


The primary focus of the article titled "10 Key Medical Device Labeling Requirements for FDA Compliance" is to delineate the essential labeling requirements that manufacturers must adhere to in order to ensure compliance with FDA regulations. It underscores that compliance with these requirements—such as providing accurate product information and avoiding misleading claims—is paramount for patient safety and regulatory approval. Non-compliance can result in serious ramifications for both manufacturers and users, highlighting the critical nature of these guidelines.
Navigating the complex landscape of medical device labeling requirements is crucial for manufacturers aiming to achieve FDA compliance. With approximately 30% of medical devices failing to meet these standards, understanding the essential guidelines is not just beneficial—it's imperative for patient safety and market success. As the stakes rise, manufacturers must ensure their labels convey accurate information while adhering to the stringent regulations set forth by the FDA. This article delves into the ten key labeling requirements that every Medtech firm must master to thrive in the competitive healthcare landscape.
bioaccess® is dedicated to empowering Medtech firms as they navigate the intricate medical device labeling requirements FDA for healthcare product identification. By emphasizing the acceleration of compliance, bioaccess® leverages its profound understanding of regulatory frameworks, particularly the medical device labeling requirements fda, to ensure that clients efficiently meet all necessary guidelines.
With its global-first clinical flexibility and provision of FDA/EMA/MDR-ready datasets under centralized oversight, bioaccess® enables faster market entry for groundbreaking healthcare products. This includes:
This ensures that documentation requirements are met promptly while delivering tailored solutions and expert support for clinical trials in diverse regions.
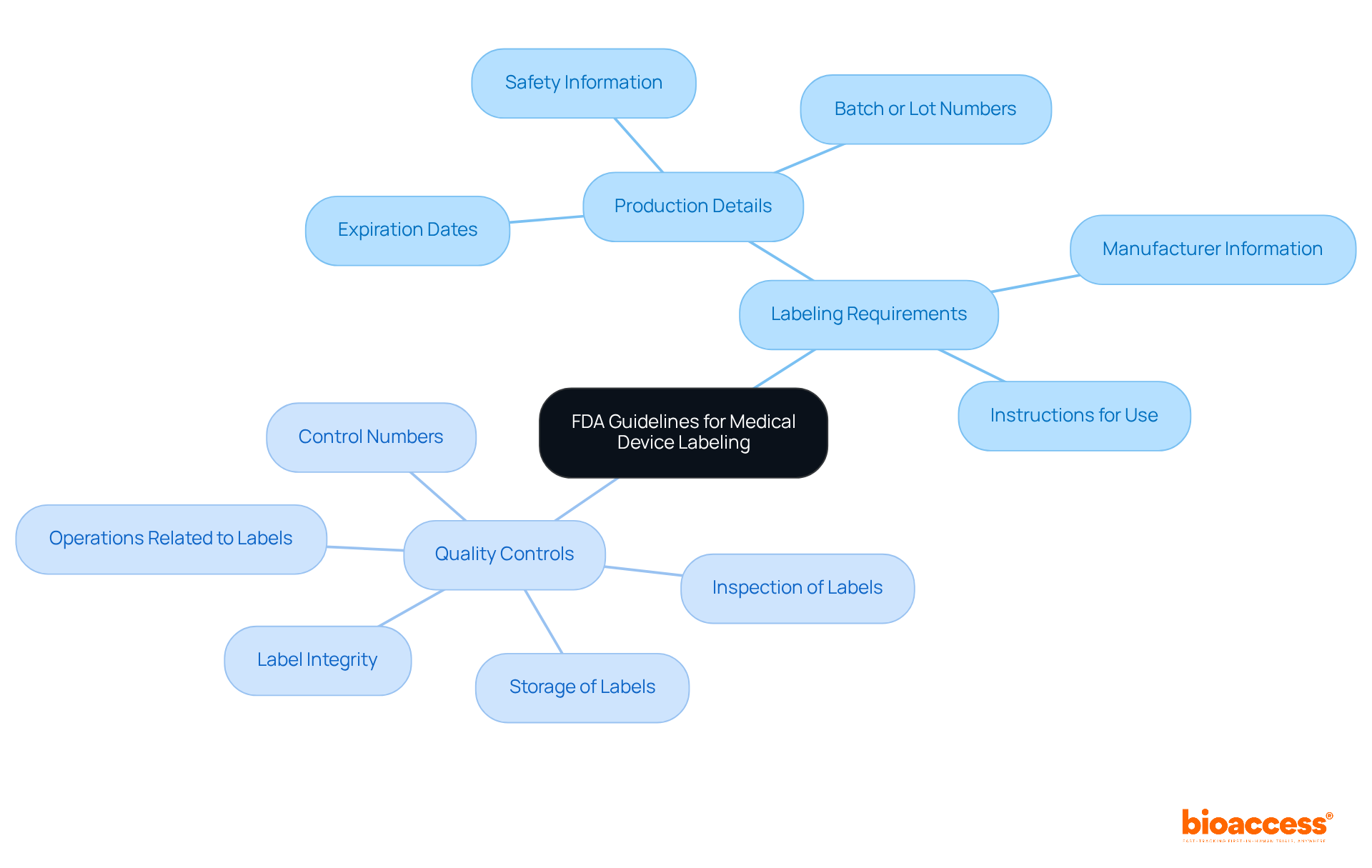
The FDA mandates that the medical device labeling requirements ensure that medical instrument labels are accurate and non-deceptive, a fundamental principle that underpins patient well-being and regulatory compliance. Central to these regulations are general marking provisions, which require that the label includes the name and location of the manufacturer, packer, or distributor, along with sufficient instructions for use. These guidelines are essential, ensuring users possess the necessary information to operate the equipment safely and effectively, which is critical for patient safety and adherence to regulations.
Notably, research indicates that approximately 30% of medical devices fail to meet FDA marking standards, leading to compliance issues and potential delays in market entry. Manufacturers must concentrate on five key quality controls:
to guarantee compliance with these guidelines. Moreover, the medical device labeling requirements set forth by the FDA's specific marking regulations under 21 CFR 801 and 820.120 highlight the necessity of including accurate production details such as expiration dates, safety information, and batch or lot numbers. Accurate labeling not only safeguards patients but also sustains the integrity of the healthcare system, underscoring the imperative for manufacturers to prioritize clear and honest communication in their labeling practices.
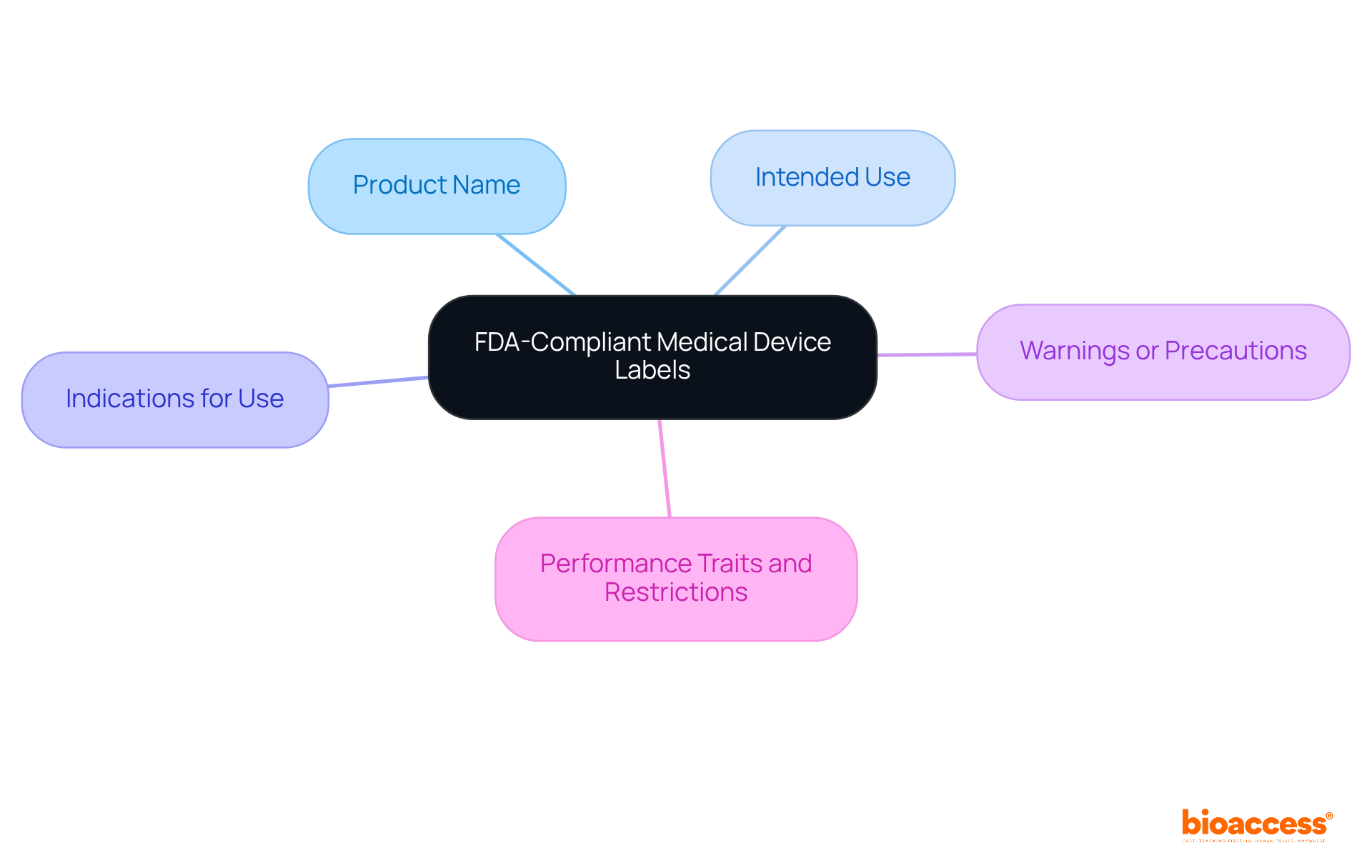
The medical device labeling requirements FDA mandate that compliant medical equipment labels must include several essential elements, such as:
Additionally, labels are required to provide details on the equipment's performance traits and any applicable restrictions. The inclusion of these elements is crucial; it not only helps manufacturers comply with medical device labeling requirements FDA but also enhances user comprehension of the product.
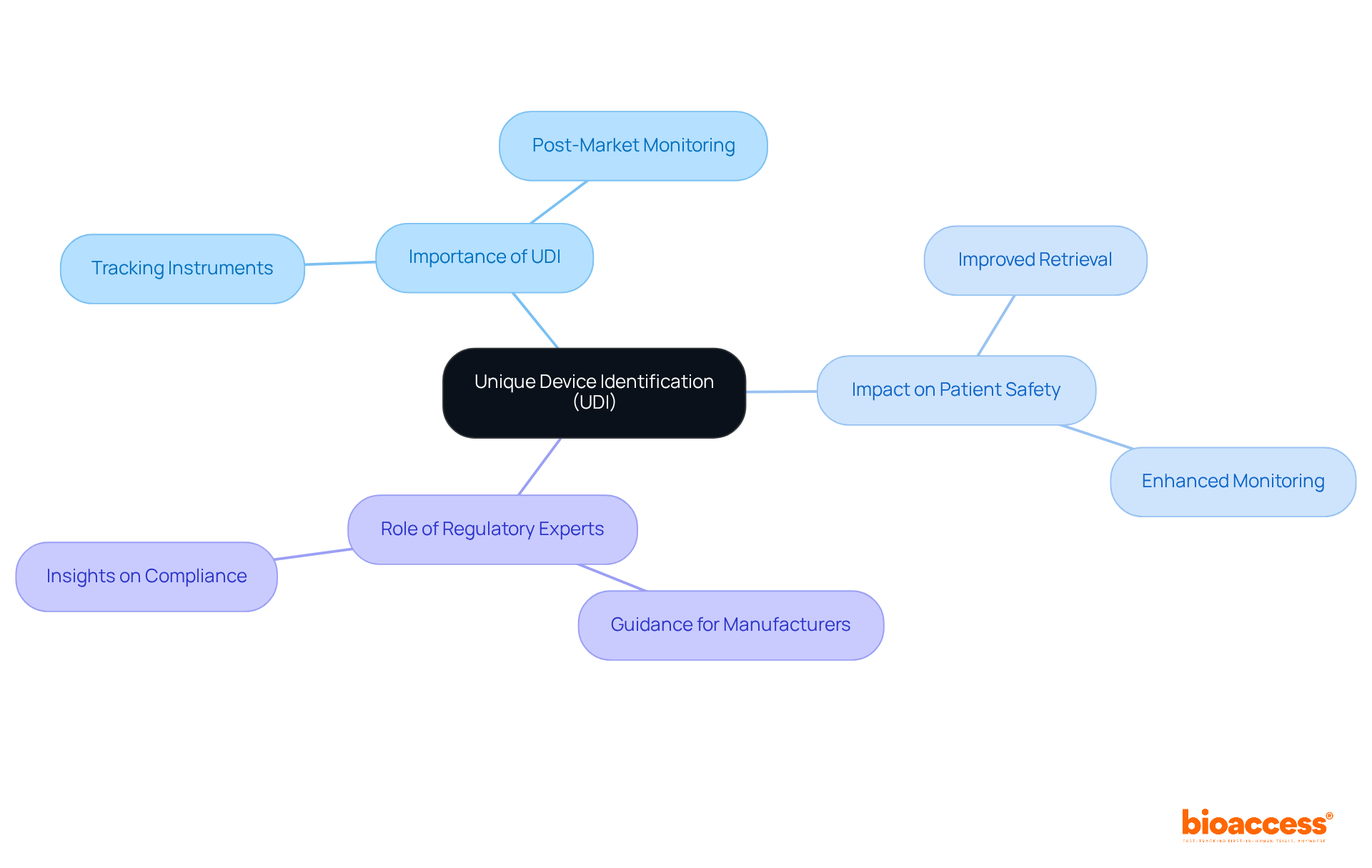
The Unique Device Identification (UDI) system mandates that most healthcare instruments carry a unique identifier, facilitating easier tracking and recognition. This system significantly enhances patient well-being by aiding in the retrieval of instruments and improving post-market monitoring. Compliance with the medical device labeling requirements FDA and UDI requirements is essential for manufacturers to meet FDA standards, ensuring that their products can be effectively monitored throughout their lifecycle.
Experts in regulatory affairs, such as Ana Criado, who possesses extensive experience at Colombia’s regulatory agency INVIMA and serves as a consultant for global companies, play a crucial role in guiding manufacturers through these requirements. Her insights are instrumental in ensuring that medical equipment adheres to UDI standards, ultimately contributing to enhanced patient protection and efficient market supervision.
Medical equipment labels must provide clear and concise instructions for use, which are crucial for ensuring security and compliance with the medical device labeling requirements FDA. These instructions should encompass all necessary steps for safe operation, including preparation, usage, and maintenance. Comprehensive and understandable directions not only facilitate adherence to medical device labeling requirements FDA but also bolster user confidence and safety.
A notable example is the collaboration between Lionbridge and Boston Scientific, which demonstrated that centralizing language translations for Summaries of Safety and Clinical Performance (SSCPs) and Instructions for Use (IFUs) across 17 translation projects in 24 languages resulted in significant efficiencies and enhanced language outcomes throughout the product life cycle. This situation underscores the significance of clarity in healthcare product markings, especially for high-risk items, where precise instructions can mitigate hazards and improve user experience.
Regulatory specialists emphasize that well-organized guidelines are essential for complying with the medical device labeling requirements FDA, thereby enhancing clarity and ensuring that users can operate equipment safely and effectively.
As Pia Windelov, VP of Life Sciences Strategy and Product Marketing, stated, 'We’re eager to understand your needs and share how our innovative capabilities can empower you to break barriers and expand your global reach.' This statement highlights the importance of comprehending customer requirements in the realm of medical equipment identification.
Furthermore, strategic thinking regarding the SSCP and its interdependent documents is vital for ensuring comprehensive documentation that supports effective labeling.
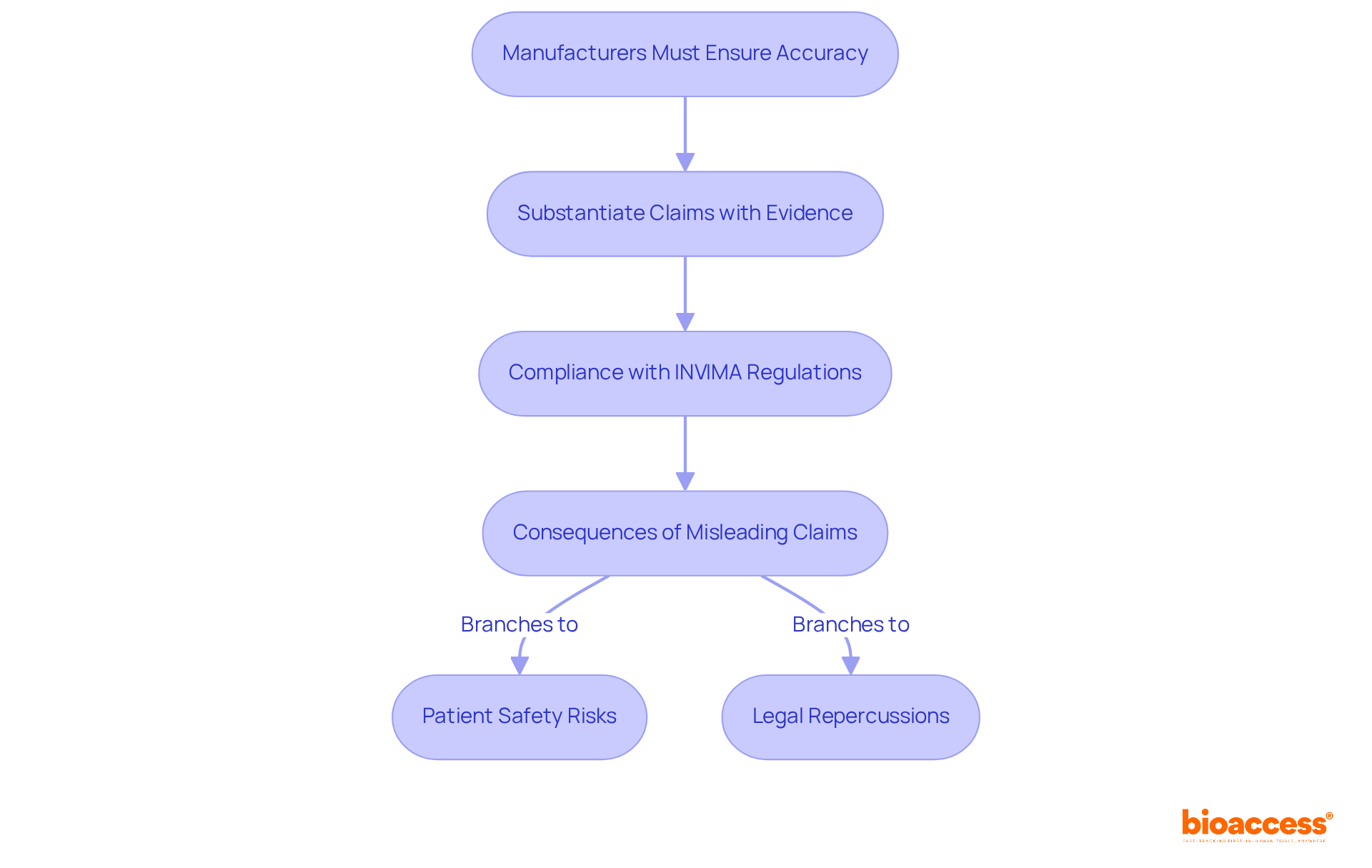
Labels must avoid any inaccurate or deceptive claims regarding the product's capabilities or performance. This requirement is not only essential for regulatory compliance but also vital for upholding the integrity of the healthcare product industry. In Colombia, the INVIMA (Colombia National Food and Drug Surveillance Institute) plays a crucial role in this oversight, ensuring that all health products, including therapeutic instruments, adhere to rigorous quality and effectiveness standards. Manufacturers must substantiate all claims made on labels with evidence, as misleading information can result in serious consequences for patient safety and legal repercussions. The classification of INVIMA as a Level 4 health authority by the Pan American Health Organization/World Health Organization underscores its capacity to enforce these regulations, further emphasizing the significance of precise labeling in the healthcare sector.
The FDA mandates that medical device labeling requirements ensure critical statements on labels are presented in a manner that maximizes visibility and comprehensibility. This involves selecting appropriate font sizes, colors, and placements to ensure that essential information is easily discernible. For instance, employing bold fonts for warnings and contrasting colors for important instructions can significantly enhance user awareness. By emphasizing the importance of these statements, manufacturers enable faster recognition of usage guidelines, ultimately improving compliance and enhancing user protection.
Integrating user engagement tactics, such as straightforward subscription methods for updates on compliance requirements, can further improve awareness. The case study on slips, trips, and falls prevention in the Irish healthcare sector illustrates how effective communication of safety information can lead to improved outcomes. Furthermore, staying updated on current trends and news regarding medical device labeling requirements assists manufacturers in effectively adjusting their packaging strategies.
To ensure compliance, manufacturers should also consider actionable tips, such as conducting user testing to gather feedback on the clarity and visibility of product information. This approach can lead to continuous improvement in practices regarding labels.
Certain medical instruments may be exempt from specific marking requirements under FDA guidelines. For instance, items classified as low-risk often benefit from simplified labeling requirements. Understanding these exemptions is crucial for manufacturers, as it helps them navigate the medical device labeling requirements FDA while avoiding being overwhelmed by unnecessary regulations and still adhering to essential quality standards. With experts like Ana Criado, Director of Regulatory Affairs at bioaccess, who possesses extensive experience in regulatory affairs and biomedical engineering, manufacturers can effectively navigate these complexities.

Standardized symbols on healthcare equipment labels serve as a crucial tool for conveying essential information quickly and efficiently. The FDA advocates for their use, particularly in multilingual environments, to enhance user understanding. However, it is imperative that these symbols are accompanied by clear explanations to ensure accurate interpretation by all users.
Efficient application of symbols can greatly enhance the usability of healthcare product labels, ultimately aiding in better patient safety and care. For instance, the integration of symbols has been shown to reduce the risk of misuse by health professionals and caregivers, thereby preventing serious health consequences.
As the worldwide healthcare apparatus sector, valued at around 577 billion and consisting of about 2 million various types of healthcare instruments, continues to grow, the significance of clear and standardized marking practices becomes increasingly vital. By adopting these symbols, manufacturers can not only comply with the medical device labeling requirements FDA sets but also promote better understanding among users, ensuring that healthcare instruments are utilized safely and effectively.
As noted by Sarah Moore, "the primary benefit of medical device markings is that it enhances the security of the product." Furthermore, challenges such as the requirement for labels in national languages may lead to inaccuracies in translations, posing safety risks. Therefore, tackling these complexities is crucial for effective categorization.
Under the FDA's Quality System Regulation (QSR), it is imperative for manufacturers to implement stringent medical device labeling requirements FDA. These controls ensure that all labeling remains accurate and compliant with the medical device labeling requirements FDA throughout the product lifecycle. Regular reviews and updates to labels are not merely best practices; they are essential, particularly when new information emerges or modifications are made to the device. Ongoing compliance is crucial—not only for maintaining regulatory approval but also for safeguarding patient safety.
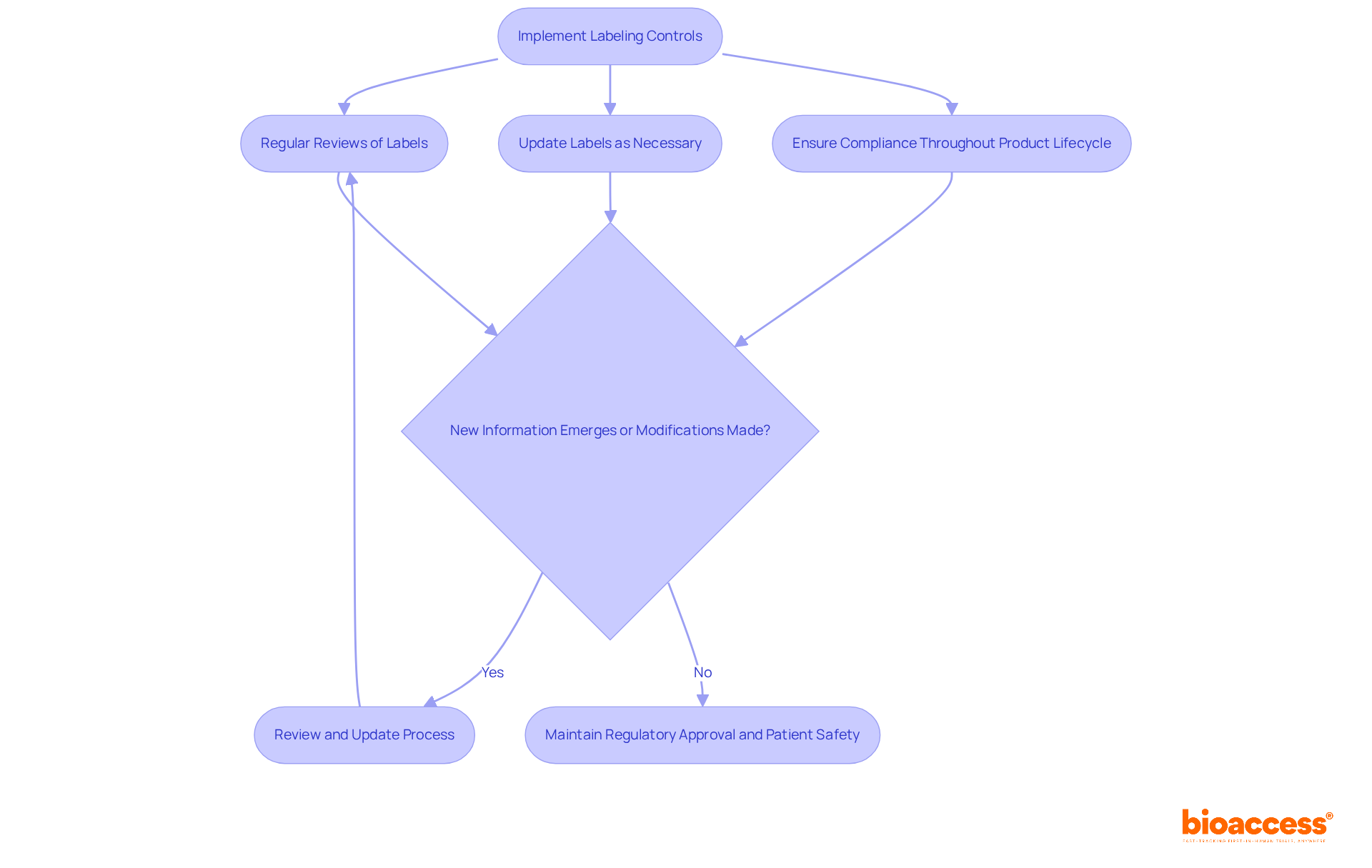
Navigating the intricate landscape of FDA medical device labeling requirements is essential for manufacturers striving to ensure compliance and promote patient safety. This article has delineated the critical elements and guidelines that must be adhered to, underscoring the necessity of precise and clear labeling practices. By grasping these requirements, manufacturers can effectively convey vital information regarding their products, thereby enhancing user comprehension and safeguarding health outcomes.
Key insights from the article emphasize the imperative of including fundamental elements such as:
Furthermore, the discussion highlights the significance of providing adequate directions for use and avoiding false or misleading statements, all of which contribute to a transparent and trustworthy healthcare environment. Manufacturers are urged to implement continuous labeling controls to maintain compliance throughout the product lifecycle, ensuring that updates and changes are promptly reflected.
The importance of these labeling requirements cannot be overstated, as they not only fulfill regulatory obligations but also play a crucial role in protecting patients and healthcare providers alike. As the medical device landscape continues to evolve, remaining informed about FDA guidelines and best practices is vital. Manufacturers should prioritize compliance, leverage expert insights, and adopt innovative labeling strategies to enhance clarity and effectiveness, ultimately fostering a safer healthcare ecosystem for all.
What is bioaccess® and how does it assist Medtech firms?
bioaccess® helps Medtech firms navigate FDA medical device labeling requirements by accelerating compliance and providing a deep understanding of regulatory frameworks, ensuring that clients meet necessary guidelines efficiently.
What are the benefits of using bioaccess® for medical device labeling?
bioaccess® offers global-first clinical flexibility, FDA/EMA/MDR-ready datasets, and enables faster market entry for healthcare products, including site activation in under 8 weeks and simultaneous submissions across multiple regions.
What are the key FDA guidelines for medical device labeling?
The FDA mandates that medical device labels must be accurate and non-deceptive, including the manufacturer's name and location, as well as sufficient instructions for use, which are essential for patient safety and regulatory compliance.
What are the common compliance issues with medical device labeling?
Approximately 30% of medical devices fail to meet FDA marking standards, which can lead to compliance issues and delays in market entry.
What quality controls should manufacturers focus on to ensure compliance?
Manufacturers should concentrate on five key quality controls: label integrity, inspection of labels, storage of labels, operations related to labels, and control numbers.
What specific information must be included on FDA-compliant medical device labels?
FDA-compliant medical device labels must include the product name, intended use, indications for use, warnings or precautions, performance traits, and any applicable restrictions.
Why is accurate labeling important for medical devices?
Accurate labeling is crucial for safeguarding patients, sustaining the integrity of the healthcare system, and ensuring clear communication about the product, which enhances user comprehension and compliance with regulations.