


This article provides a comprehensive overview of the key stages involved in medical device product development, underscoring the critical role of effective project management throughout the entire process. It delineates essential phases such as:
By highlighting strategies aimed at enhancing efficiency and reducing time-to-market, the article ensures a pathway for the successful commercialization of medical devices. In the rapidly evolving Medtech landscape, understanding these stages is imperative for stakeholders seeking to navigate the complexities of clinical research effectively.
The journey of bringing a medical device from concept to market is fraught with complexities, where each stage presents unique challenges and opportunities. Understanding the key stages in medical device product development is crucial for innovators aiming to navigate this intricate landscape effectively. This article explores the essential phases of development—from ideation and regulatory compliance to design validation and market entry—highlighting how strategic approaches can streamline processes and enhance outcomes.
As the industry evolves, we must ask: what are the critical factors that will determine success in this competitive field?
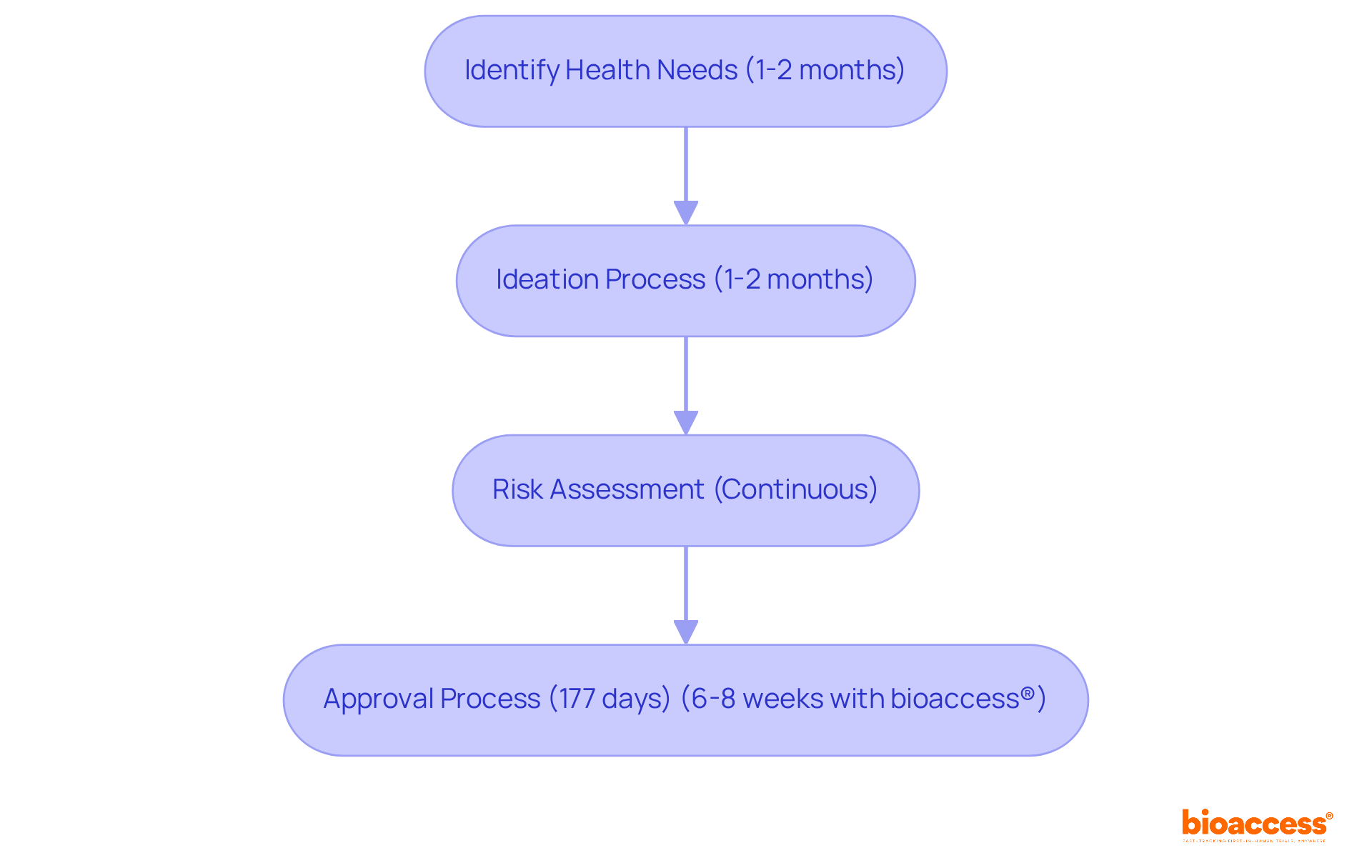
At bioaccess®, we recognize that swift ideation and thorough risk assessment are critical in the medical device product development process. This preliminary stage is essential for identifying unfulfilled health needs and creating innovative solutions while concurrently evaluating potential risks associated with the equipment.
Leveraging our extensive knowledge of compliance frameworks and diverse patient demographics, we empower clients to refine their ideation processes. This not only ensures that concepts are viable and market-aligned but also significantly reduces the time spent in this vital phase.
For instance, the preliminary phase of medical device creation typically lasts 1-2 months, while the standard approval duration for Class 2 medical devices is approximately 177 days. However, with bioaccess®'s innovative sprint method, we can secure approval in just 6-8 weeks, a considerable improvement compared to the usual 6-12 months required in the US and EU. This acceleration allows clients to advance more swiftly to design and production, ultimately enhancing their market journey.
As we approach 2025, the emphasis on efficient risk assessment strategies will be more crucial than ever, as producers strive to navigate complex compliance frameworks and foster creativity in medical device product development. Implementing strategies such as comprehensive opportunity analysis and continuous risk assessment will be vital for success, particularly in overcoming challenges posed by established competitors and recruitment issues in clinical trials.
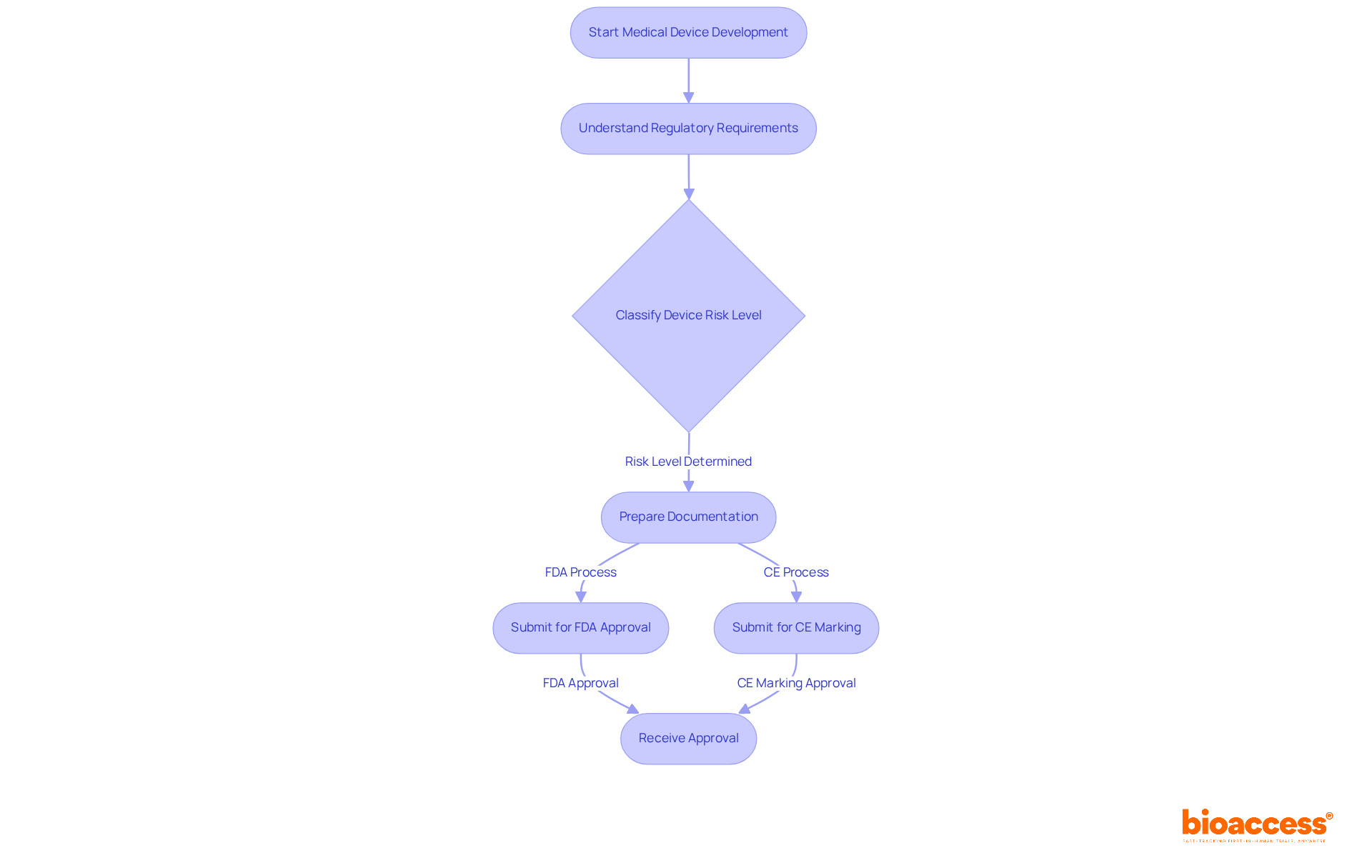
Navigating the regulatory environment presents a significant challenge in medical device product development. At bioaccess®, we provide expert guidance on compliance with both local and international regulations, including the stringent FDA and CE marking requirements. The FDA classifies medical equipment into three risk levels, with Class III items undergoing the most rigorous approval procedures. This includes pre-market authorization (PMA), which demands extensive scientific evidence to ensure safety and efficacy. In the EU, the CE marking process requires thorough documentation and evaluations by Notified Bodies, ensuring that products adhere to high safety and efficacy standards.
Current approval timelines can vary considerably; for example, the FDA's PMA process can span several months to years, while CE marking may require a similar duration based on the device's classification. Effectively navigating these complexities necessitates the integration of compliance considerations early in the medical device product development process. This proactive approach in medical device product development not only mitigates risks but also helps avoid costly delays, thereby facilitating faster market access.
Industry leaders emphasize the importance of staying informed about compliance changes. Regular training for staff on documentation practices is essential for maintaining compliance and efficiency. By leveraging technology solutions, businesses can automate data gathering and streamline compliance processes, ultimately enhancing their ability to meet legal requirements. At bioaccess®, we are committed to ensuring our clients are well-prepared for submissions, allowing them to focus on innovation while confidently navigating the compliance landscape.
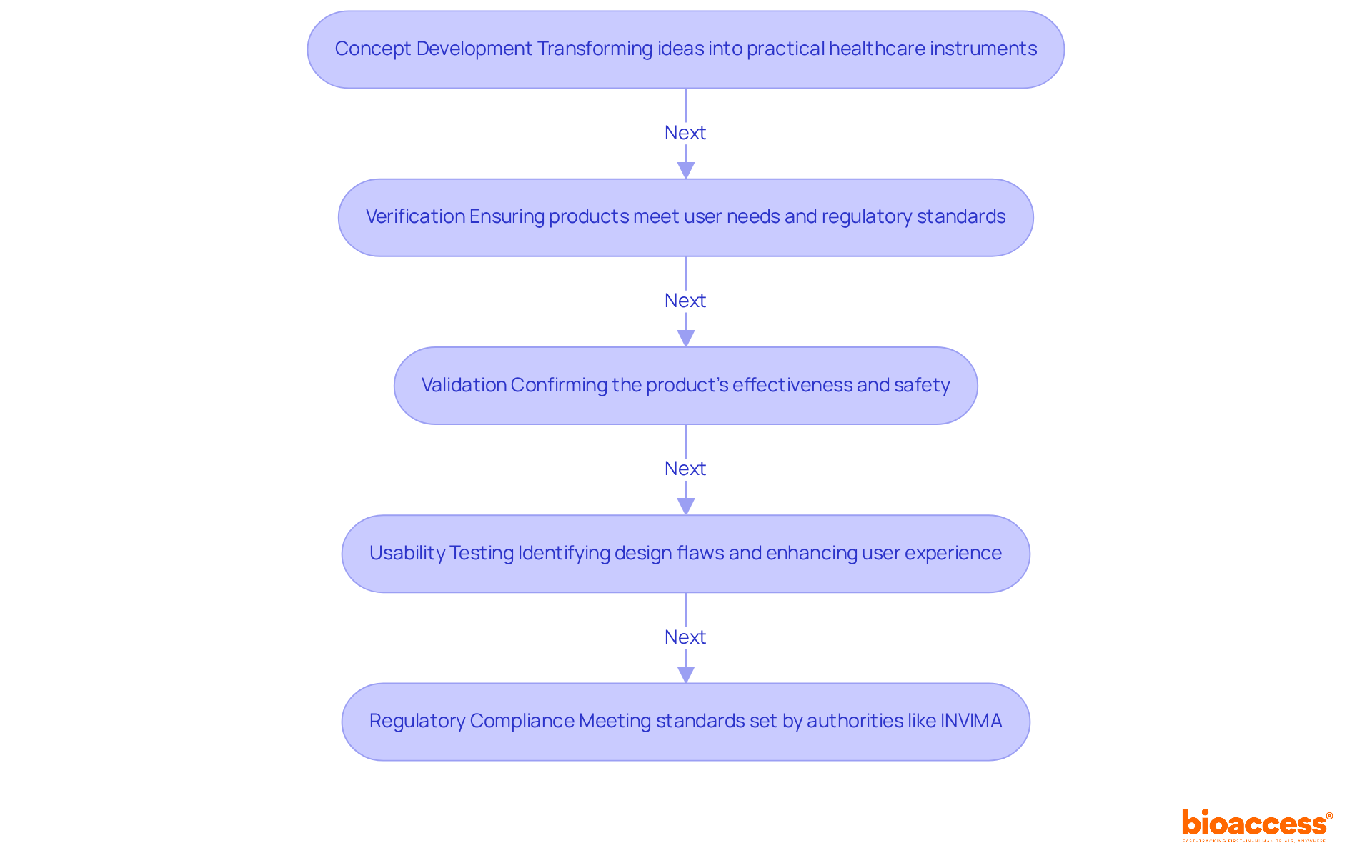
The design development stage is pivotal in medical device product development, as it transforms concepts into practical healthcare instruments. At bioaccess®, we emphasize rigorous verification and validation processes to guarantee that our products not only fulfill user needs but also adhere to regulatory standards established by authorities such as INVIMA, the Colombia National Food and Drug Surveillance Institute.
INVIMA is instrumental in examining and overseeing the promotion and production of health products, regulating healthcare instruments, and ensuring compliance with safety, efficacy, and quality standards. This process of medical device product development encompasses comprehensive design reviews, usability testing, and performance evaluations.
Recent advancements in usability testing have shown that design flaws account for over 33% of healthcare tool recalls, underscoring the critical importance of early identification of usage errors. By adopting a structured approach, we can detect potential issues early in medical device product development, significantly diminishing the risk of costly redesigns later on. In fact, the projected loss without further usability testing was estimated at €390,815.
A recent study indicated that an optimal sample size for usability testing is 100 participants, maximizing the expected net benefit of sampling (ENBS) as noted by Alexandre Caron. This proactive strategy not only enhances patient safety but also expedites market access in medical device product development, ensuring that innovative healthcare products are both effective and reliable.
Furthermore, incorporating human factor engineering (HFE) processes within the scope of medical device product development is vital to mitigate usability-induced errors, reinforcing our commitment to quality and safety. Additionally, INVIMA's designation as a Level 4 health authority highlights its proficiency and effectiveness in executing health regulation responsibilities, which is essential for ensuring the safety and quality of healthcare products.
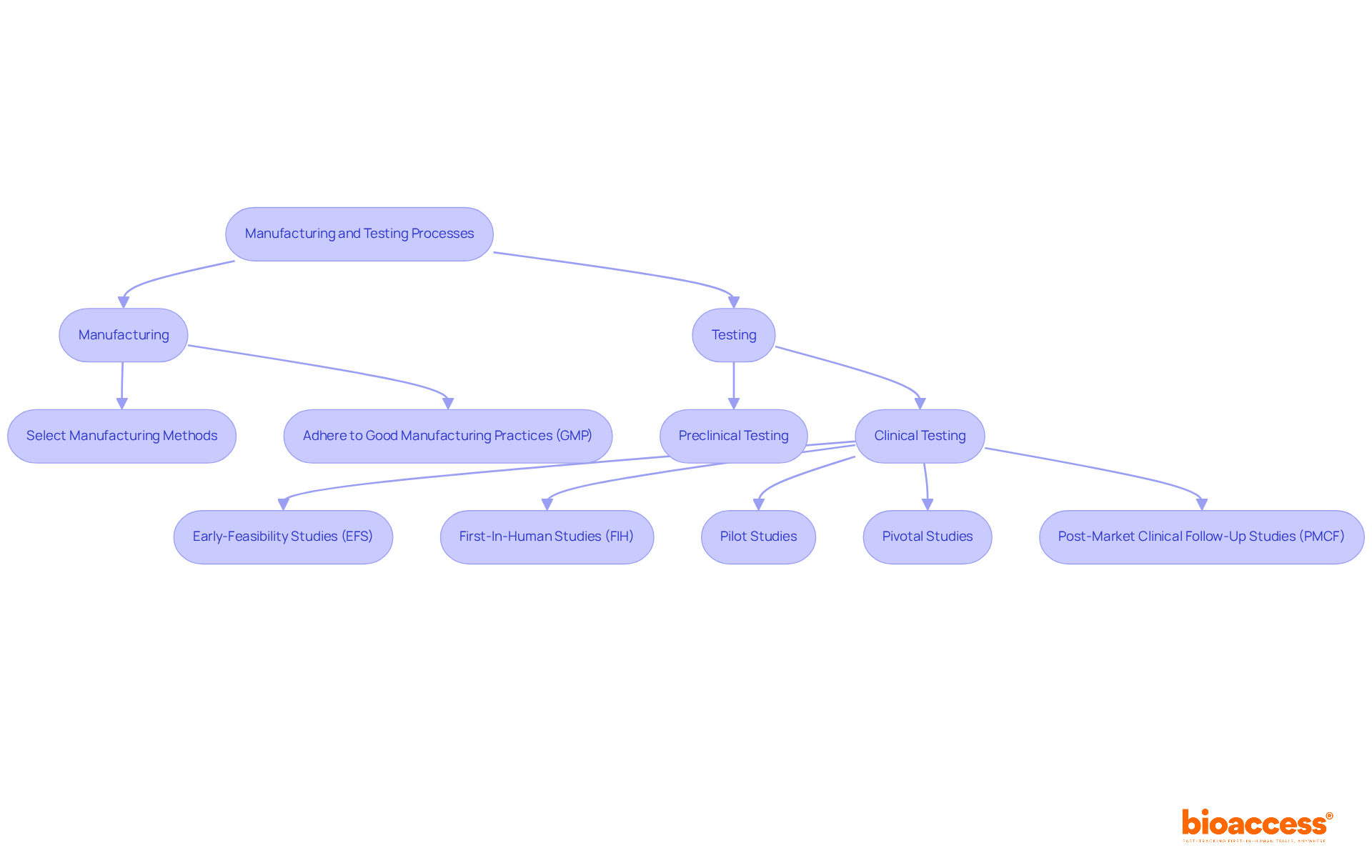
Manufacturing and testing are critical phases in the medical equipment development lifecycle. At bioaccess®, we guide clients through the complexities of medical device product development, including selecting appropriate manufacturing methods and establishing stringent testing protocols. Adhering to Good Manufacturing Practices (GMP) is vital, as it guarantees that products are consistently produced and controlled according to high-quality standards. This compliance not only enhances the safety and efficacy of the equipment but also builds trust with regulatory bodies and consumers. Our unwavering commitment to quality control throughout the manufacturing process ensures that products undergo essential preclinical and clinical testing, validating their performance and readiness for market entry.
Furthermore, our comprehensive clinical trial management services—including:
ensure meticulous handling of every aspect of clinical evaluation. Present adherence rates with GMP in the healthcare product sector serve as crucial indicators of industry standards, reflecting the ongoing dedication to excellence in product manufacturing.
Clinical trials serve as a cornerstone for securing official approval of medical devices. At bioaccess®, we specialize in designing and executing clinical trials that not only comply with regulatory standards but also address the distinct needs of our clients. Our comprehensive approach encompasses:
This ensures that trials are conducted efficiently and ethically. By leveraging our extensive experience, clients can navigate the complexities of the approval process with ease, significantly reducing average timelines and enhancing their prospects for timely market entry. With ethical approvals obtained in just 4-6 weeks and enrollment processes that are 50% faster than conventional markets, bioaccess® empowers creators to launch their healthcare products swiftly and effectively.
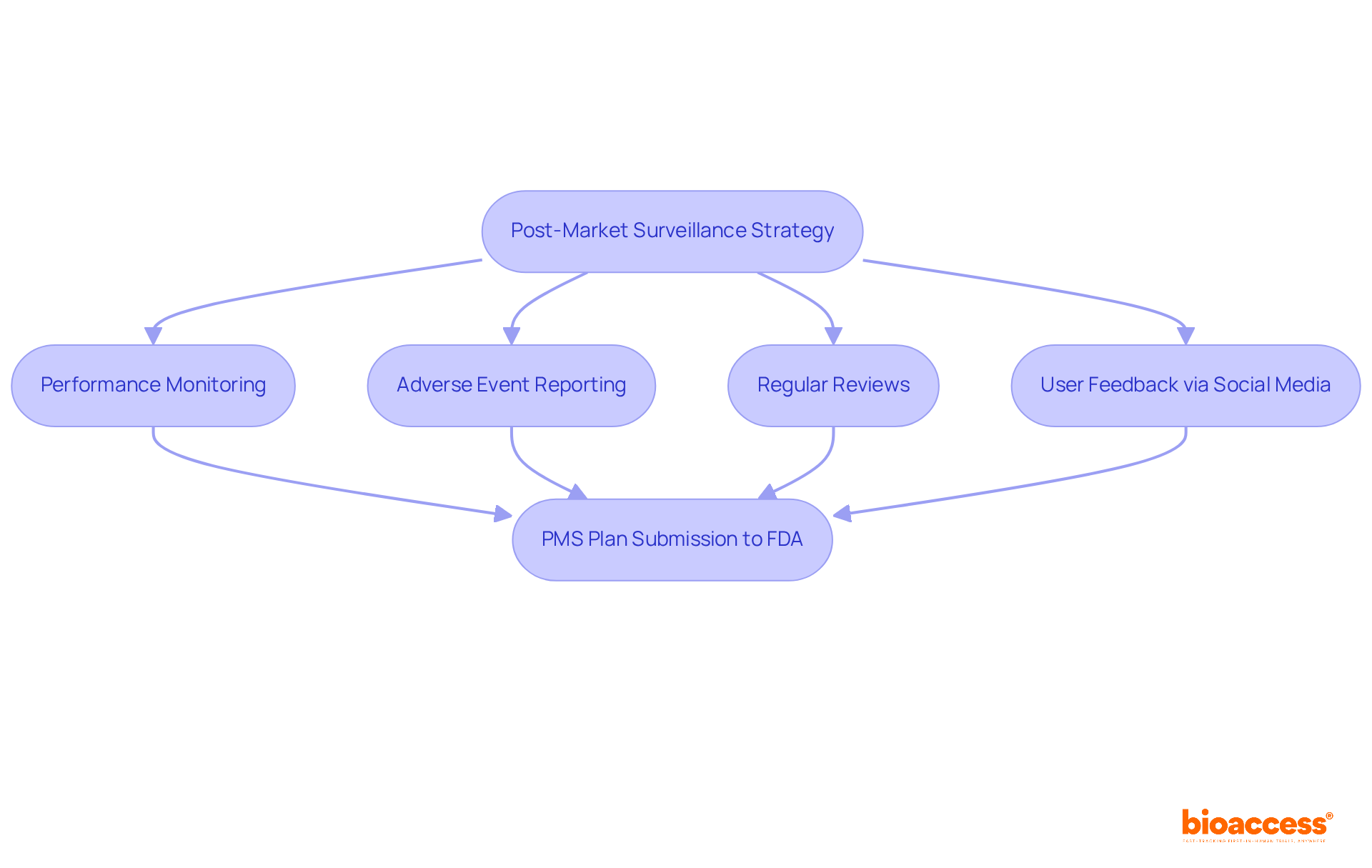
Post-market activities are essential for ensuring the ongoing safety and efficacy of medical products once they enter the market. A robust post-market surveillance (PMS) strategy is crucial, particularly for class II and class III products that may pose significant health risks. At bioaccess®, we support clients in developing comprehensive PMS strategies that encompass performance monitoring, adverse event reporting, and regular reviews. This proactive approach not only facilitates the early identification of potential safety concerns but also ensures that corrective actions can be swiftly implemented to protect patients and maintain compliance with regulatory standards.
Current trends underscore the growing dependence on social media as an invaluable resource for gathering user feedback, enabling manufacturers to assess public sentiment and address concerns effectively. By incorporating social media insights into PMS systems, companies can refine their complaint management processes and enhance marketing strategies by highlighting positive customer experiences. This integration aligns seamlessly with bioaccess®'s commitment to utilizing innovative strategies in clinical study services.
Furthermore, the FDA's 21 CFR Part 822 delineates specific PMS requirements, obligating manufacturers to submit a PMS plan as part of their premarket approval applications. This plan must outline data collection methodologies, analytical procedures, and strategies for addressing safety issues, ensuring that the PMS system is tailored to the associated risks.
Effective monitoring strategies encompass continuous data collection through user surveys, product registries, and post-market clinical follow-up studies, including Early-Feasibility Studies and First-In-Human Studies. These initiatives not only comply with legal standards but also facilitate the identification of long-term safety and performance issues, ultimately leading to product design improvements based on real-world usage. As the regulatory landscape for healthcare equipment evolves, establishing a structured PMS system is increasingly vital for ensuring product safety and effectiveness in the marketplace.
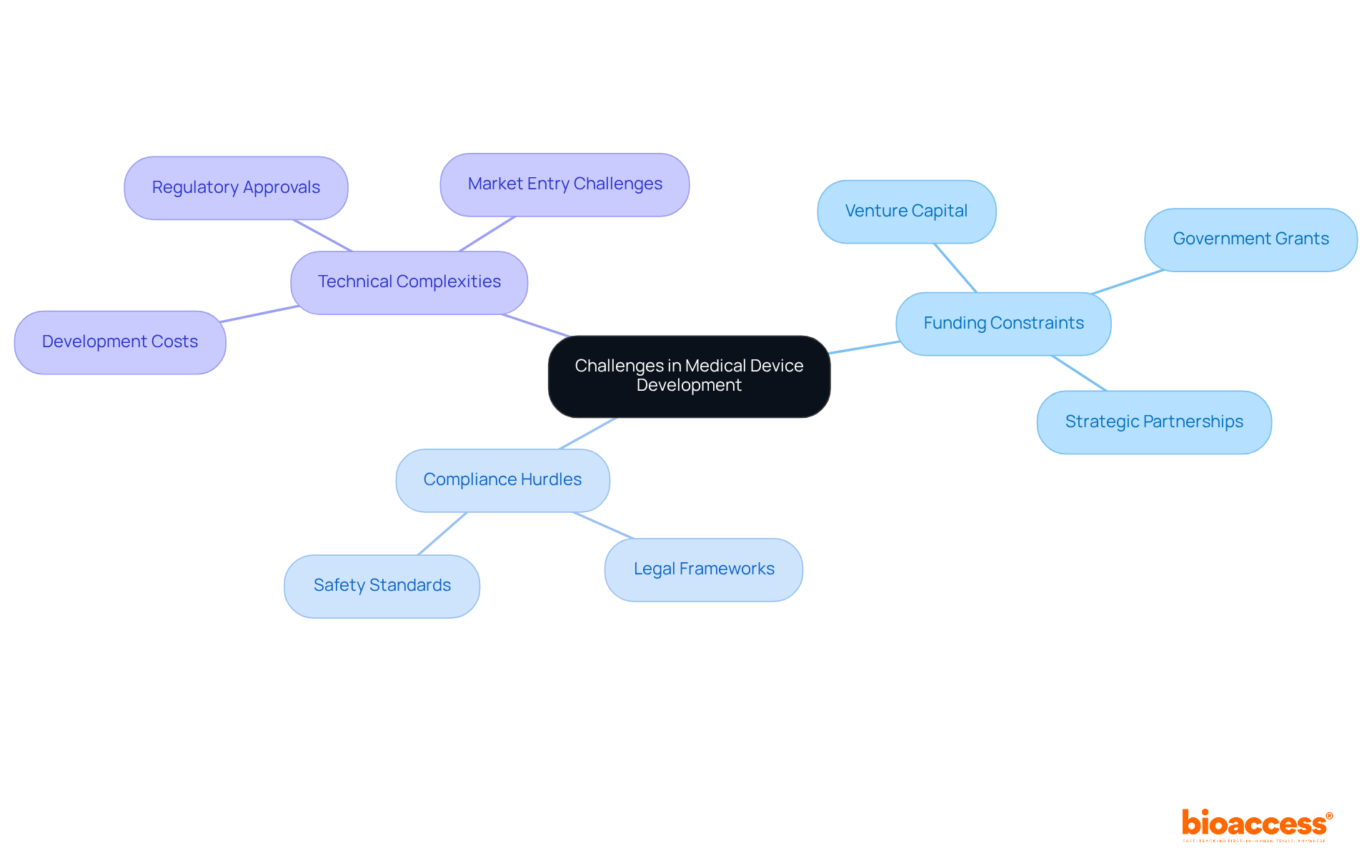
The challenges of medical device product development include compliance hurdles, funding constraints, and technical complexities. Particularly in 2025, funding constraints can significantly hinder innovation, as the costs of developing a medical device can range from hundreds of thousands to several million dollars. To address these financial challenges, Medtech startups should adopt successful funding strategies such as:
Common challenges in medical device product development involve navigating intricate legal frameworks and ensuring adherence to safety standards. At bioaccess®, we assist clients in identifying these challenges early in the development process. Our comprehensive clinical trial management services directly address these issues:
Our project management and reporting services further enhance project efficiency and reduce time to market by keeping stakeholders informed and aligned.
Moreover, promoting a cooperative atmosphere is essential for addressing compliance challenges. By collaborating closely with governing organizations and utilizing resources like the Unique Device Identification (UDI) system, manufacturers can enhance traceability and compliance, ultimately enabling smoother market entry. As Carolina Amaral highlights, "To ensure the safety and effectiveness of instruments intended for use on patients, the creation of suitable regulatory frameworks is essential." Through our assistance, clients can confidently navigate the intricacies of medical device product development, ensuring that their innovations reach the market quickly and securely.
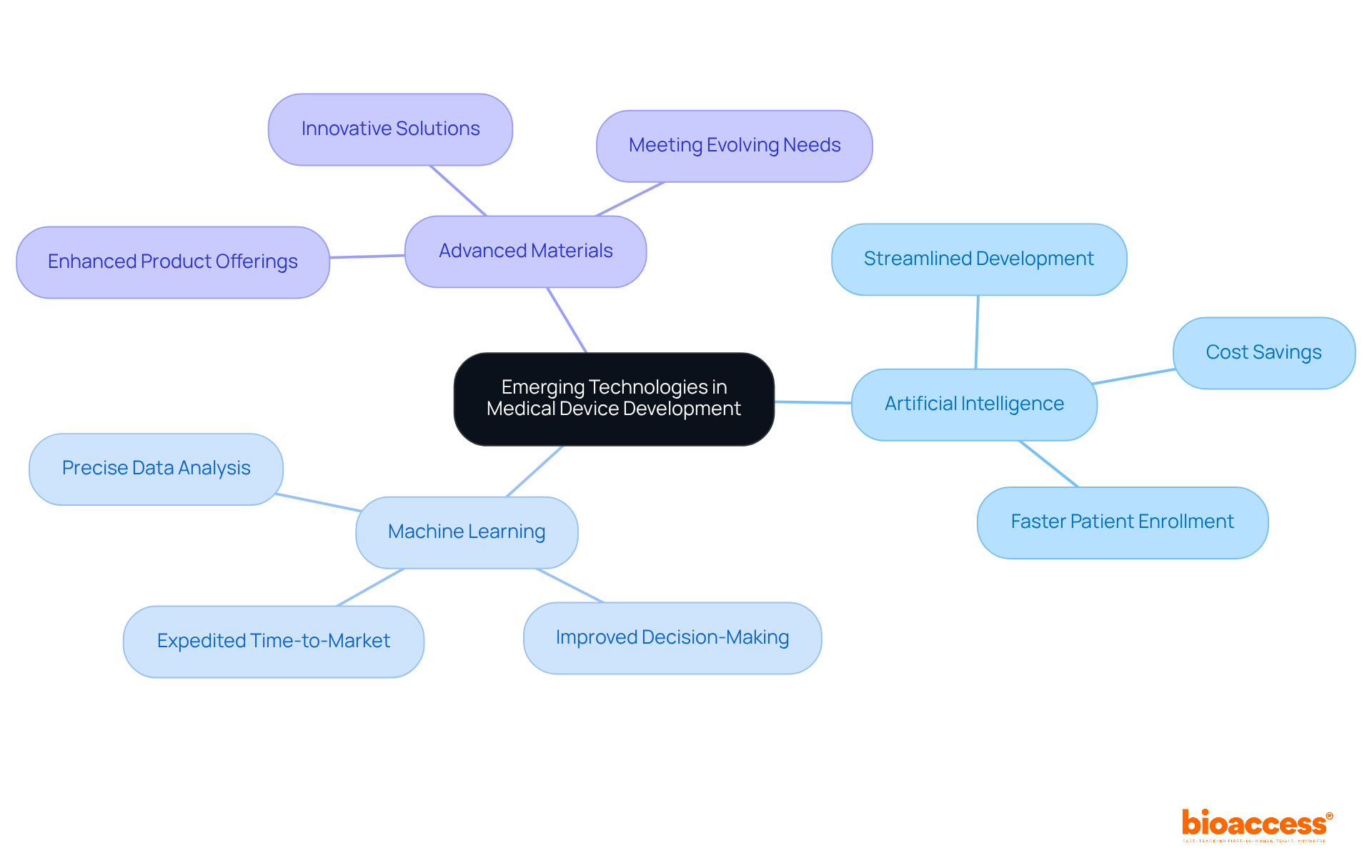
Emerging technologies, particularly artificial intelligence (AI), machine learning, and advanced materials, are revolutionizing medical device product development in the healthcare sector. At bioaccess®, we empower our clients to leverage these innovations, enhancing their product offerings and improving patient outcomes. The integration of AI and machine learning streamlines the medical device product development process and facilitates precise data analysis, leading to better decision-making and expedited time-to-market. Notably, bioaccess® achieves patient enrollment for cardiology and neurology cohorts 50% faster than Western sites, resulting in substantial savings of $25K per patient with FDA-ready data—no rework, no delays. This capability illustrates how advanced technologies can effectively address patient recruitment challenges in early-phase clinical trials.
Furthermore, collaborations such as that between bioaccess® and Caribbean Health Group position Barranquilla as a premier destination for clinical trials in Latin America, bolstered by support from Colombia's Minister of Health. As we approach 2025, adopting these technologies becomes increasingly vital for maintaining competitiveness in a rapidly evolving healthcare landscape. By embracing these advancements in medical device product development, our clients can effectively meet the evolving needs of healthcare providers and patients, ensuring their products remain at the forefront of innovation in the field.
Effective market entry strategies are crucial for the success of healthcare products in today’s competitive environment. bioaccess® empowers clients to identify target markets and navigate the intricacies of local regulations, which are critical for successful commercialization. Understanding the nuances of pricing and reimbursement strategies is equally vital, as these elements directly influence market entry and long-term sustainability. By utilizing our knowledge, clients can take advantage of worldwide opportunities, ensuring their innovations not only enter the market quickly but also create a lasting influence in the healthcare equipment sector.
Effective project management stands as a cornerstone in the medical device creation landscape, particularly in 2025, where the complexity of regulations and market demands is ever-evolving. Understanding the progression timeline in medical device product development—from ideation and design through clinical trials to market entry—is essential for successful project execution. At bioaccess®, our team of specialists, boasting over 20 years of experience, outlines the key phases of progress, enabling clients to allocate resources efficiently and establish realistic timelines for their projects.
Incorporating comprehensive clinical trial management services, bioaccess® excels in:
Our expertise allows us to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, leading to significant cost savings of $25K per patient with FDA-ready data—eliminating rework and delays.
Statistics reveal that effective resource allocation significantly influences project outcomes, with management strategies yielding a 30% reduction in time-to-market. By implementing robust project management practices, including risk assessment and timeline management, we empower innovators in the Medtech sector to navigate the intricate development process while remaining aligned with their strategic objectives. Adhering to standards such as ISO 13485 for quality management and ISO 14971 for risk management ensures that our clients can achieve their goals with greater agility and precision, effectively overcoming regulatory challenges along the way.
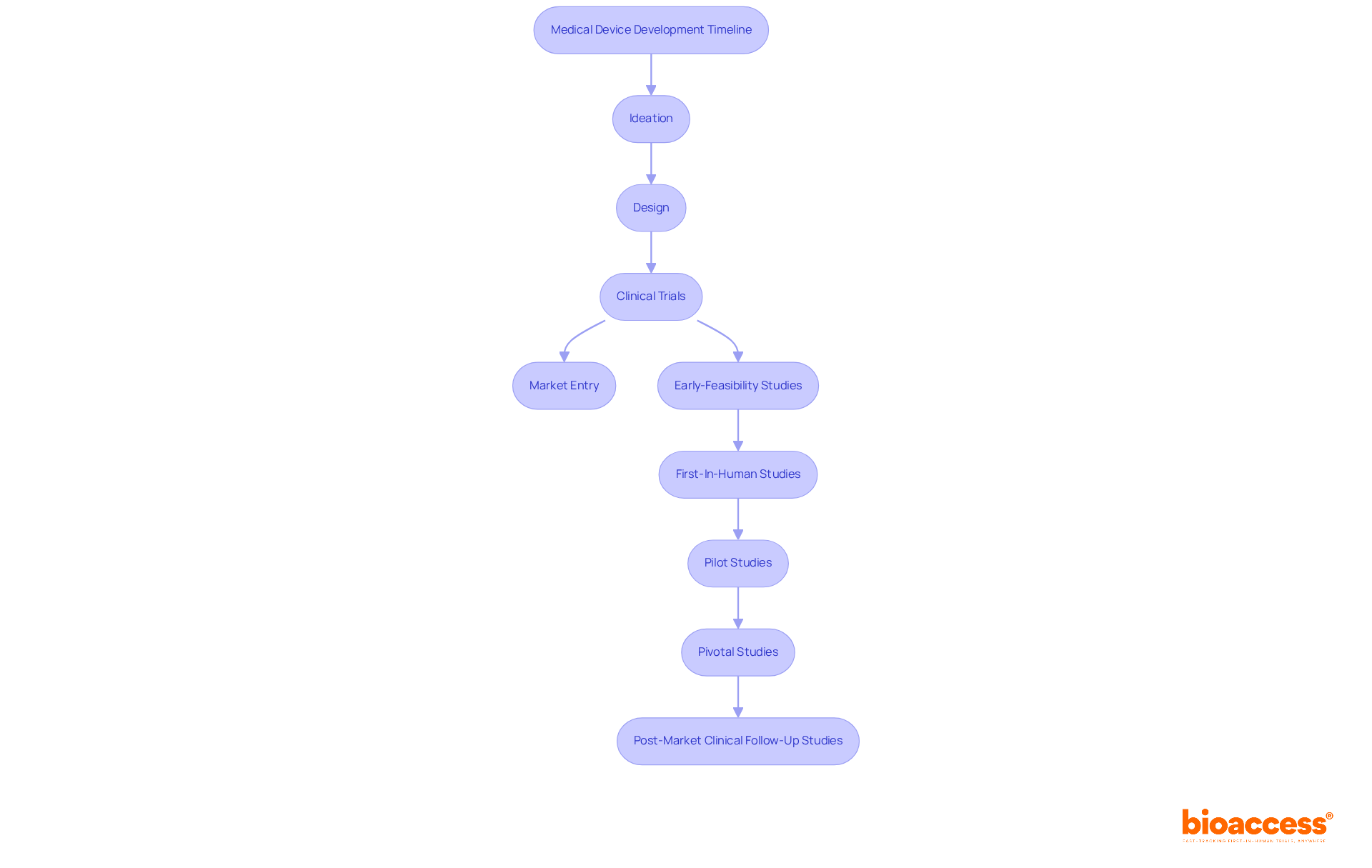
The journey of medical device product development is intricate and multifaceted, encompassing critical stages that range from initial ideation to post-market surveillance. Each phase plays a pivotal role in ensuring that innovative healthcare solutions not only meet regulatory standards but also address the real needs of patients and healthcare providers. By understanding and effectively navigating these stages, companies can significantly enhance their product offerings and improve patient outcomes.
Key insights from this exploration highlight the importance of a structured approach throughout the development process. From accelerated ideation and rigorous regulatory compliance to meticulous design validation and comprehensive clinical trials, each step is essential for mitigating risks and ensuring market readiness. Additionally, leveraging emerging technologies and effective market access strategies can empower innovators to capitalize on global opportunities, ultimately leading to successful commercialization.
In a rapidly evolving healthcare landscape, the significance of adhering to best practices in medical device development cannot be overstated. Companies are encouraged to embrace these insights and strategies, fostering a culture of innovation while ensuring compliance and safety. By doing so, they not only contribute to advancing healthcare solutions but also position themselves as leaders in the competitive medical device market.
What is the focus of bioaccess® in medical device development?
bioaccess® focuses on accelerating ideation and risk analysis in medical device development, emphasizing the importance of identifying unmet health needs and evaluating potential risks.
How does bioaccess® improve the ideation process for medical devices?
bioaccess® leverages extensive knowledge of compliance frameworks and diverse patient demographics to help clients refine their ideation processes, ensuring concepts are viable and market-aligned while reducing the time spent in this phase.
What is the typical duration for the preliminary phase of medical device creation?
The preliminary phase typically lasts 1-2 months, while the standard approval duration for Class 2 medical devices is approximately 177 days.
How does bioaccess®'s sprint method impact approval timelines?
bioaccess®'s sprint method can secure approval in just 6-8 weeks, significantly faster than the usual 6-12 months required in the US and EU.
What challenges do producers face in medical device product development as 2025 approaches?
Producers will face challenges in navigating complex compliance frameworks and fostering creativity, making efficient risk assessment strategies, comprehensive opportunity analysis, and continuous risk assessment vital for success.
What role does regulatory compliance play in medical device development?
Regulatory compliance is crucial as it helps navigate local and international regulations, including FDA and CE marking requirements, mitigating risks and avoiding costly delays.
What are the FDA's classifications for medical equipment?
The FDA classifies medical equipment into three risk levels, with Class III items undergoing the most rigorous approval procedures, including pre-market authorization (PMA).
What is the significance of CE marking in the EU?
CE marking requires thorough documentation and evaluations by Notified Bodies to ensure that medical devices meet high safety and efficacy standards.
How can businesses enhance their compliance processes?
Businesses can leverage technology solutions to automate data gathering and streamline compliance processes, which enhances their ability to meet legal requirements.
What is the importance of the design development stage in medical device product development?
The design development stage transforms concepts into practical healthcare instruments, emphasizing rigorous verification and validation processes to ensure compliance with regulatory standards.
What is the role of INVIMA in medical device regulation?
INVIMA oversees the promotion and production of health products in Colombia, ensuring compliance with safety, efficacy, and quality standards.
Why is usability testing critical in medical device development?
Usability testing is vital as design flaws account for over 33% of healthcare tool recalls, highlighting the need for early identification of usage errors to avoid costly redesigns.
What is the optimal sample size for usability testing according to recent studies?
An optimal sample size for usability testing is 100 participants, which maximizes the expected net benefit of sampling.
How does human factor engineering (HFE) contribute to medical device development?
Incorporating HFE processes helps mitigate usability-induced errors, reinforcing the commitment to quality and safety in medical device product development.