


The article presents an overview of ten pivotal randomized control trials (RCTs) that have profoundly influenced healthcare practices. It highlights their critical role in establishing causal relationships and validating therapeutic efficacy. Notable trials, such as the Diabetes Control and Complications Trial and the Women's Health Initiative, exemplify how rigorous scientific evaluation can lead to enhanced treatment protocols and improved patient outcomes. This underscores the significance of RCTs as the gold standard in clinical research, demonstrating their indispensable contribution to advancing medical knowledge and practice.
Groundbreaking research in healthcare is often driven by randomized control trials (RCTs), which serve as the gold standard for establishing the efficacy of medical interventions. This article explores ten pivotal RCT examples that have not only transformed clinical practices but also reshaped patient care across various medical fields. While these studies showcase remarkable successes, they also raise critical questions regarding the complexities of implementation and the ethical considerations involved. What lessons can be gleaned from these trials, and how can their insights propel further advancements in healthcare?
bioaccess® is at the forefront of Medtech advancement, conducting groundbreaking studies that serve as randomized control trial examples and significantly accelerate the progress of medical technologies. By adeptly navigating complex regulatory frameworks and refining clinical research procedures, bioaccess® ensures that studies are executed with both effectiveness and ethical integrity. This dedication not only hastens the introduction of innovative solutions to the market but also improves patient outcomes through thorough scientific evaluation.
Industry leaders assert that randomized control trial examples are crucial for establishing causal relationships and validating therapeutic efficacy, thereby solidifying their status as the gold standard in clinical research. With over 20 years of experience, bioaccess® has successfully executed numerous RCTs, demonstrating that approximately 75% of Phase I trials achieve success. The company's pioneering approaches and expedited ethical approval processes yield results in just 4-6 weeks, in stark contrast to Colombia's typical regulatory approval timeframe of 90-120 days, positioning bioaccess® as a leader in transforming clinical research and ensuring that new medical technologies reach patients quickly and safely.
Furthermore, bioaccess® offers comprehensive clinical research management services, including:
This establishes itself as a trusted CRO and consulting partner for U.S. medical device companies in Colombia. As Dr. Scott Podolsky remarks, "Randomized control trial examples demonstrate that RCTs are the best way to evaluate therapeutic efficacy in an empirical sense," highlighting the vital role these trials play in advancing medical technology and enhancing healthcare delivery.

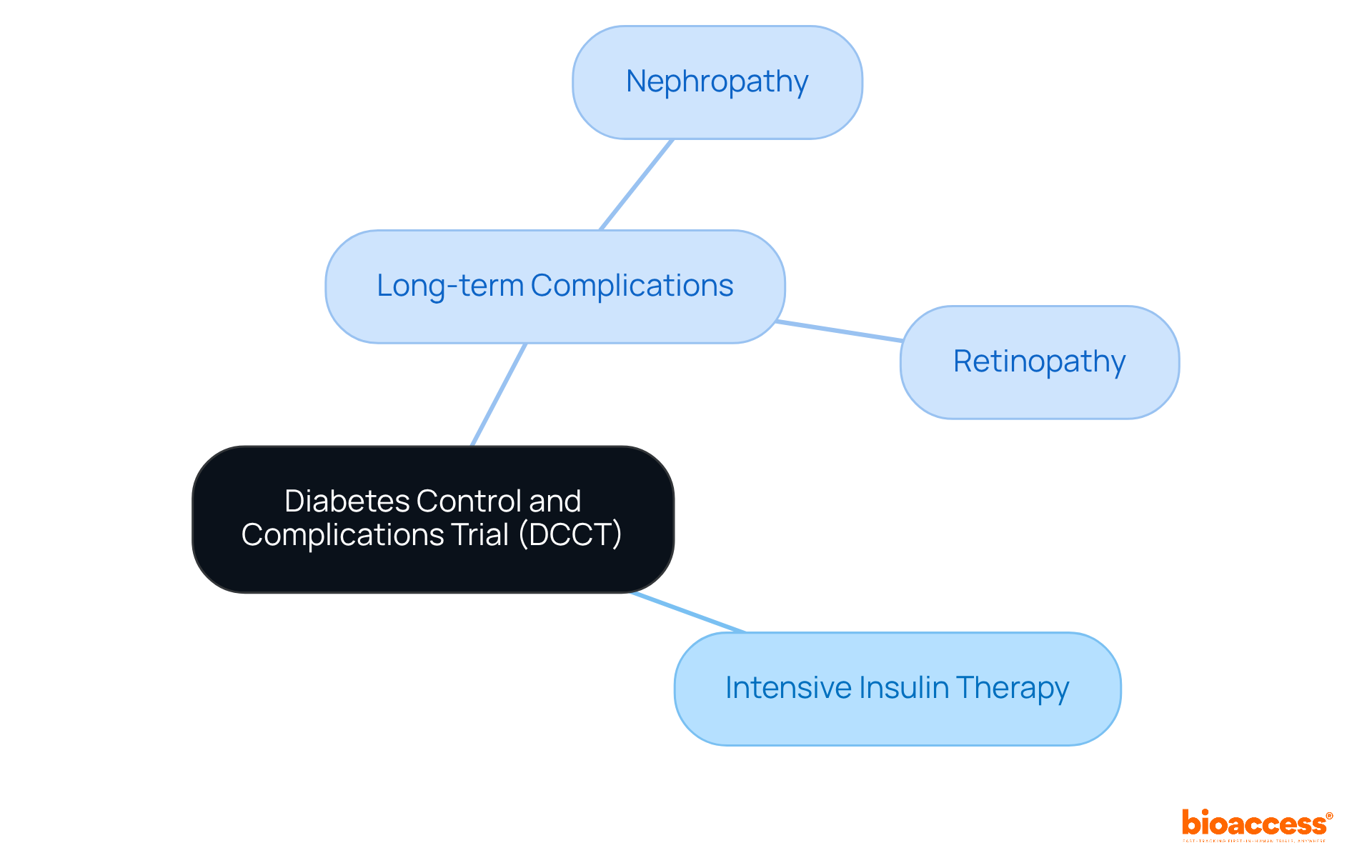
The Diabetes Control and Complications Trial (DCCT) represents a pivotal research initiative that underscored the necessity of tight glucose control in Type 1 diabetes. Over several years, this comprehensive study demonstrated that intensive insulin therapy markedly reduced the risk of long-term complications, such as:
The implications of the DCCT's findings have revolutionized diabetes management, leading to updated clinical guidelines that stress the critical importance of maintaining optimal blood glucose levels.
The Women's Health Initiative (WHI) represents a pivotal investigation into the effects of hormone replacement therapy (HRT) in postmenopausal women. Enrolling over 161,000 women, this study revealed that HRT could significantly elevate the risks of heart disease, stroke, and breast cancer, prompting a critical reassessment of its application. As a result, healthcare providers have adopted more cautious strategies regarding HRT, emphasizing patient safety and the necessity for tailored treatment plans.
The observational research followed participants for an average of eight years, underscoring the long-term implications of HRT as highlighted by the WHI findings. As of 2025, the influence of WHI continues to shape clinical practices, guiding healthcare professionals in making informed decisions about HRT and its long-term implications.
Recently, the FDA convened an Expert Panel on Menopause and Hormone Replacement Therapy, furthering the discussion on the associated risks and benefits of HRT. This evolution in practice reflects a broader trend in the medical community, where the focus is increasingly on individualized approaches to hormone therapy, ensuring that the benefits outweigh the risks for each patient.

The CALGB 9344 study was pivotal in assessing the effectiveness of adjuvant chemotherapy for women with node-positive breast cancer. Published in the Journal of Clinical Oncology, Volume 16_suppl, in June 2005, this landmark study revealed that incorporating a specific chemotherapy regimen led to a notable improvement in survival rates. This was accompanied by a significant increase in the utilization of taxane-based therapies.
The results from CALGB 9344 not only transformed therapeutic approaches for breast cancer patients but also established a standard for future oncology research. It emphasized the crucial role of randomized control trial examples in developing evidence-based care protocols. By showcasing the tangible advantages of taxane-based therapies, the study significantly influenced clinical practices, leading to a wider acceptance of these effective treatments in the management of breast cancer.
For additional reference, the PubMed ID for this article is 27946147. It is also supported by the case analysis titled 'Impact of CALGB 9344 on Adjuvant Taxane Use for Breast Cancer,' which explores the impact on clinical practices concerning taxane therapies.

The ACTG 076 trial represents a pivotal moment in the battle against mother-to-child transmission (MTCT) of HIV, demonstrating the efficacy of zidovudine in significantly reducing transmission rates. This landmark study established that with appropriate antiretroviral therapy, the risk of MTCT could be dramatically lowered, leading to its adoption as a standard practice for pregnant women living with HIV. Consequently, the MTCT rate plummeted from 32.3% in 1990 to merely 2.9% by 2000, underscoring the profound impact of effective care protocols. Notably, 97.2% of mothers received intravenous zidovudine during labor, showcasing the implementation and effectiveness of this procedure.
Current practices now emphasize the importance of early initiation of highly active antiretroviral therapy (HAART) during pregnancy, with studies indicating that longer durations of HAART usage, coupled with cesarean deliveries—92% of which were performed in the study—further mitigate transmission risks. Public health specialists underscore the importance of randomized control trial examples, such as ACTG 076, which not only provide essential data for enhancing care strategies but also highlight the necessity for comprehensive prenatal support.
Adriane M Delicio noted, 'HAART leads to undetectable viral load more rapidly in the course of treatment,' reinforcing the critical nature of adherence to these protocols. Real-world examples illustrate that following these protocols has resulted in significant reductions in MTCT rates, with analysis indicating that MTCT occurred in 3.74% of the cases examined, thereby emphasizing the ongoing need for research and the application of evidence-based practices in maternal health.

The STAR (Study of Tamoxifen and Raloxifene) research evaluated the effectiveness of tamoxifen and raloxifene in reducing breast cancer risk among women at high risk. This pivotal study demonstrated that both medications significantly decreased the incidence of breast cancer, with raloxifene presenting a notably favorable safety profile. As a result, the STAR trial has profoundly influenced clinical guidelines for breast cancer prevention, empowering women with critical options to mitigate their risk.

The National Asthma Education and Prevention Program (NAEPP) serves a pivotal role in establishing asthma treatment guidelines, grounded in extensive research, including randomized control trial examples. These studies have provided compelling evidence regarding the efficacy of various therapeutic approaches, leading to improved patient outcomes and enhanced quality of life for individuals living with asthma. The NAEPP's guidelines underscore the necessity of personalized treatment plans and continuous monitoring, thereby illustrating the transformative impact of RCTs in managing chronic diseases effectively.

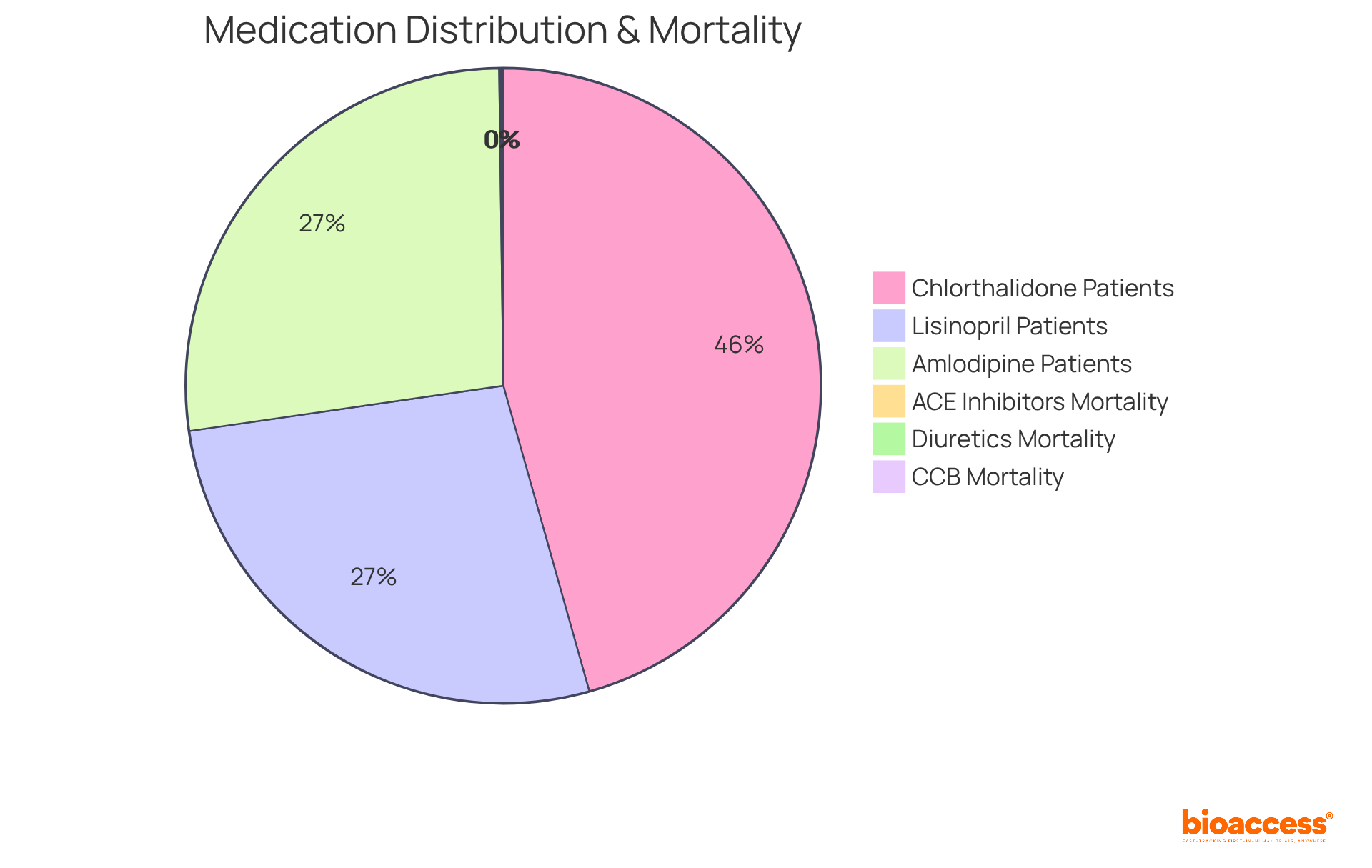
The ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) stands as a pivotal study that evaluated the effectiveness of various antihypertensive medications. It clearly demonstrated that thiazide diuretics, particularly chlorthalidone, were as effective as newer, more expensive drugs such as amlodipine and lisinopril in preventing cardiovascular events. This conclusion is underscored by the study's findings, which indicated that chlorthalidone had the lowest lifetime costs, approximately $53,500, consistently lower than the greater expenses associated with amlodipine and lisinopril.
The implications of the ALLHAT trial have significantly influenced hypertension management guidelines, advocating for cost-effective strategies that prioritize patient outcomes. Cardiologists emphasize that controlling blood pressure is paramount, regardless of the specific medication used. As Dr. Barry Davis noted, "The most important thing is to control the blood pressure," reinforcing the notion that effective management of hypertension can lead to better health outcomes without incurring unnecessary costs. Furthermore, Dr. Nwachuku emphasized that if chlorthalidone were utilized as the primary approach instead of lisinopril or amlodipine, expenses could be lowered by over $6.4 billion during the first six years.
Moreover, the study's results have prompted a shift towards utilizing thiazide diuretics as a primary approach, particularly given their demonstrated efficacy and cost-effectiveness. The analysis revealed that thiazide diuretics were consistently the most affordable option across various scenarios, making them an attractive selection for clinicians aiming to enhance management strategies in hypertension. The study involved:
This provided a substantial dataset that supports the findings. Additionally, the cardiovascular mortality rates per 100 participants were:
This showcases the relative efficacy of these interventions. The long-term follow-up findings further emphasize that managing blood pressure is more crucial than the specific medication utilized, aligning with the overarching theme of the ALLHAT study's implications for hypertension treatment.
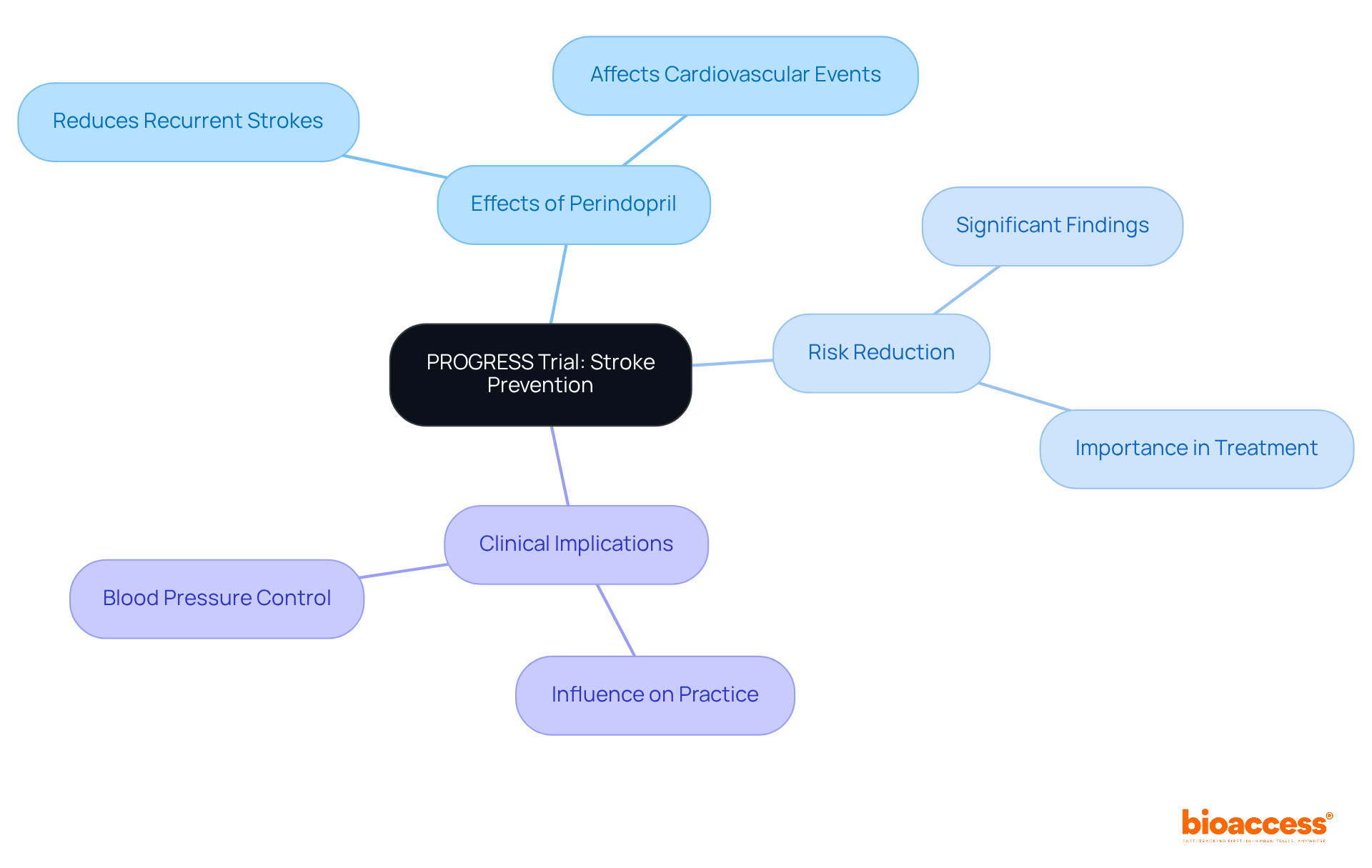
The PROGRESS (Perindopril Protection Against Recurrent Stroke Study) investigated the effects of perindopril on stroke prevention among patients with a history of stroke or transient ischemic attack. This pivotal study demonstrated that perindopril significantly reduced the risk of recurrent strokes and other cardiovascular events. Consequently, the PROGRESS study has profoundly influenced clinical practice, underscoring the critical importance of blood pressure control in preventing strokes.
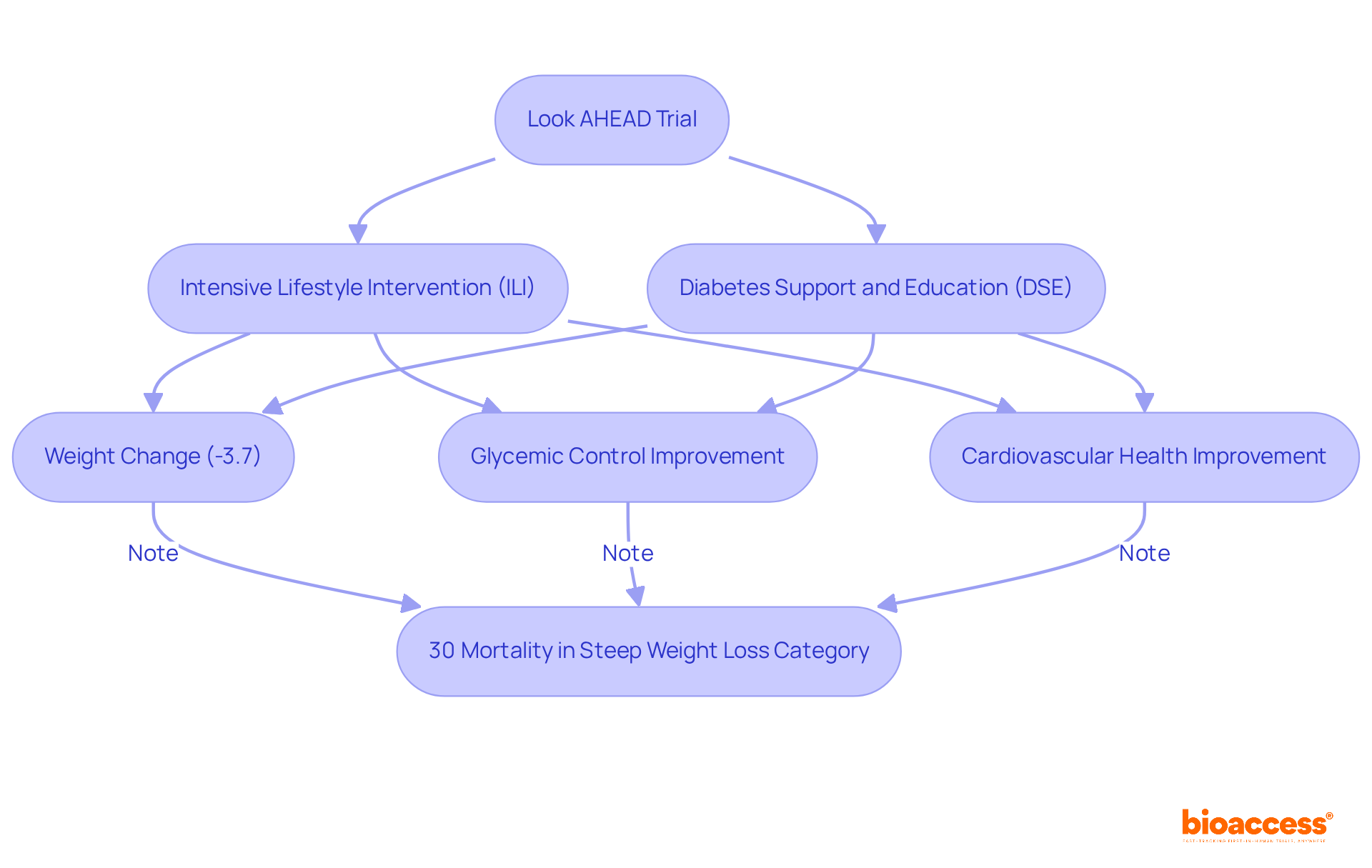
The Look AHEAD (Action for Health in Diabetes) study rigorously examined the impact of a lifestyle intervention on weight reduction and diabetes management among overweight individuals diagnosed with Type 2 diabetes. The results revealed that participants adhering to structured weight loss programs achieved substantial reductions in weight, with a postintervention average weight change of -3.7%. Furthermore, these individuals experienced significant improvements in glycemic control and cardiovascular health.
Notably, the study found that 30% of participants in the steep weight loss category died during the postintervention follow-up, underscoring the serious risks associated with excessive weight loss, particularly among older adults. Researchers, including Holly Wyatt, have stressed the importance of closely monitoring older individuals with prolonged diabetes duration and multimorbidity for excessive unintentional weight loss.
This landmark trial has fundamentally transformed obesity treatment strategies, providing randomized control trial examples that highlight the critical role of lifestyle modifications in preventing chronic diseases. The findings from Look AHEAD have prompted a reevaluation of current obesity management approaches, emphasizing the necessity for tailored interventions that consider individual health profiles and the potential risks linked to significant weight changes.
Additionally, the comparison between the intensive lifestyle intervention (ILI) and diabetes support and education (DSE) groups serves as randomized control trial examples that further illustrate the effectiveness of structured weight loss programs in enhancing health outcomes.
The exploration of randomized control trials (RCTs) in healthcare reveals their transformative power in enhancing medical practices and patient outcomes. By systematically evaluating interventions, RCTs serve as a cornerstone for evidence-based medicine, driving innovations and shaping clinical guidelines across various fields. The examples highlighted in this article, from bioaccess®'s pioneering trials to landmark studies like the Diabetes Control and Complications Trial, underscore the critical role these trials play in validating therapeutic efficacy and improving healthcare delivery.
Key insights from the discussed trials illustrate the profound impact of rigorous research on treatment strategies:
Each example reinforces the necessity of RCTs in guiding clinical practice and ensuring patient safety.
As healthcare continues to evolve, the importance of randomized control trials cannot be overstated. They not only provide vital data that informs clinical decisions but also pave the way for innovative treatments that can change lives. Embracing the lessons learned from these trials encourages a commitment to evidence-based practices and highlights the ongoing need for research that prioritizes patient care. The future of healthcare depends on the continued integration of RCTs, ensuring that advancements are grounded in solid scientific evidence for the benefit of all patients.
What is bioaccess® and what role does it play in Medtech innovation?
bioaccess® is a leader in Medtech advancement, conducting randomized controlled trials (RCTs) that accelerate the progress of medical technologies while ensuring ethical integrity and effective execution of studies.
Why are randomized control trials important in clinical research?
Randomized control trials are crucial for establishing causal relationships and validating therapeutic efficacy, making them the gold standard in clinical research.
What is the success rate of Phase I trials conducted by bioaccess®?
Approximately 75% of Phase I trials conducted by bioaccess® achieve success.
How does bioaccess® expedite the regulatory approval process for clinical studies?
bioaccess® has expedited ethical approval processes that yield results in just 4-6 weeks, compared to the typical regulatory approval timeframe of 90-120 days in Colombia.
What clinical research management services does bioaccess® offer?
bioaccess® offers services including feasibility assessments, site selection, compliance reviews, research setup, import permits, project management, and reporting.
What was the focus of the Diabetes Control and Complications Trial (DCCT)?
The DCCT focused on the necessity of tight glucose control in Type 1 diabetes and demonstrated that intensive insulin therapy significantly reduced the risk of long-term complications such as retinopathy and nephropathy.
What impact did the DCCT findings have on diabetes management?
The findings from the DCCT revolutionized diabetes management, leading to updated clinical guidelines emphasizing the importance of maintaining optimal blood glucose levels.
What did the Women's Health Initiative (WHI) study reveal about hormone replacement therapy (HRT)?
The WHI study revealed that HRT could significantly elevate the risks of heart disease, stroke, and breast cancer in postmenopausal women, prompting a reassessment of its application.
How has the WHI influenced clinical practices regarding hormone replacement therapy?
The WHI has led healthcare providers to adopt more cautious strategies regarding HRT, emphasizing patient safety and the need for tailored treatment plans.
What recent developments have occurred regarding the discussion on hormone replacement therapy?
The FDA convened an Expert Panel on Menopause and Hormone Replacement Therapy, furthering the discussion on the associated risks and benefits of HRT, reflecting a trend towards individualized approaches in hormone therapy.