research viability.

Technology plays a pivotal role in enhancing the study feasibility of clinical trials of research studies. Electronic data capture (EDC) systems, patient enrollment platforms, and project management software significantly streamline processes and improve data accuracy. For instance, EDC systems enable real-time data collection and analysis, empowering researchers to make informed decisions swiftly.
Recent studies reveal that:
By leveraging patient enrollment platforms, researchers can effectively identify and engage potential participants for study feasibility clinical trials, thereby reducing timelines and improving overall trial outcomes.
The integration of EDC technologies has been shown to stabilize error rates in data collection to below 7.3% within 1.5 years post-implementation, underscoring their impact on data quality and operational efficiency in research.

Effective patient engagement strategies are pivotal in clinical research, relying on the utilization of social media, partnerships with healthcare providers, and the execution of community outreach programs. Engaging potential participants through social media networks not only increases awareness about clinical studies but also fosters interest among diverse groups.
The collaboration with healthcare providers is essential; their ability to refer eligible patients directly significantly enhances recruitment efficiency. Furthermore, community outreach initiatives play a critical role in engaging underrepresented groups, thereby ensuring inclusivity in participation. Providing clear and accessible information about the study's benefits, while addressing potential concerns, can greatly enhance participant readiness to join.
Notably, bioaccess™ has been instrumental in positioning Barranquilla as a leading hub for medical studies in Latin America, thanks to its partnership with Caribbean Health Group, which has garnered support from Colombia's Minister of Health. Additionally, collaborations such as that between GlobalCare Clinical Trials and bioaccess™ have resulted in a remarkable 50% reduction in recruitment time and an impressive 95% retention rate, underscoring the effectiveness of these strategies.

Regulatory and ethical evaluations of study feasibility clinical trials are paramount for ensuring adherence to both local and international regulations. This critical process encompasses obtaining necessary approvals from ethics committees and regulatory bodies, alongside securing informed consent from participants.
Recent studies reveal that the average duration for ethics review among various Institutional Review Boards (IRBs) can vary significantly, ranging from 1 to 396 days. This underscores the importance of prompt approvals for the successful initiation of study feasibility clinical trials.
Researchers must remain alert to the latest regulations and guidelines to maintain compliance and safeguard participant rights. Furthermore, establishing comprehensive training programs for research personnel is vital for upholding ethical standards throughout the study lifecycle. Such training not only enhances understanding of regulatory requirements but also fosters a culture of respect for participant rights, ultimately supporting the integrity and success of research studies.

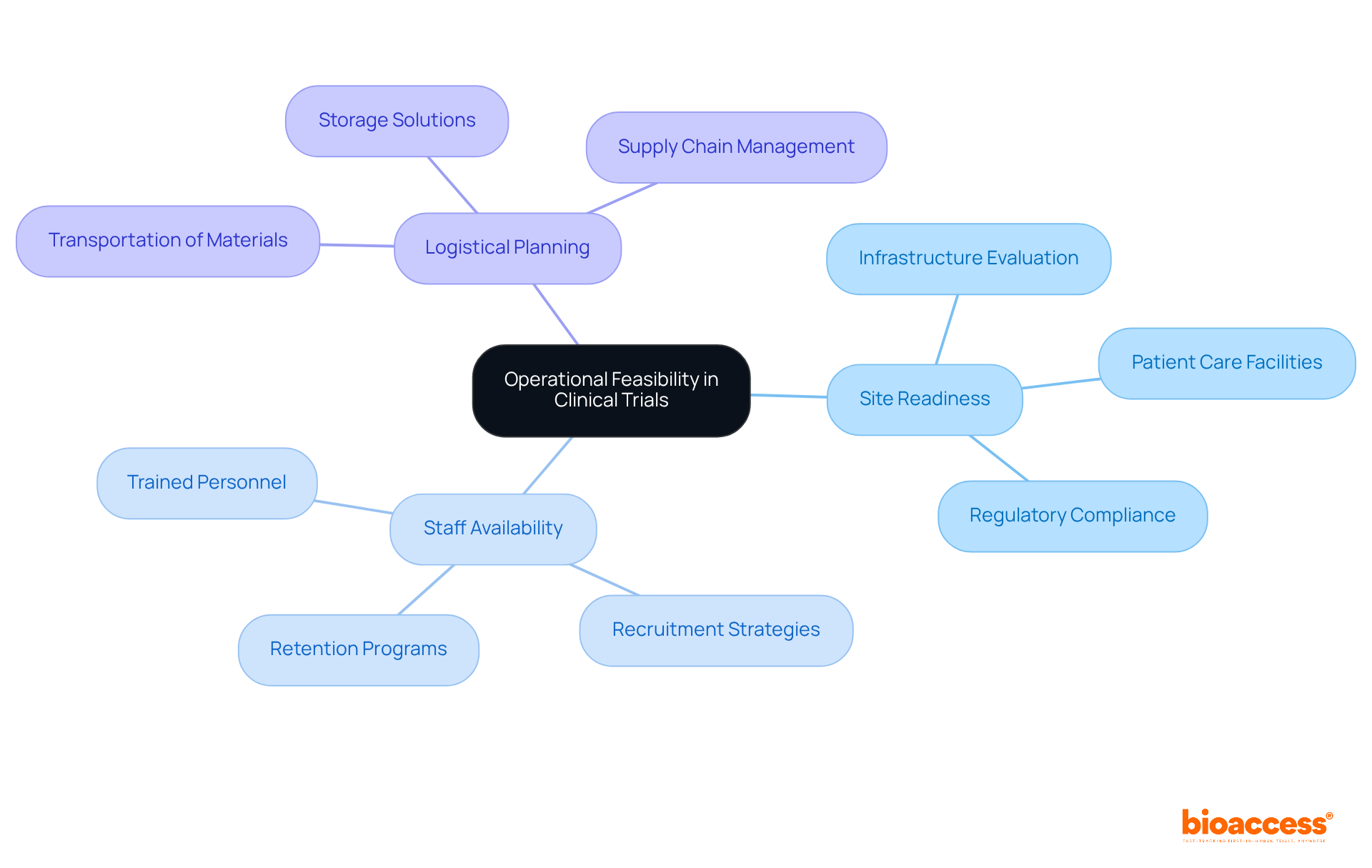
The study feasibility clinical trials is fundamentally anchored in three pivotal factors:
To assess site preparedness, an extensive evaluation of the infrastructure and resources at each location is essential, ensuring they align with the project's specific requirements. A well-prepared site must possess adequate facilities for patient care, data collection, and compliance with regulatory standards.
Moreover, staff availability is critical for sustaining the program's momentum; having trained personnel ready to engage with participants can significantly enhance recruitment and retention rates.
Effective logistical planning is equally vital, facilitating the seamless transportation and storage of study materials, which may include sensitive biological samples or specialized equipment.
By meticulously addressing these operational elements, researchers can substantially mitigate risks and bolster the likelihood of achieving successful outcomes in the study feasibility clinical trials.
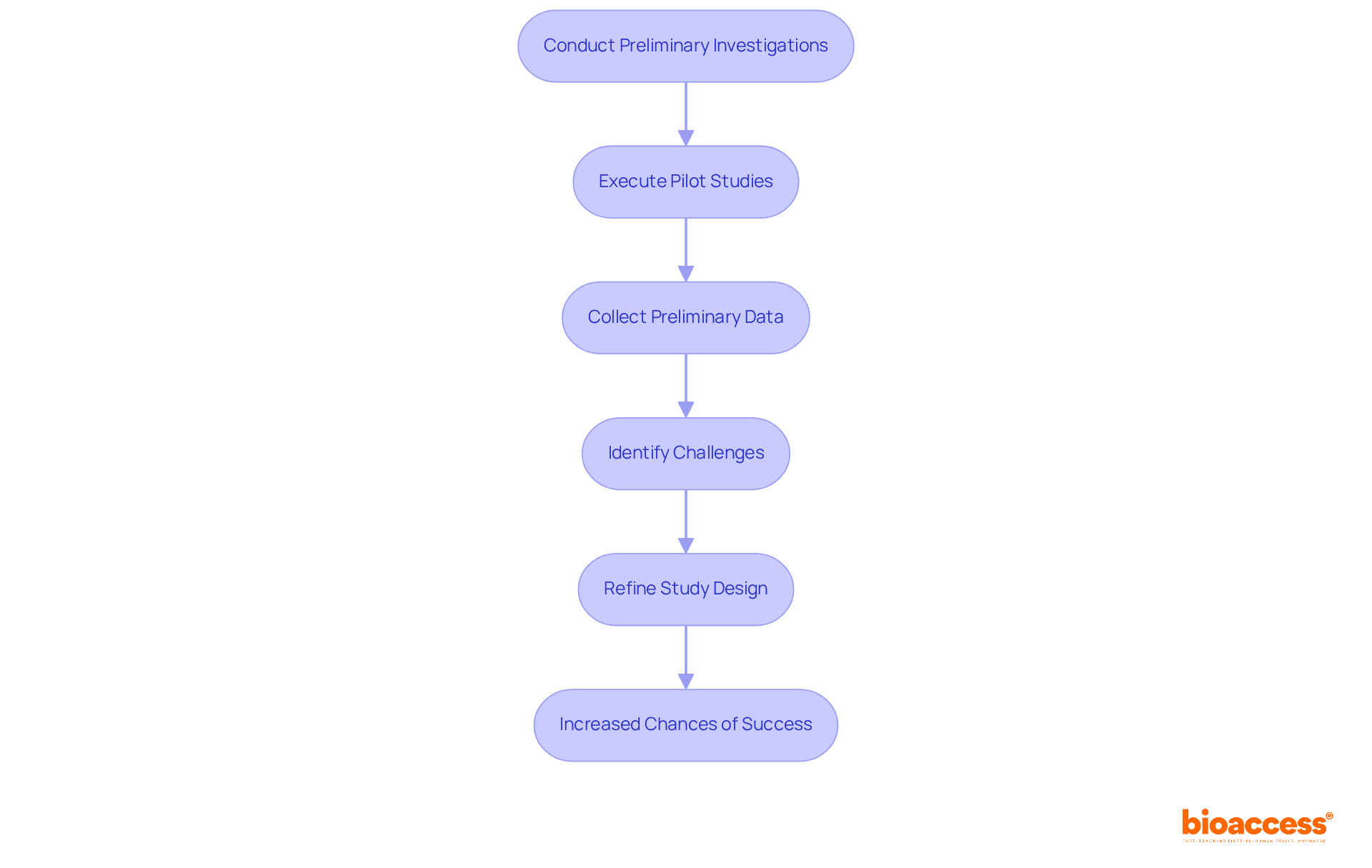
Preliminary investigations and pilot studies are critical components in the study feasibility of clinical trials. These studies enable researchers to rigorously test protocols, identify potential challenges, and refine methodologies prior to launching a full-scale experiment. By executing pilot studies, researchers can collect essential preliminary data on enrollment rates, participant adherence, and logistical hurdles, which are vital for informing modifications to the primary study design. This proactive strategy not only enhances the study feasibility of clinical trials but also significantly increases the likelihood of success.
For instance, pilot studies can reveal unforeseen issues that may arise during participant recruitment or data collection, allowing for timely adjustments that bolster overall study efficiency. A notable case is Avantec Vascular's first-in-human research study in Latin America, supported by bioaccess™, which encompasses crucial assistance such as the selection of a principal investigator and the submission of regulatory dossiers for ministry of health approvals. This highlights the necessity of comprehensive preliminary investigations in securing successful outcomes.
Ultimately, the insights derived from these preliminary investigations are indispensable in crafting robust research designs that enhance the study feasibility of clinical trials and are better positioned to achieve their objectives.
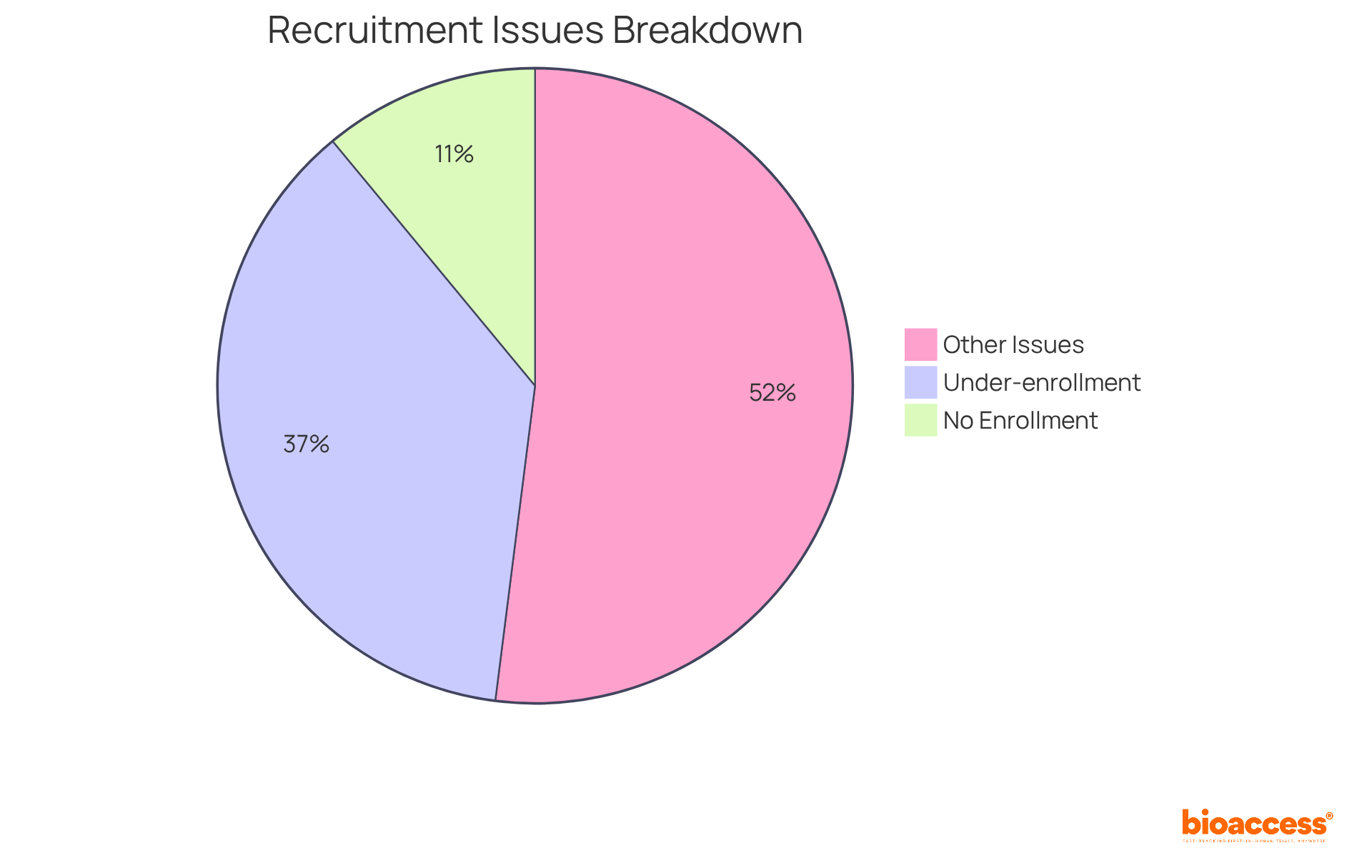
Comprehensive evaluations of study feasibility clinical trials are crucial for the success of research studies. Insufficient assessments can lead to significant hiring failures, budget overruns, and regulatory non-compliance, ultimately jeopardizing the integrity and outcomes of the study. For instance, approximately 80% of research studies face delays or shutdowns due to recruitment issues, with:
Conversely, thorough assessments enhance planning, streamline processes, and boost participant engagement, resulting in more successful study outcomes. A notable case is ReGelTec's Early Feasibility Study on HYDRAFIL™, where eleven patients with degenerative disc disease were effectively treated in Colombia. The procedures were supervised remotely via Zoom, and the patented hydrogel was injected into the nucleus of the degenerated disc using a 17-gauge needle, showcasing the efficacy of comprehensive evaluations in medical settings.
Successful organizations, such as bioaccess, routinely employ extensive viability evaluations to study feasibility clinical trials, validating scientific and operational elements to ensure tests are well-defined and adequately funded. Stakeholders must prioritize these assessments related to study feasibility clinical trials to position their clinical trials for success, as the financial ramifications of recruitment delays can range from $600,000 to $8 million per day. By investing in thorough feasibility evaluations, sponsors can mitigate risks and enhance the likelihood of achieving favorable outcomes.
The insights presented on study feasibility in clinical trials underscore the critical importance of thorough assessments for successful research outcomes. By understanding and addressing operational, regulatory, and recruitment viability, stakeholders can significantly enhance the likelihood of clinical trial success. This comprehensive approach not only streamlines processes but also ensures that innovative therapies reach the market more rapidly and efficiently.
Key arguments highlight the necessity of meticulous site selection, budget analysis, and proactive planning to overcome common challenges such as participant enrollment and regulatory hurdles. Leveraging technology, such as electronic data capture systems and patient enrollment platforms, further optimizes these processes, leading to improved data accuracy and participant engagement. The collaboration between organizations, like bioaccess™ and Caribbean Health Group, exemplifies how strategic partnerships can expedite recruitment and enhance overall trial feasibility.
As the landscape of clinical research continues to evolve, prioritizing feasibility assessments becomes essential. By investing in these evaluations, sponsors can mitigate risks, avoid significant financial repercussions, and ultimately contribute to the advancement of healthcare. Embracing these insights and best practices will not only strengthen the integrity of clinical trials but also ensure that they meet the urgent demands of the healthcare environment.
What is bioaccess® and how does it benefit clinical trials?
bioaccess® is a platform that leverages the regulatory speed of Latin America, diverse patient populations in the Balkans, and Australia’s efficient pathways to secure ethical approvals in just 4 to 6 weeks. This accelerates studies for Medtech, Biopharma, and Radiopharma innovators, achieving enrollment rates that are 50% quicker than conventional markets.
How does bioaccess® impact the length of research studies?
The typical length of research studies is around 574 days; however, with bioaccess®, companies can expect a streamlined process that enhances their competitive advantage, allowing for quicker introductions of innovative solutions to the market.
What are the financial benefits of using bioaccess®?
bioaccess® ensures FDA-ready data and saves approximately $25,000 per patient without rework or delays, addressing urgent healthcare demands while optimizing costs.
What are the critical aspects of clinical trial feasibility?
Clinical trial feasibility includes operational practicality, regulatory viability, and patient recruitment viability, all of which are essential for the success of clinical studies.
Why is operational practicality important in clinical trials?
Operational practicality focuses on the logistical components necessary for executing a trial, ensuring that resources, timelines, and site capabilities align with study requirements.
What is the significance of regulatory viability in clinical trials?
Regulatory viability assesses compliance with legal and ethical standards, which is increasingly important due to heightened scrutiny from regulatory authorities and the demand for transparency and efficiency in medical research.
How does patient recruitment viability affect clinical studies?
Patient recruitment viability evaluates the capacity to recruit sufficient participants, which is crucial as approximately 80% of studies fail to meet initial enrollment targets, leading to significant financial losses.
What components are essential in clinical trial feasibility assessments?
Key components include site selection, budget analysis, timeline projections, and resource availability, all of which require thorough evaluation for effective execution of trials.
Can you provide an example of effective site selection in clinical trials?
A dermatology study achieved its enrollment targets six weeks ahead of schedule by selecting locations with robust patient groups, saving approximately $500,000 in additional expenses.
How does a Clinical Research Assessment (CRFA) contribute to clinical trial success?
A CRFA verifies the viability of a study and aligns stakeholder expectations early in the planning process, ensuring that all logistical and regulatory factors are thoroughly considered, which enhances the likelihood of success.