


The article delineates ten essential steps in the product development process for medical devices, underscoring the necessity of structured ideation, adherence to regulatory standards, and comprehensive verification and validation. Each step—from identifying hazards and navigating clinical trials to executing post-market surveillance—is vital for guaranteeing product safety, efficacy, and market success. This is substantiated by the detailed processes and strategies articulated throughout the article.
The landscape of medical device development is increasingly complex, characterized by rapid innovations and evolving regulatory demands. Navigating this intricate process necessitates a strategic approach that encompasses ideation, risk analysis, compliance, and post-market activities. As the industry grapples with mounting challenges, including stringent regulations and the imperative for swift market entry, companies must consider how to effectively streamline their product development while ensuring safety and efficacy.
This article delves into ten key steps designed to empower innovators in successfully navigating the medical device development journey, ultimately enhancing patient outcomes and driving market success.
At bioaccess®, we recognize that a structured ideation process is crucial for the successful product development of medical devices, especially regarding comprehensive risk assessment. This process begins with the identification of potential hazards associated with the healthcare concept, evaluating their impacts, and formulating effective mitigation strategies.
Leveraging our extensive expertise in compliance and clinical research, we empower innovators to navigate the complexities of product development medical devices during the early-phase development. This not only ensures that their ideas are groundbreaking but also market-ready.
As emphasized in ISO 14971, understanding the intended application of a healthcare instrument is vital for effective risk analysis, guiding the identification of hazards and the assessment of associated risks. Our methodology aligns with industry standards, guaranteeing that risk management is systematic and thorough, ultimately enhancing patient safety and product efficacy.
Navigating the compliance environment is essential for product development of medical devices; adherence to standards established by governing bodies such as the FDA and EMA is crucial for ensuring the safety and effectiveness of products intended for patient use. Recent updates from the FDA and EMA underscore the importance of strict compliance in product development of medical devices, especially for high-risk products, which now require thorough assessments and expert insights before market introduction.
At bioaccess®, we specialize in guiding clients through the complexities of compliance submissions, ensuring they understand the necessary documentation and procedures for successful approval. Our extensive expertise in this area allows us to streamline compliance efforts, significantly reducing time to market while upholding the highest safety standards. By leveraging our knowledge, clients can navigate the evolving regulatory landscape with confidence, facilitating the successful product development of medical devices.
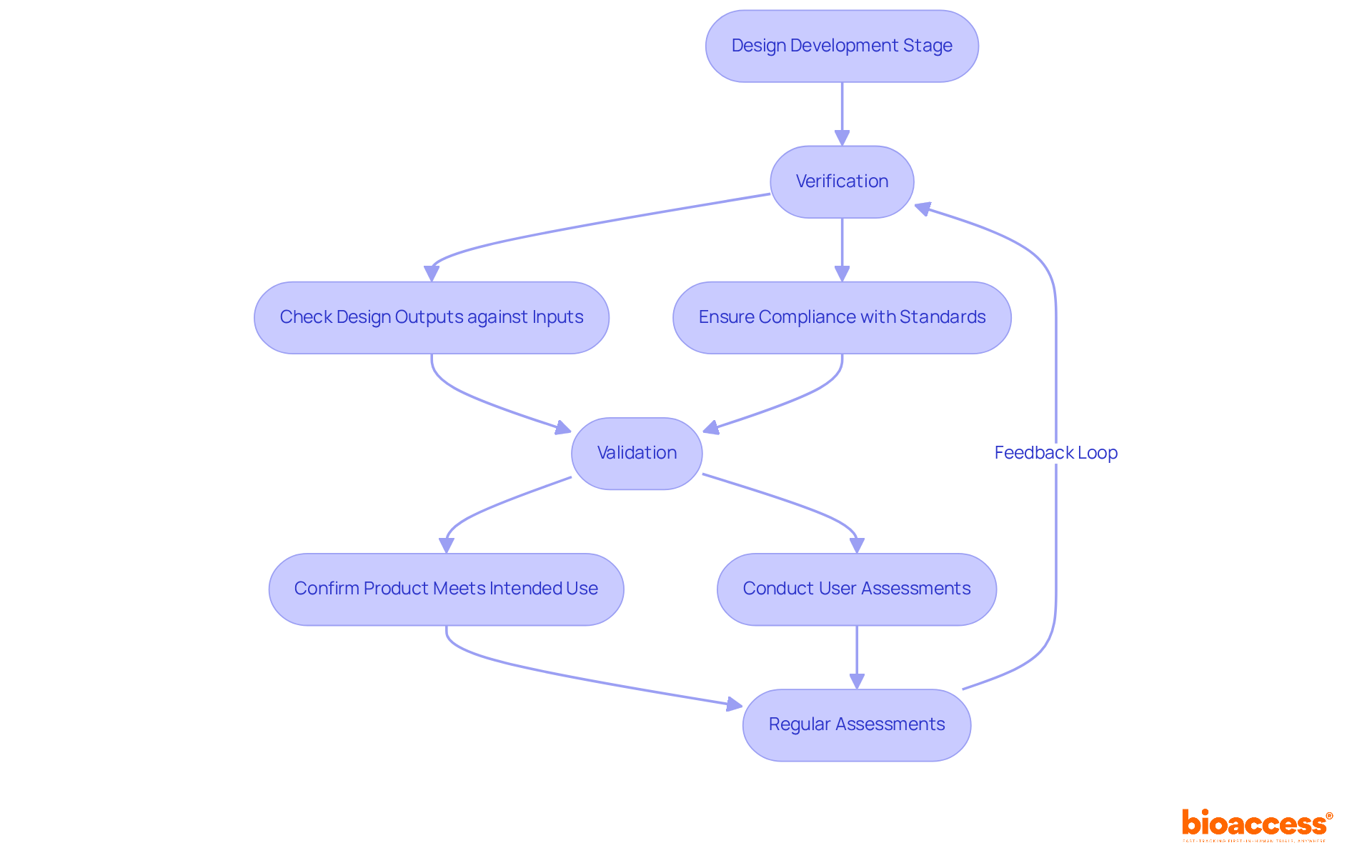
The design development stage in product development medical devices is crucial, incorporating thorough verification and validation procedures to ensure that medical instruments meet all specifications and user needs. Verification confirms that design outputs align with design inputs, while validation ensures the product fulfills its intended use.
At bioaccess®, we apply best practices in product development medical devices, leveraging our extensive expertise to guide clients through these essential processes, particularly in adherence to standards established by authorities such as INVIMA, the Colombia National Food and Drug Surveillance Institute, recognized as a Level 4 health authority by PAHO/WHO.
INVIMA plays a vital role in overseeing the marketing and manufacturing of health products, particularly in the product development of medical devices, ensuring compliance with safety, efficacy, and quality standards. For instance, Medtronic's user interface redesign for RF generators exemplifies how effective design can enhance user experience and product efficiency, in alignment with INVIMA's oversight.
By utilizing tools like Altia, which streamline development processes, we can significantly enhance product quality and safety. Ultimately, this approach not only benefits manufacturers involved in product development medical devices but also improves patient outcomes.
To implement these insights, consider conducting regular assessments of your design controls to ensure they conform to industry standards and user needs, alongside the requirements set by INVIMA.
Selecting appropriate manufacturing methods is crucial for product development of medical devices that ensure high quality. Key considerations encompass:
The choice of materials significantly influences performance, longevity, and compliance with standards. For example, materials such as ABS, PLA, and PEI are frequently utilized in additive manufacturing, whereas injection molding typically employs polypropylene and medical-grade silicones due to their consistency and repeatability.
At bioaccess®, we assist clients in identifying the most effective manufacturing methods tailored to their unique requirements. Our expertise guarantees that these methods not only fulfill regulatory criteria but also adhere to the highest quality standards. Comprehensive testing protocols are implemented to validate the performance and safety of the equipment, instilling confidence among stakeholders. By leveraging advanced production techniques, including Selective Laser Sintering (SLS) and Direct Metal Laser Sintering (DMLS), we enable the creation of intricate geometries and durable components suitable for functional testing and end-use applications. This strategic approach to material selection and manufacturing techniques is vital for efficiently and effectively advancing product development of medical devices.

Conducting clinical trials represents a pivotal phase in the evolution of medical devices, delivering essential data to validate their safety and efficacy for regulatory bodies. The average duration of clinical trials has been on the rise, with the median time frame for:
At bioaccess®, we excel in the design and management of clinical trials that not only comply with standards but also optimize patient recruitment and data collection processes. Our profound understanding of the clinical trial approval landscape empowers clients to adeptly navigate complex regulations, ensuring their products enter the market more expediently. By leveraging our expertise, clients can refine their trial designs to meet compliance standards, significantly enhancing their chances of successful approval.

Post-market surveillance is vital for the long-term success of health products. It entails ongoing monitoring of performance in real-world environments and the organized gathering of information on adverse events. Recent trends reveal that over 4 million adverse event reports were submitted to the FDA's MAUDE database from September 2019 to December 2022, highlighting the critical need for effective reporting mechanisms.
At bioaccess®, we empower our clients to develop comprehensive post-market monitoring strategies for product development medical devices that not only fulfill compliance standards but also enhance risk management and product quality throughout the product's lifecycle. Our expertise in managing Post-Market Clinical Follow-Up Studies (PMCF) ensures that clients navigate the complexities of post-market tasks efficiently, upholding the safety and effectiveness of their products. This commitment promotes long-term success in the competitive healthcare market.
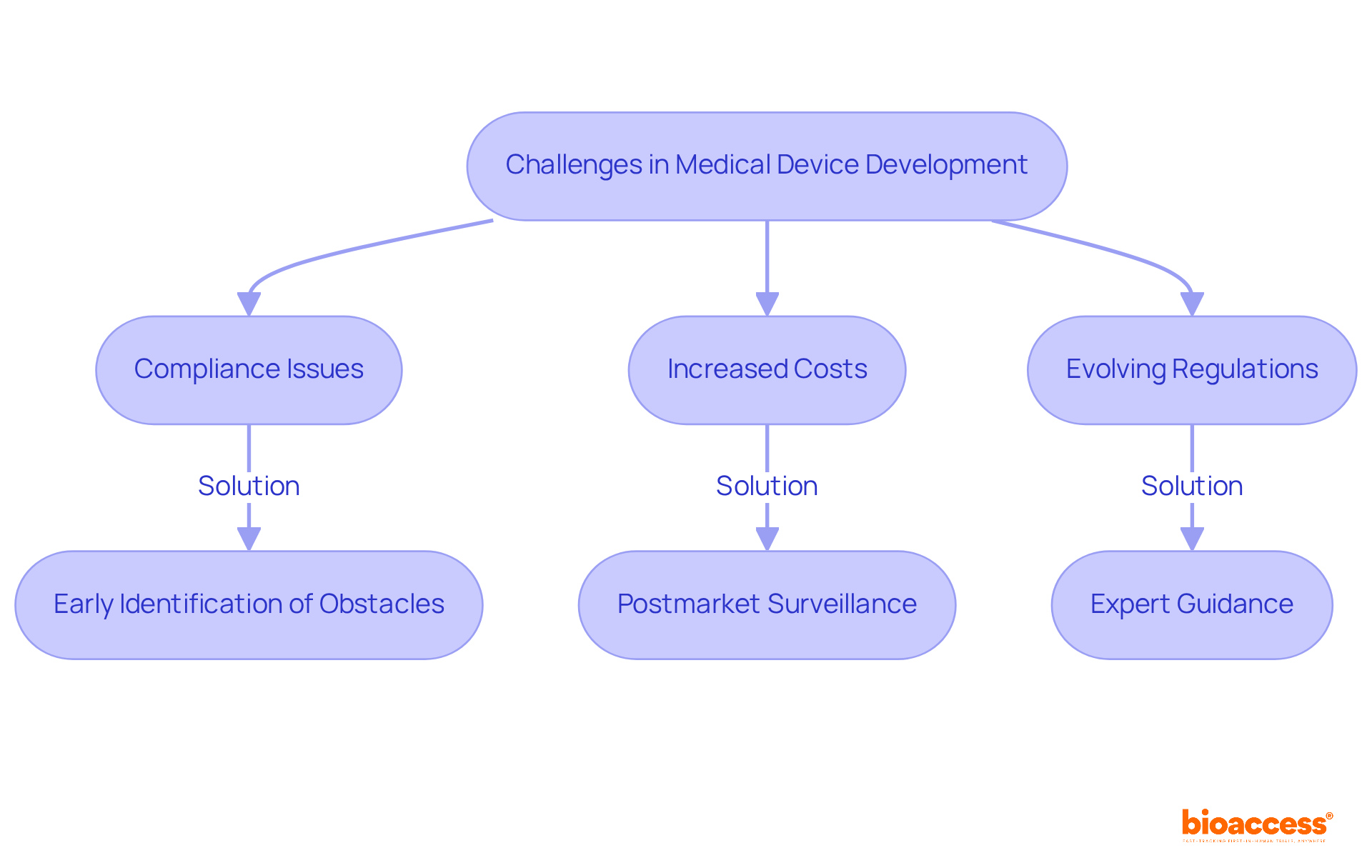
The healthcare equipment development environment presents numerous obstacles, particularly compliance issues that can significantly hinder innovation and market access. With global annual sales in the medical device industry projected to approach nearly US$800 billion by 2030, the pressure to innovate is immense. However, navigating the intricate web of regulations can prove daunting. For instance, producers often face substantial costs associated with compliance, with an anticipated 50% increase in expenses during the certification period due to evolving legal requirements.
At bioaccess®, we empower our clients to identify potential obstacles early in the development process. Our extensive experience in clinical research management equips us with the tools necessary to effectively mitigate risks. By integrating postmarket surveillance into the quality management system, we help ensure compliance while allowing our clients to focus on innovation. This proactive approach is crucial, as companies with marketed products spend an average of 52 hours per month on reactive remediation activities, compared to just 17 hours for pre-commercial entities.
Moreover, the oversight environment is continually evolving, with new frameworks emerging globally. For example, the EU's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) introduce stringent requirements that manufacturers must navigate to maintain market access. Our expertise in these areas enables us to guide clients through the complexities of compliance, ensuring they remain competitive in a rapidly changing landscape.
In summary, while the regulatory obstacles in the healthcare equipment sector can be considerable, strategic planning and expert advice regarding product development medical devices can facilitate successful navigation of these challenges, ultimately promoting innovation and enhancing patient outcomes.
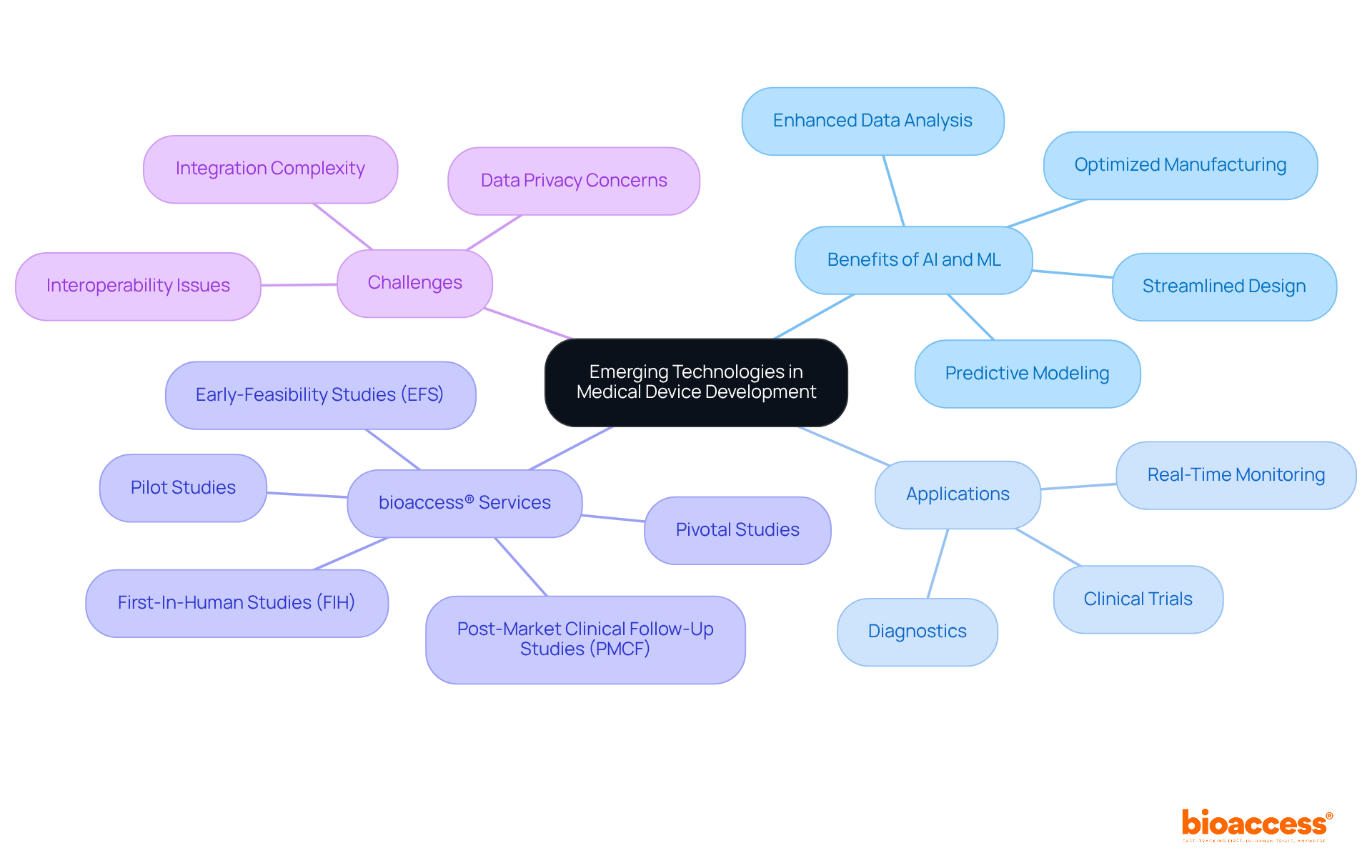
Innovative technologies, particularly artificial intelligence (AI) and machine learning (ML), are revolutionizing the healthcare equipment sector. These advancements streamline design processes, optimize manufacturing techniques, and enhance data analysis during clinical trials. AI facilitates predictive modeling, allowing developers to foresee potential challenges and improve product outcomes. For instance, AI algorithms can analyze extensive datasets to identify trends and insights that inform design decisions, ultimately resulting in more efficient medical instruments. Furthermore, machine learning applications in real-time monitoring and diagnostics are proving invaluable, enabling adaptive responses to patient needs.
At bioaccess®, we are committed to leveraging these advancements, empowering our clients to harness innovative technologies that enhance product development of medical devices and accelerate time-to-market. Notably, bioaccess® enables treatment-naive cardiology or neurology cohorts to enroll 50% faster than Western sites, achieving $25K savings per patient with FDA-ready data—no rework, no delays. Our comprehensive clinical trial management services encompass:
This ensures a customized approach to each project.
However, it is essential to acknowledge the challenges associated with integrating AI and ML into healthcare, including data privacy concerns and the need for interoperability among systems. By addressing these challenges, we can fully unlock the potential of these innovations to transform product development of medical devices.
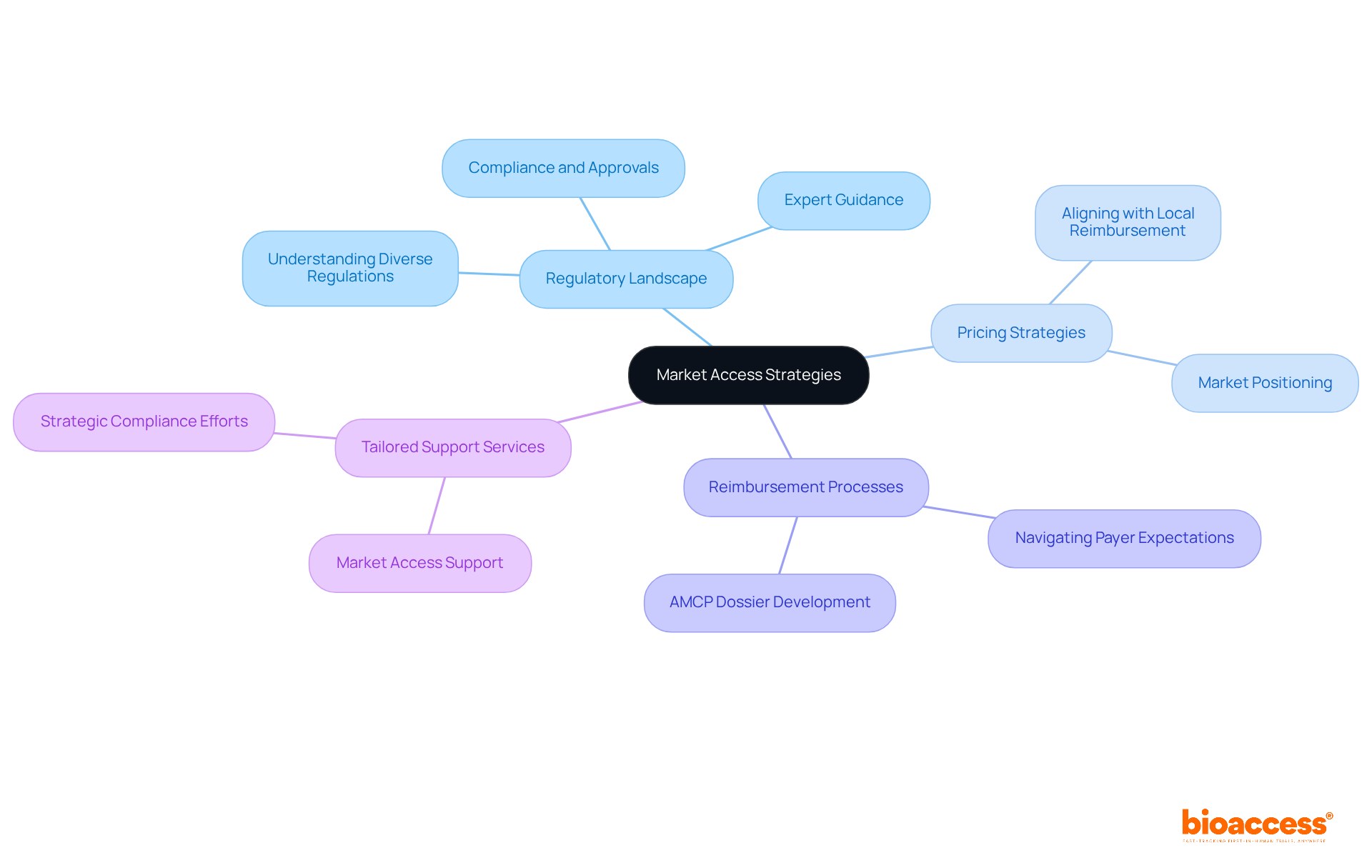
To effectively capitalize on global opportunities in 2025, healthcare equipment firms must develop robust market access strategies. A comprehensive understanding of the regulatory landscape is paramount, as it directly influences market entry and product success. Navigating diverse regulations across regions requires strategic foresight and expert guidance, ensuring compliance and facilitating smoother approvals. Additionally, pricing strategies play a critical role in market success; they must align with local reimbursement processes to enhance product adoption.
At bioaccess®, we specialize in providing tailored market access support, empowering clients to navigate these complexities and ensuring their innovative products reach the right audience efficiently. By utilizing our knowledge, companies can place themselves favorably in the competitive healthcare equipment market. Our expertise not only helps in overcoming key challenges but also positions our clients for sustained success in a dynamic landscape.
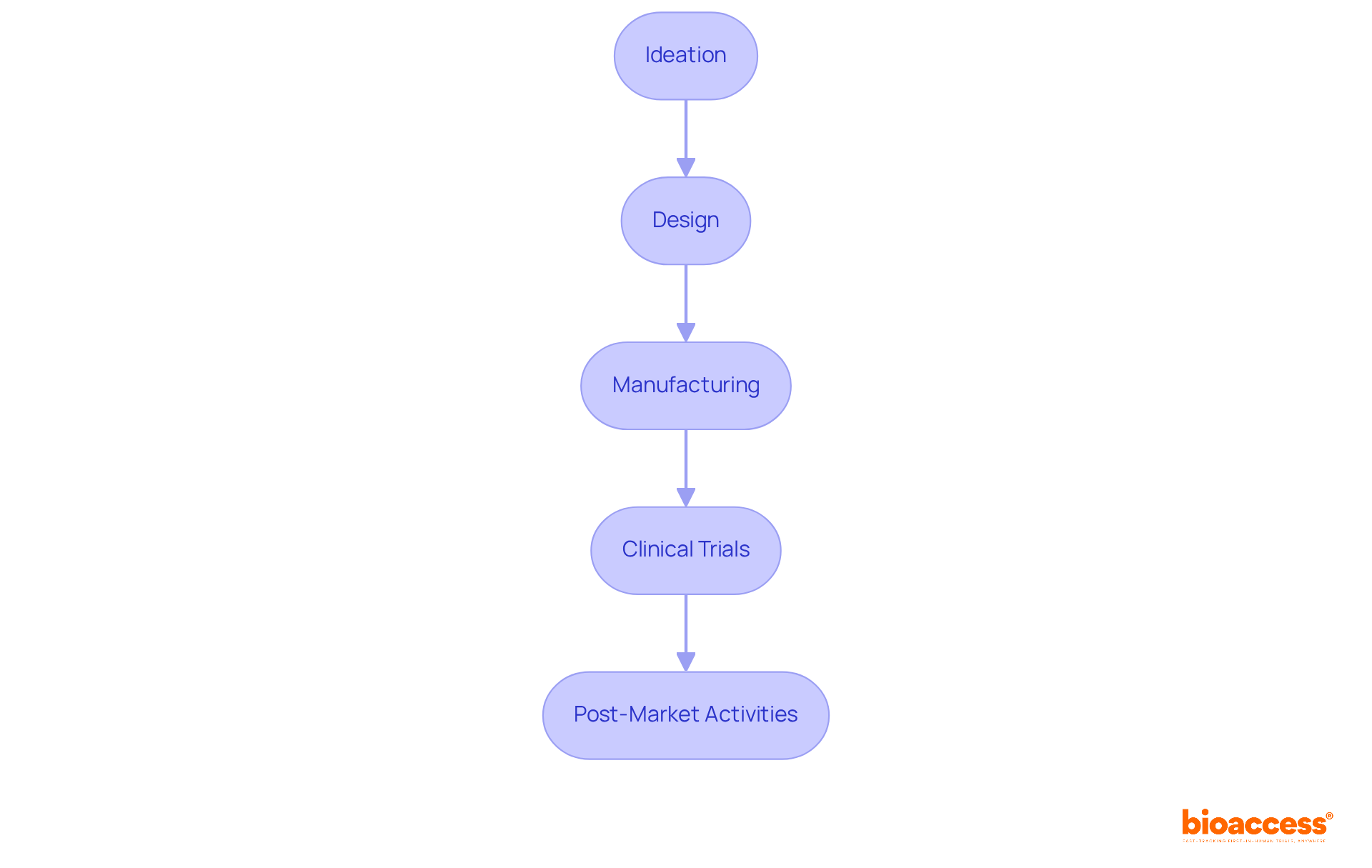
The timeline of medical device creation is structured around several critical phases:
Each phase presents unique requirements and timelines that necessitate careful management to ensure successful product creation. Research indicates that efficient time management can decrease total project duration by as much as 30%, significantly influencing market entry and competitive edge.
At bioaccess®, we empower clients to establish achievable timelines for each phase, ensuring they remain on course and efficiently accomplish their objectives. By concentrating on the effective management of these phases, we assist innovators in navigating the complexities of medical device development, from initial concept to post-market success.
The journey of product development for medical devices is intricate and multifaceted, encompassing various critical steps that ensure compliance with regulatory standards and the safety and efficacy of the final product. A structured approach—from ideation and risk analysis to post-market activities—is essential for navigating the complexities of this sector. By following these key steps, innovators can transform groundbreaking ideas into market-ready solutions that enhance patient care.
Throughout this article, pivotal elements in the medical device development lifecycle—such as regulatory compliance, design development, manufacturing processes, and clinical trials—have been highlighted. Each stage requires meticulous attention to detail, expert guidance, and a commitment to quality to successfully bring a product to market. The integration of emerging technologies, alongside strategic market access planning, underscores the importance of innovation and adaptability in this rapidly evolving field.
Ultimately, the significance of a well-defined product development process cannot be overstated. It facilitates timely market entry and fosters long-term success in a competitive landscape. Stakeholders are encouraged to embrace these practices, ensuring that their medical devices not only meet regulatory requirements but also effectively address real-world healthcare challenges. The future of medical device development lies in the hands of those who prioritize comprehensive planning and continuous improvement, paving the way for advancements that can significantly impact patient outcomes.
What is the purpose of the bioaccess® structured ideation process in medical device development?
The bioaccess® structured ideation process aims to facilitate successful product development by identifying potential hazards, evaluating their impacts, and formulating effective mitigation strategies, especially regarding comprehensive risk assessment.
How does bioaccess® assist innovators in medical device development?
bioaccess® leverages its expertise in compliance and clinical research to empower innovators, helping them navigate the complexities of early-phase product development, ensuring their ideas are both groundbreaking and market-ready.
Why is understanding the intended application of a healthcare instrument important?
Understanding the intended application is vital for effective risk analysis, guiding the identification of hazards and the assessment of associated risks, as emphasized in ISO 14971.
What role do regulatory bodies like the FDA and EMA play in medical device development?
Regulatory bodies such as the FDA and EMA establish standards that ensure the safety and effectiveness of medical devices, particularly for high-risk products that require thorough assessments and expert insights before market introduction.
How does bioaccess® support clients with regulatory compliance?
bioaccess® specializes in guiding clients through compliance submissions, helping them understand necessary documentation and procedures for successful approval, which streamlines compliance efforts and reduces time to market while maintaining high safety standards.
What is the significance of verification and validation in the design development stage of medical devices?
Verification confirms that design outputs align with design inputs, while validation ensures the product fulfills its intended use, both of which are crucial for ensuring that medical instruments meet specifications and user needs.
How does bioaccess® ensure adherence to standards in design development?
bioaccess® applies best practices and leverages extensive expertise to guide clients through verification and validation processes, ensuring compliance with standards established by authorities such as INVIMA.
What is the role of INVIMA in medical device development?
INVIMA oversees the marketing and manufacturing of health products in Colombia, ensuring compliance with safety, efficacy, and quality standards in the product development of medical devices.
Can you provide an example of effective design in medical device development?
An example is Medtronic's user interface redesign for RF generators, which enhanced user experience and product efficiency in alignment with INVIMA's oversight.
What tools does bioaccess® utilize to enhance product quality and safety?
bioaccess® utilizes tools like Altia to streamline development processes, which significantly enhances product quality and safety, ultimately benefiting manufacturers and improving patient outcomes.