


The primary focus of the article titled "10 Key Steps in the Investigational New Drug IND Process" is to delineate the essential steps involved in the IND submission process for new drugs. This article underscores that meticulous preparation and strict adherence to regulatory guidelines are vital for successful IND submissions. It highlights the structured approach to documentation and the critical importance of engaging with regulatory bodies, such as the FDA and IRBs, throughout the process. By following these steps, stakeholders can navigate the complexities of clinical research more effectively.
The journey from a groundbreaking medical innovation to its availability on the market is fraught with challenges, particularly in the realm of Investigational New Drug (IND) applications. This intricate process is essential for ensuring the safety and efficacy of new therapies; yet, it remains a daunting task for many Medtech and Biopharma companies. By delving into the ten key steps involved in the IND process, readers will uncover strategies that can significantly enhance their chances of success.
How can companies effectively streamline their IND submissions and overcome the hurdles that often impede progress?
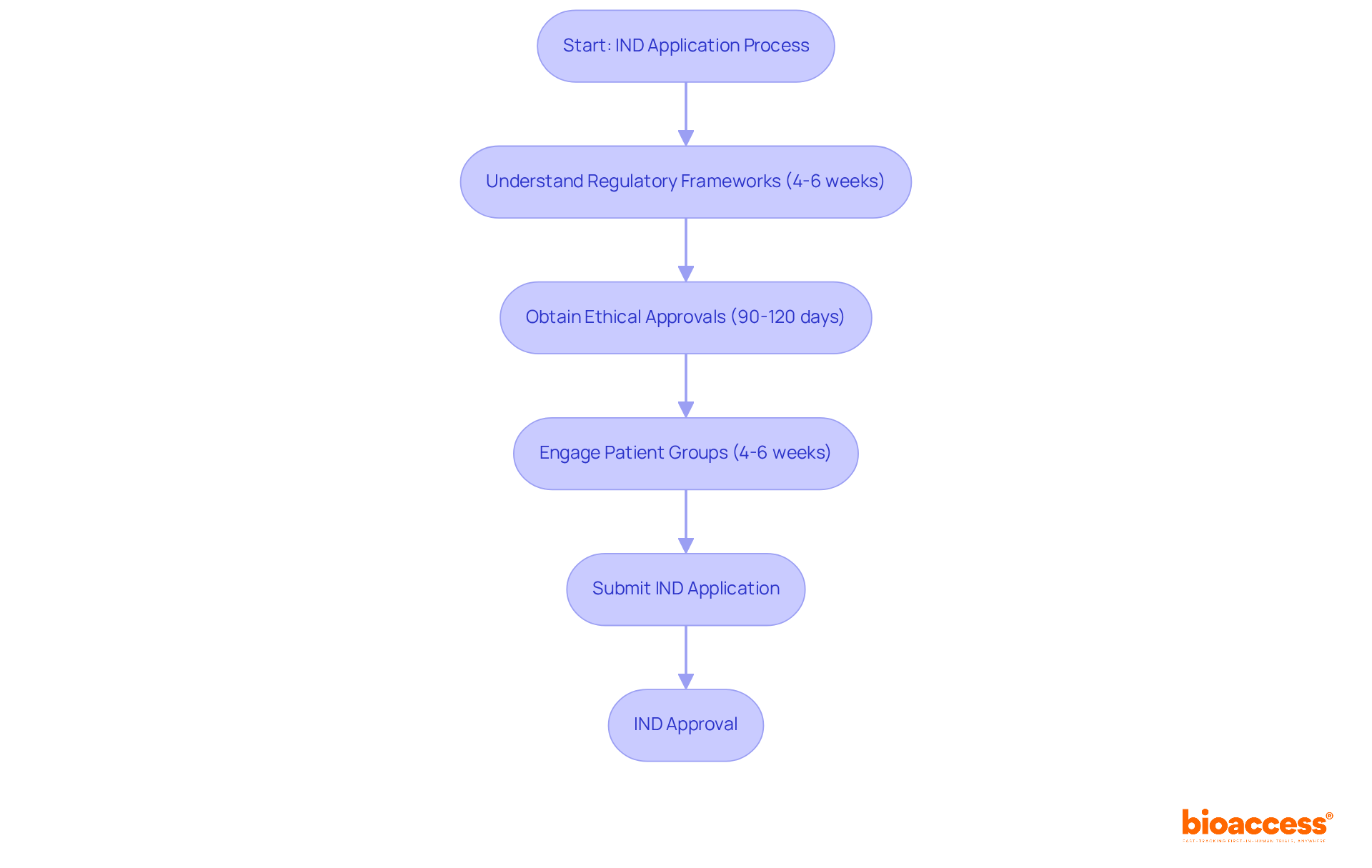
bioaccess® excels in expediting Investigational New Drug (IND) submissions by harnessing its deep understanding of regulatory frameworks and the fast-tracked ethical approval processes available in Latin America, particularly in Colombia. With an average approval timeline of just 4-6 weeks, bioaccess® significantly reduces the time to market for Medtech innovations, allowing companies to bring their products to patients more swiftly. Colombia provides exceptional cost effectiveness, with savings exceeding 30% compared to studies in North America or Western Europe, along with a regulatory pace that allows total IRB/EC and MoH (INVIMA) evaluations to be finalized in merely 90-120 days.
Regulatory experts emphasize that ethical approvals are crucial for maintaining public trust and ensuring the safety and efficacy of new medical technologies. As Dr. Michael Carome, a physician with public health expertise, notes, "The incentive to do really properly conducted trials—after the device is already approved? It's so low." By engaging with the varied patient groups in Colombia, where approximately 95% of the populace has access to universal healthcare, and the efficient processes in Australia that enable faster regulatory evaluations, bioaccess® not only speeds up investigational new drug (IND) submissions but also improves the overall quality of clinical studies.
As the Medtech landscape evolves in 2025, bioaccess® remains at the forefront, leveraging regulatory speed and the robust healthcare system in Colombia to facilitate timely access to groundbreaking therapies and improve patient outcomes.
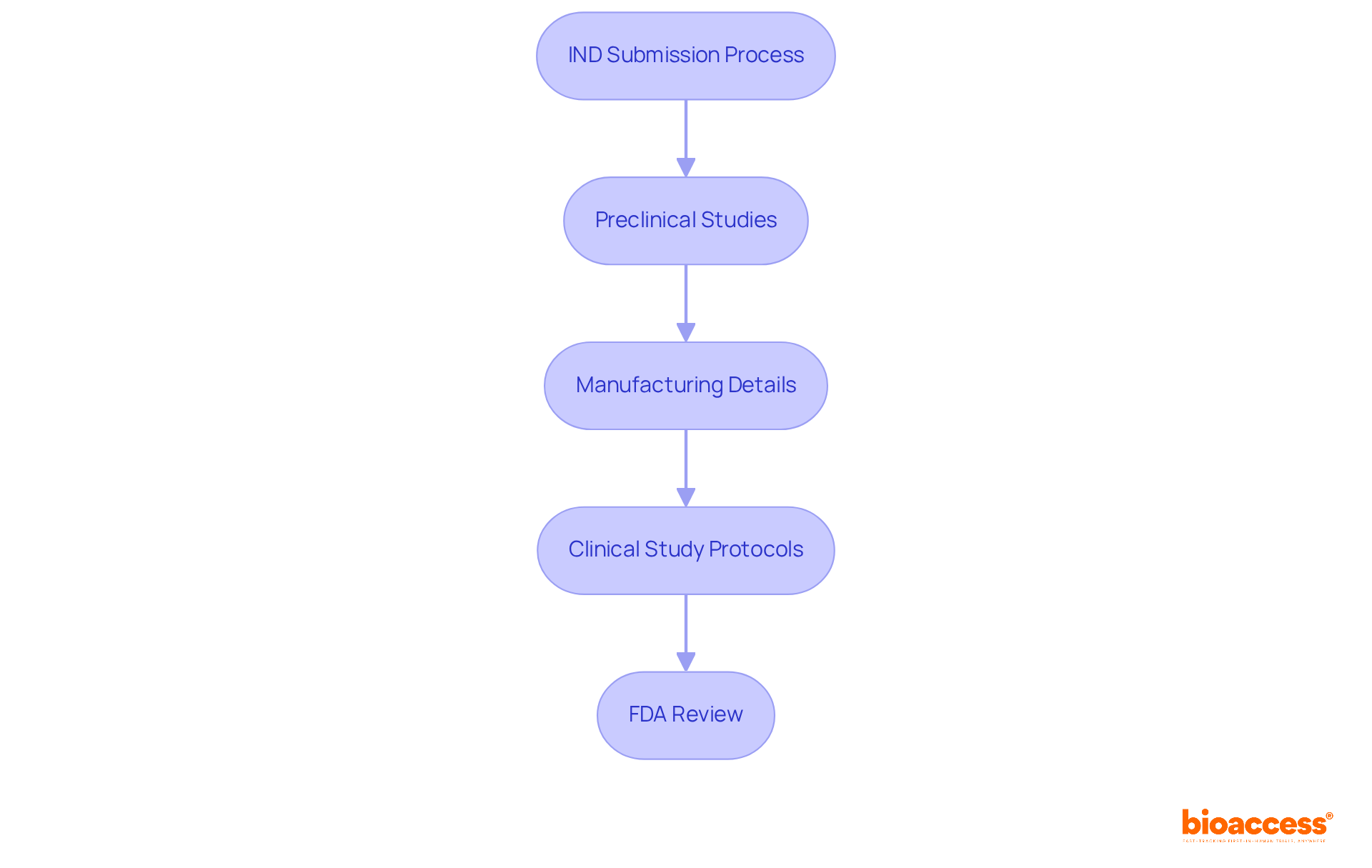
The investigational new drug IND submission represents a pivotal request made to the FDA, allowing Medtech and Biopharma firms to initiate human clinical studies for new medications. This comprehensive program encompasses data from preclinical studies, manufacturing details, and proposed clinical study protocols. The investigational new drug IND procedure is vital for confirming the safety and efficacy of new drugs before their market introduction, making it an essential step for any company operating within these sectors.
Success rates for IND submissions in the Medtech sector reveal a notable trend: only about 12% of drugs entering clinical trials ultimately secure FDA approval. This statistic underscores the critical importance of meticulous preparation and adherence to regulatory requirements throughout the investigational new drug IND process. In 2019, the pharmaceutical industry invested approximately $83 billion in research and development, demonstrating a substantial commitment to advancing new therapies, despite the challenges posed by IND submissions.
FDA officials emphasize the significance of the investigational new drug IND procedure, indicating that it is designed to ensure investigational drugs undergo thorough safety assessments prior to human use. Practical examples illustrate the complexities involved; for instance, the submission of FDA Form 1571, which serves as the cover page for IND submissions, is a key component that establishes a contractual agreement between the sponsor and the FDA.
As we approach 2025, the importance of the investigational new drug IND submissions in clinical trials continues to be paramount, particularly as the industry increasingly focuses on developing specialty medications for complex conditions. The investigational new drug IND procedure not only aids in the transition from laboratory to clinical environments but also plays a crucial role in upholding public health standards by ensuring that new therapies are rigorously tested and monitored.
The investigational new drug IND process encompasses three primary categories: Commercial INDs, Research INDs, and Expanded Access INDs.
Commercial INDs are submitted for drugs intended for sale, constituting a significant portion of IND submissions. In 2025, approximately 70% of IND submissions were Commercial INDs, underscoring the industry's focus on introducing new therapies to the market.
Research INDs are tailored for investigational studies that are not aimed at commercial distribution, frequently submitted by academic institutions or researchers. These INDs represented about 25% of submissions, highlighting their crucial role in advancing medical knowledge.
Expanded Access INDs allow patients access to investigational drugs outside of clinical trials, typically for those with serious or life-threatening conditions who have exhausted other treatment options. This pathway emphasizes the ethical commitment to patient care while navigating regulatory requirements.
Instances of successful investigational new drug INDs illustrate how medications have transformed treatment protocols in oncology and rare diseases, showcasing the potential impact of these uses. Industry leaders stress the importance of comprehending the nuances of each investigational new drug IND type to adeptly navigate the regulatory landscape. As one specialist noted, 'Navigating the IND pathways necessitates a strategic approach to ensure compliance and accelerate the development phase.' This insight underscores the essential nature of investigational new drug IND submissions within the drug development lifecycle.

The IND submission procedure serves as a critical pathway for introducing new medications to the market, encompassing several essential steps:
Each of these steps demands meticulous attention to detail and strict adherence to regulatory guidelines to facilitate a seamless evaluation.
To achieve a successful IND submission in 2025, it is imperative that the documentation is well-organized and clear. A poorly structured IND can lead to confusion and delays, with common reasons for submission setbacks including:
For example, the NDA for remdesivir was submitted in August 2020 and received approval just 76 days later, illustrating the significance of thorough preparation and responsiveness to regulatory requirements.
Successful IND preparation often includes engaging in Pre-IND consultations with the FDA, enabling sponsors to clarify expectations and refine their development plans. This proactive approach significantly enhances the application's credibility and streamlines the review process. Moreover, the case of Elexacaftor, which required 1,043 days from IND opening to approval, underscores how prior experience in similar therapeutic areas can expedite the timeline.
Regulatory consultants stress the importance of clarity in submissions, noting that concise and well-structured documents improve the likelihood of favorable reviews. As industry experts emphasize, "Clear and concise IND submissions allow reviewers to quickly find pertinent information, reducing the risk of clinical holds."
In conclusion, navigating the IND submission process necessitates a strategic approach that prioritizes clarity, thoroughness, and proactive engagement with regulatory bodies. By recognizing common pitfalls and employing effective preparation strategies, sponsors can significantly enhance their chances of a timely and successful IND approval. Notably, the average duration for new drug approval in the U.S. is 12 years, while device approval takes approximately 7 years, highlighting the critical importance of efficient IND submissions.

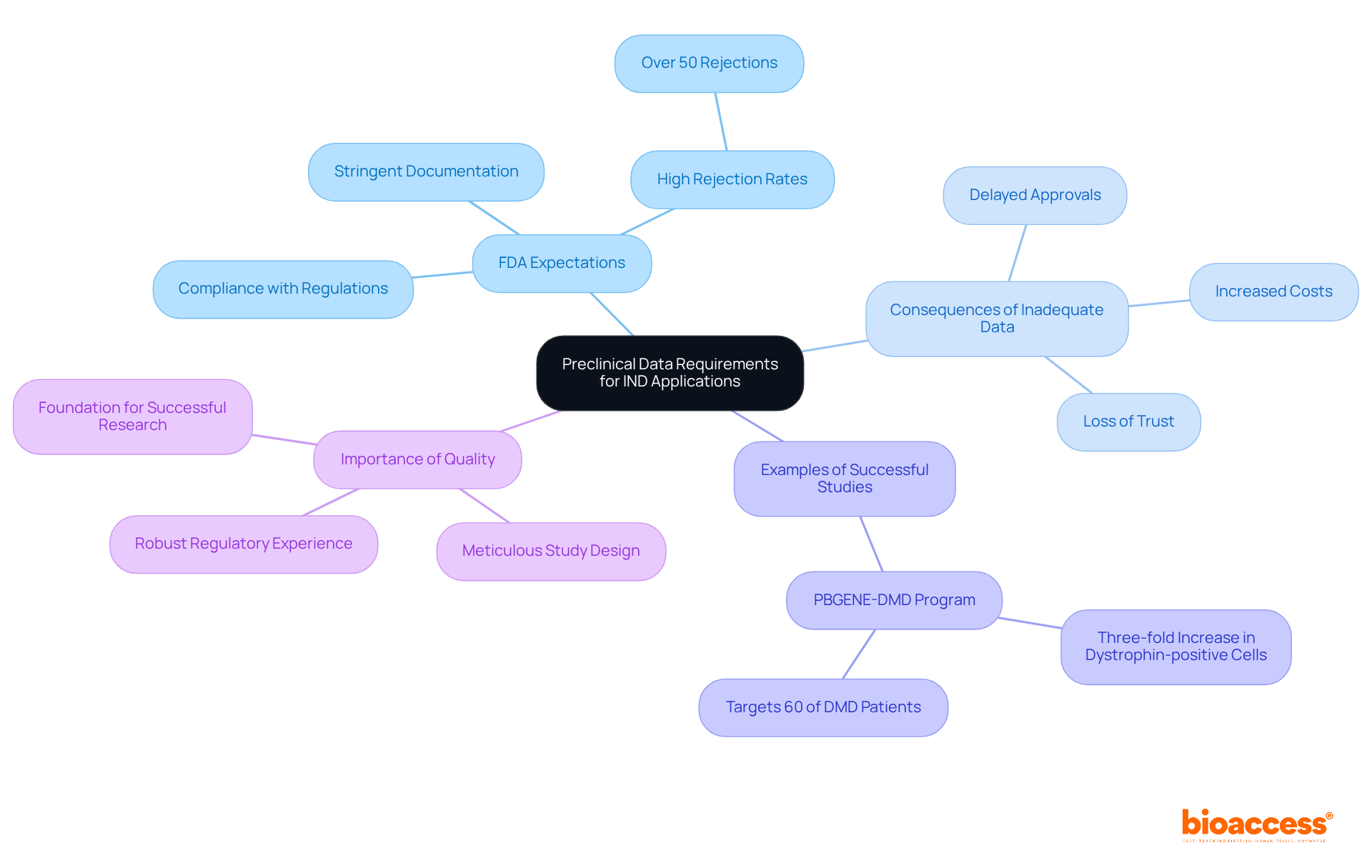
The requirements for preclinical data in investigational new drug (IND) applications are crucial, comprising results from laboratory and animal studies that substantiate the drug's safety and biological activity. The FDA maintains stringent expectations, with rejection rates for preclinical data often surpassing 50% due to inadequate rigor or documentation. Comprehensive and well-documented data is vital to support proposed clinical research protocols and ensure regulatory compliance.
Notable examples, such as the PBGENE-DMD program, demonstrate how robust preclinical studies can facilitate expedited investigational new drug (IND) submissions and foster innovative therapies. In a long-term study utilizing a DMD mouse model, researchers noted up to a three-fold increase in dystrophin-positive muscle cells, underscoring the significance of thorough preclinical data.
Corey Leet emphasizes that quality is foundational in preclinical research, highlighting the necessity of meticulous study design and execution. To enhance the probability of regulatory approval, researchers should design their preclinical studies with these insights in mind.
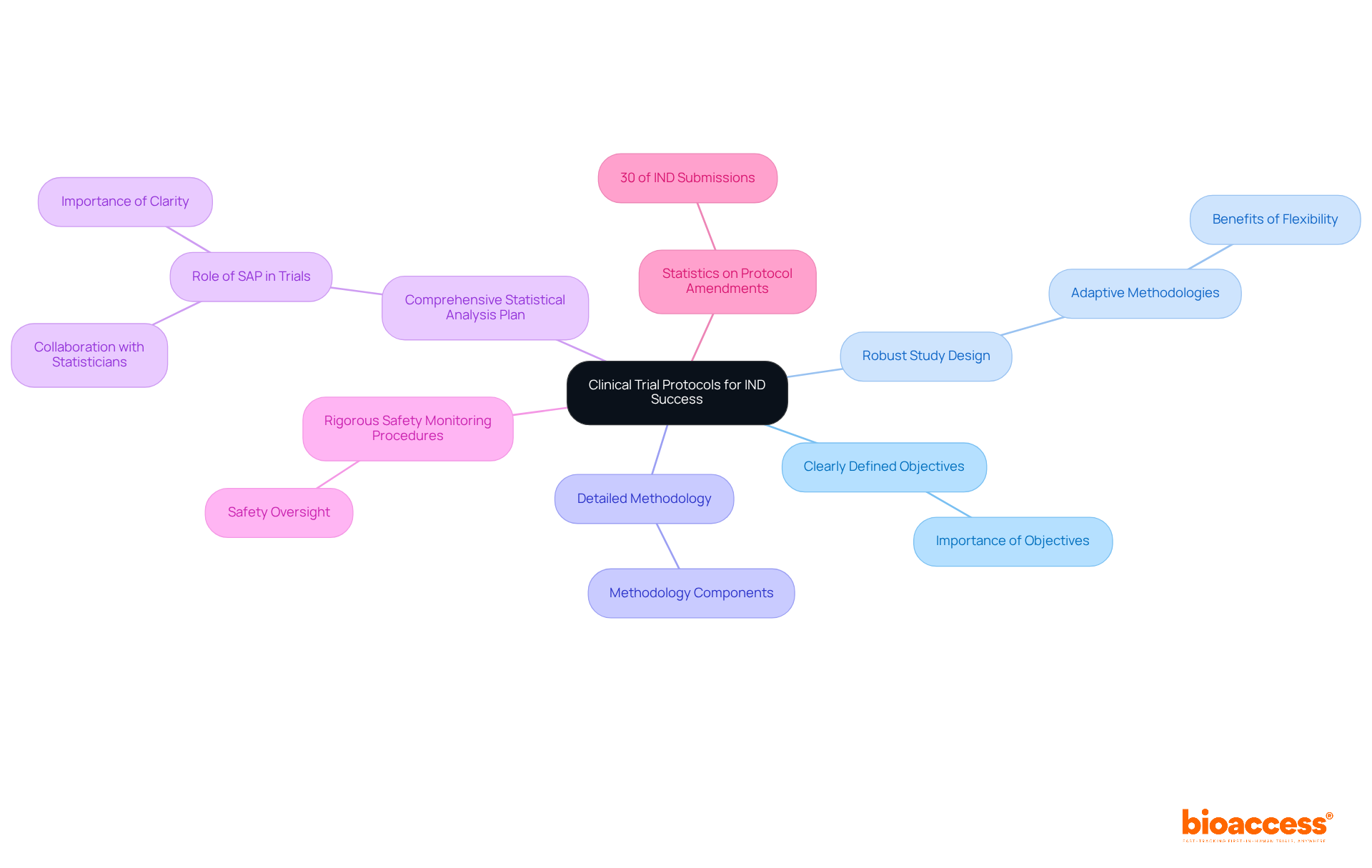
Clinical trial protocols serve as foundational documents that must encompass several critical components to ensure ethical and effective trial conduct. Key elements include:
These components not only direct the research activities but also conform to regulatory requirements essential for Investigational New Drug (IND) approval.
Statistics indicate that poorly prepared protocols can lead to significant delays in the investigational new drug (IND) process, often requiring amendments to address gaps or inaccuracies. For instance, studies reveal that nearly 30% of IND submissions necessitate protocol amendments during the review phase, underscoring the importance of comprehensive initial planning.
Effective study designs in investigational new drug (IND) applications frequently incorporate adaptive methodologies, allowing for modifications based on interim results. This flexibility can enhance the process's efficiency and relevance, ultimately resulting in quicker approvals. Experts emphasize that a well-organized protocol, which includes a detailed SAP, is crucial for maintaining the integrity of the study and ensuring that the results are both trustworthy and replicable.
As we approach 2025, developing clinical study protocols for investigational new drug (IND) submissions requires a keen awareness of evolving regulatory environments and optimal practices. Integrating insights from clinical study experts can significantly elevate protocol quality, ensuring that all necessary elements are addressed from the outset. This proactive approach not only facilitates smoother regulatory interactions but also bolsters the overall success of the clinical study. Notably, bioaccess® accelerates the regulatory approval process, achieving it in just 6-8 weeks compared to the typical 6-12 months in the US and EU. This expedited timeline is particularly advantageous for enrolling treatment-naive cardiology or neurology cohorts, allowing for a 50% faster enrollment rate than Western sites. By addressing these essential components within the framework of bioaccess®'s innovative approach, clinical studies can navigate the complexities of regulatory challenges more effectively, ultimately enhancing market access for medical devices in Latin America.
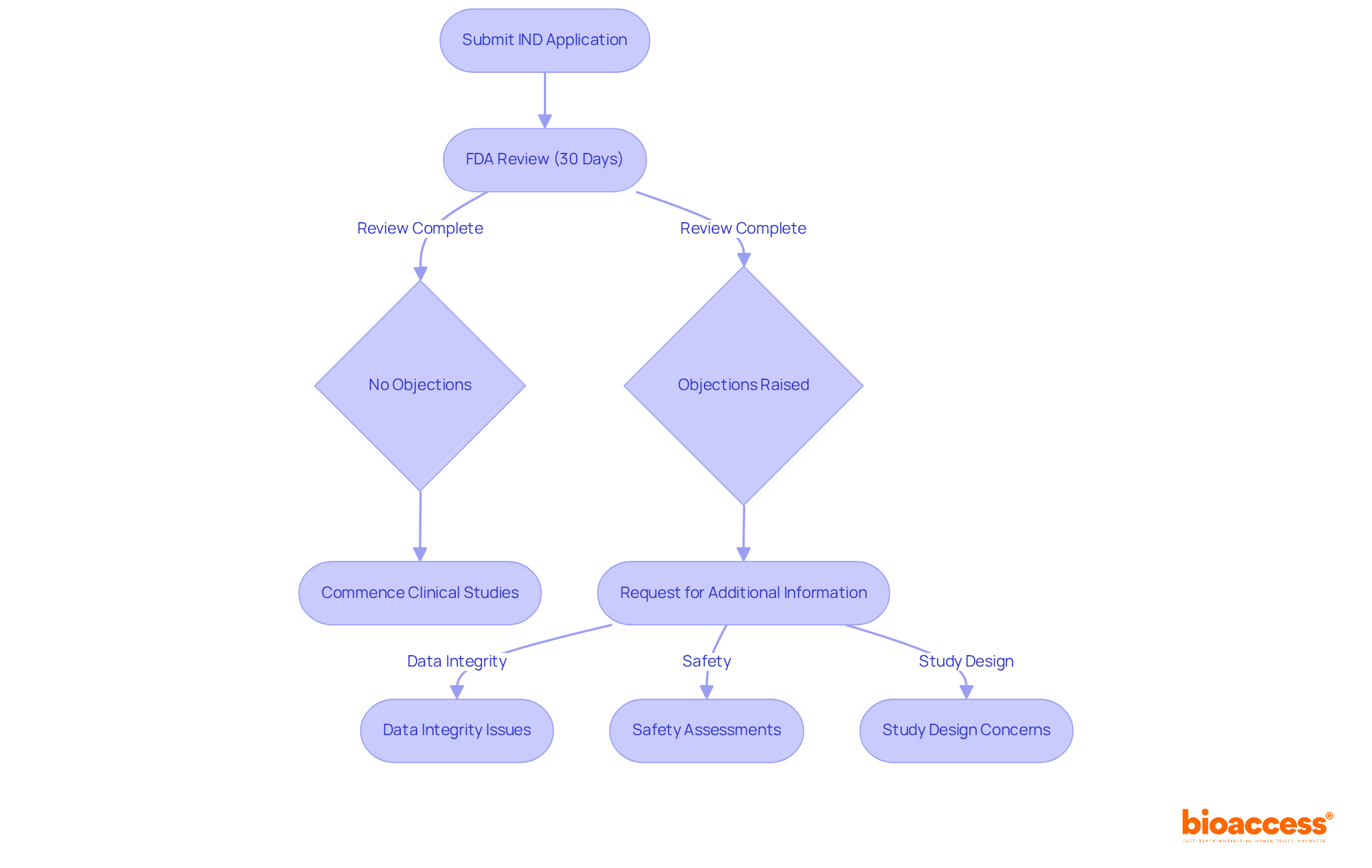
Upon submission of an IND application, the FDA undertakes a thorough review within a 30-day timeframe. This crucial period encompasses the assessment of safety information and the proposed clinical study protocols. If the FDA identifies no objections, the applicant is authorized to commence clinical studies. However, should any concerns arise—such as issues related to data integrity, safety assessments, or study design—the FDA may request additional information or modifications. Historically, the FDA raises an average of several objections during IND reviews, underscoring the necessity for meticulous preparation by sponsors.
Successful IND applications often stem from proactive communication with the FDA, including pre-IND meetings that clarify expectations and address potential issues early on. Bioaccess® enhances this process by providing professional services such as:
This ensures adherence to regulatory standards. As noted by FDA representatives, "Close communication with the FDA during drug development can reduce the likelihood of multiple review cycles." This collaborative approach not only streamlines the evaluation but also increases the chances of a favorable outcome, facilitating prompt progression into clinical studies.
Katherine Ruiz, a specialist in regulatory affairs for medical devices and in vitro diagnostics in Colombia, plays a vital role in guiding clients through this intricate process.
Institutional Review Boards (IRBs) play a crucial role in overseeing clinical trials, ensuring that protocols adhere to ethical standards and protect participant rights. Their approval is not merely a regulatory requirement; it is a fundamental step that can shape the entire research process. Research indicates that 59% of IND submissions gain approval on the initial attempt, underscoring the significance of comprehensive and well-prepared protocols.
The average duration for IRB evaluations of IND submissions typically spans from 26 to 30 days, although this can vary significantly based on the investigator's experience. Less experienced researchers often encounter longer approval times, averaging 97 days, compared to 29 days for their more seasoned counterparts. This discrepancy highlights the pressing need for training and resources to streamline the IRB application procedure.
IRBs not only evaluate the ethical implications of research but also influence the design of clinical trial protocols. Their feedback can lead to modifications that enhance participant safety and ensure compliance with ethical guidelines. As one IRB member noted, the review procedure aims to ensure that the rights and welfare of research subjects are adequately safeguarded.
Successful examples of IRB approvals illustrate the effectiveness of this oversight. For instance, multicenter studies have benefited from centralized IRB procedures, which can reduce redundancy and expedite approvals. However, challenges persist, particularly in managing the complexities of multicenter studies where variations in IRB responses can lead to delays.
In summary, the role of IRBs in the investigational new drug IND application procedure is indispensable. Their commitment to ethical oversight not only protects participants but also enhances the integrity of clinical research, ultimately contributing to the advancement of medical innovations.

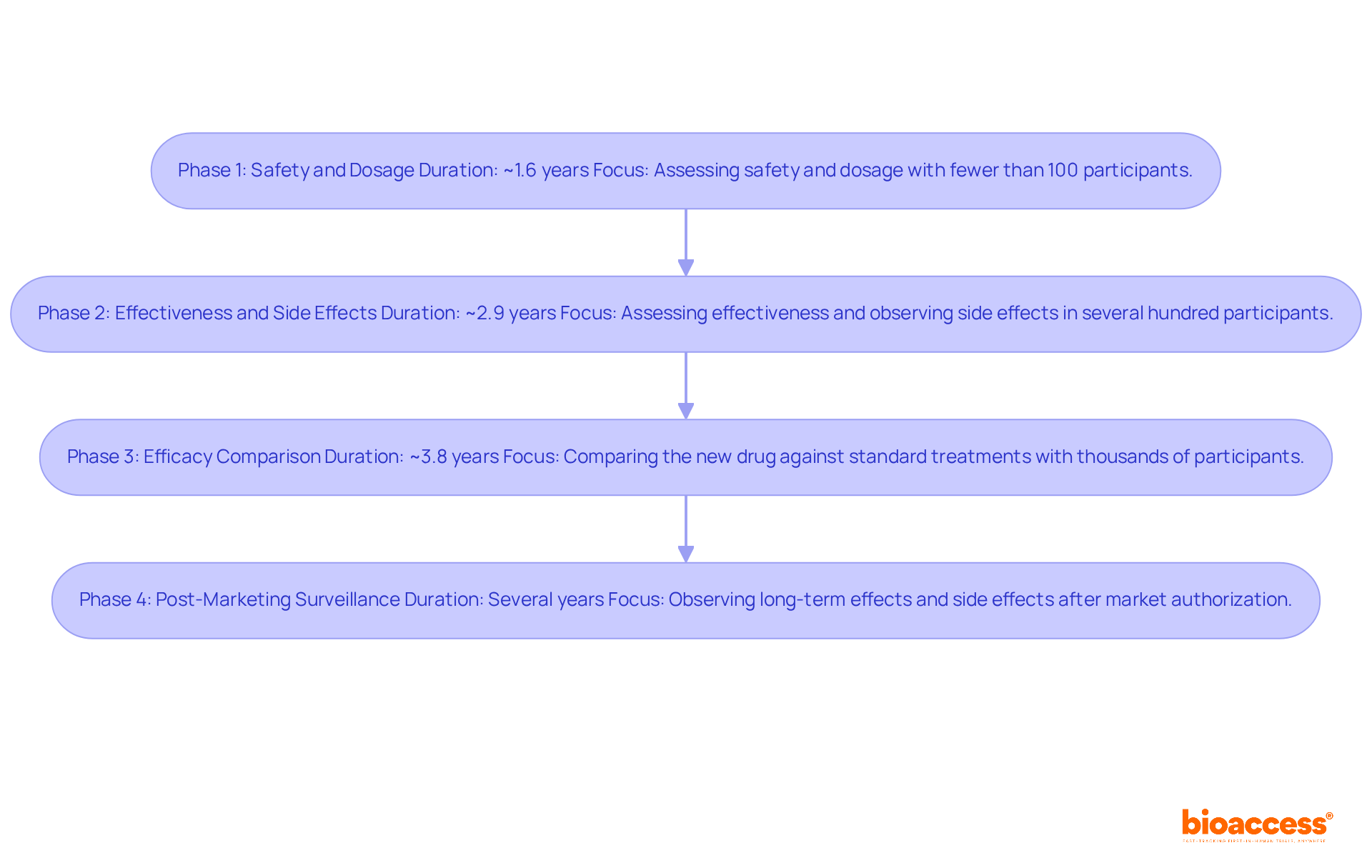
Clinical studies are systematically categorized into four distinct phases, each serving a vital role in the drug development process.
Phase 1: This initial phase primarily focuses on assessing the safety and dosage of the investigational drug. Typically involving fewer than 100 participants, Phase 1 studies aim to identify common side effects and determine the optimal dosing regimen. The average duration for this phase is approximately 1.6 years.
Phase 2: Following the successful conclusion of Phase 1, Phase 2 studies assess the drug's effectiveness and observe side effects in a larger cohort, often consisting of several hundred participants. This phase is crucial for refining research plans and setting goals for subsequent studies, with an average duration of around 2.9 years.
Phase 3: In this pivotal phase, thousands of participants are involved to compare the new drug against standard treatments. Phase 3 studies provide robust information regarding the medication's efficacy and safety, usually requiring approximately 3.8 years to finalize. These tests are crucial for ensuring varied representation among participants, which is vital for the generalization of the results. With bioaccess®, clinical studies can achieve patient enrollment 50% quicker than conventional locations, significantly improving the efficiency of this phase.
Phase 4: After a drug obtains market authorization, Phase 4 studies, or post-marketing surveillance, are conducted to observe long-term effects and side effects that may not have been apparent in earlier phases. This phase can last for several years and is vital for assessing the drug's impact on patients' quality of life and exploring new uses or combinations with other treatments.
Each phase of clinical trials builds upon the previous one, ensuring thorough testing and evaluation of investigational treatments before they reach the market. Understanding these stages is crucial for navigating the IND pathway and achieving successful market entry, particularly when utilizing bioaccess®'s FDA-ready information that can save $25K per patient and enhance the overall workflow.
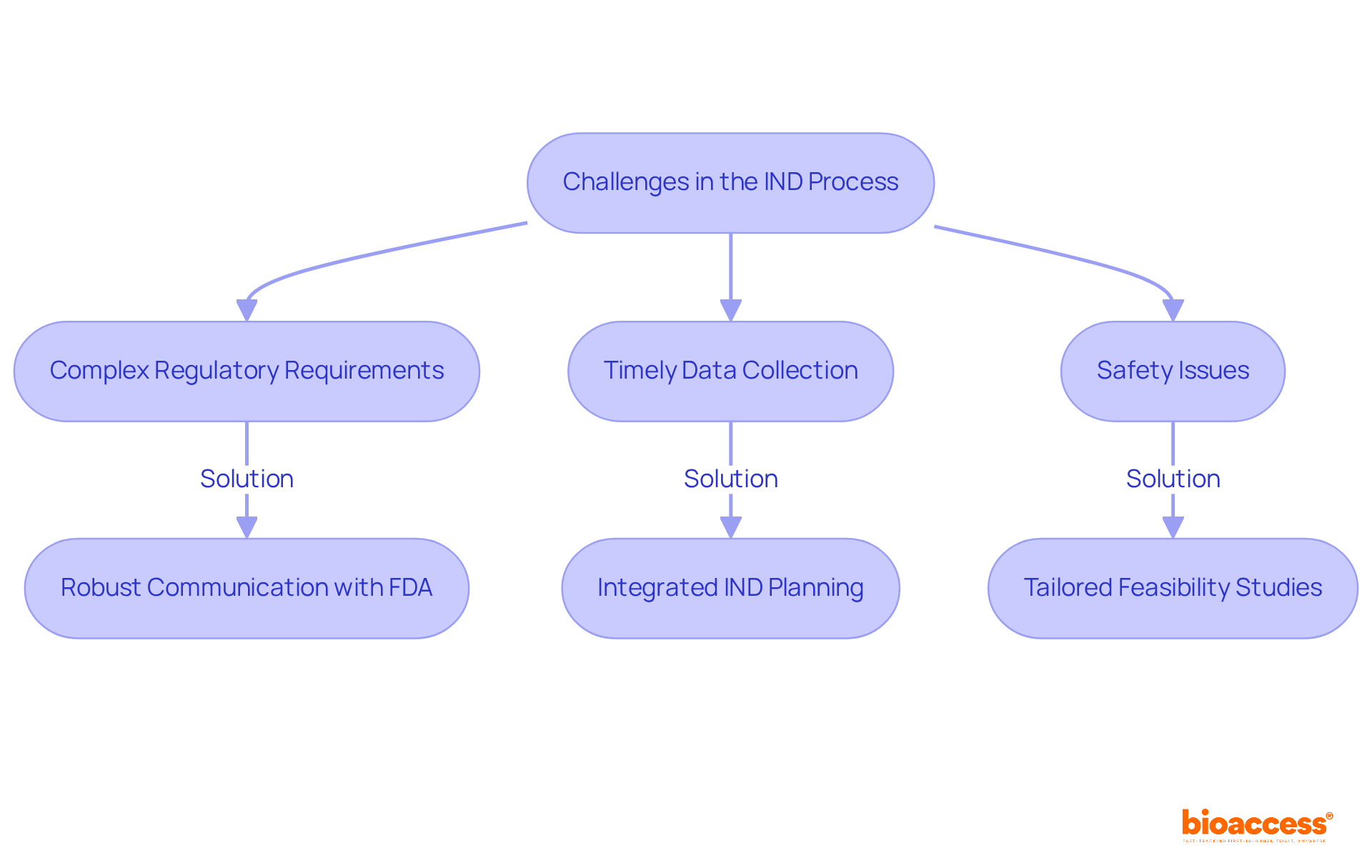
Navigating the investigational new drug IND process presents several challenges, primarily due to complex regulatory requirements and the necessity for timely data collection. A major concern is the safety issues raised during the investigational new drug IND reviews, with statistics indicating that clinical study efficacy and safety data are crucial for regulatory approval, scoring 66.6 on impact assessments. Companies must remain agile, adapting their strategies to address these concerns while ensuring robust communication with the FDA and Institutional Review Boards (IRBs).
Industry leaders emphasize that a tightly integrated approach to investigational new drug IND planning can significantly enhance the likelihood of success. For instance, bioaccess® provides tailored feasibility studies and site selection services that help identify optimal research sites, ensuring compliance with regulatory standards. Additionally, their expertise in managing Chemistry, Manufacturing, and Controls (CMC) challenges is crucial, as failure to meet FDA standards can lead to delays in the investigational new drug IND development timeline.
By fostering open lines of communication and being responsive to feedback, bioaccess® enables companies to navigate these regulatory hurdles more effectively, ultimately facilitating a smoother path to clinical trial initiation.
Understanding the intricacies of the Investigational New Drug (IND) process is essential for Medtech and Biopharma companies aiming to bring innovative therapies to market. This article highlights the critical steps involved in this complex journey, emphasizing the importance of thorough preparation, regulatory compliance, and strategic engagement with authorities such as the FDA and Institutional Review Boards (IRBs). By leveraging platforms like bioaccess®, companies can significantly reduce timelines and costs, paving the way for faster access to groundbreaking treatments.
Key insights from the discussion reveal the significance of:
The various types of IND applications—Commercial, Research, and Expanded Access—each play a vital role in the development landscape, underscoring the necessity for tailored approaches to meet specific regulatory requirements. Moreover, the importance of ongoing communication with regulatory bodies cannot be overstated, as it facilitates smoother reviews and enhances the likelihood of successful submissions.
In conclusion, as the landscape of drug development continues to evolve, staying informed about the IND process and its associated challenges is paramount for any organization involved in medical innovation. Embracing a proactive strategy that prioritizes clarity, thoroughness, and ethical considerations will not only streamline the IND submission process but also ultimately contribute to improved patient outcomes. The future of Medtech innovations hinges on the ability to navigate these complexities effectively, making the insights shared in this article invaluable for all stakeholders in the industry.
What is bioaccess® and what role does it play in IND submissions?
bioaccess® is a company that accelerates Investigational New Drug (IND) submissions by leveraging its expertise in regulatory frameworks and ethical approval processes, particularly in Latin America, mainly Colombia. It significantly reduces the time to market for Medtech innovations, with an average approval timeline of just 4-6 weeks.
How does Colombia benefit companies looking to submit IND applications?
Colombia offers exceptional cost effectiveness, with savings exceeding 30% compared to studies in North America or Western Europe. The regulatory pace in Colombia allows for total IRB/EC and MoH (INVIMA) evaluations to be finalized in approximately 90-120 days.
Why are ethical approvals important in the context of IND submissions?
Ethical approvals are crucial for maintaining public trust and ensuring the safety and efficacy of new medical technologies. They ensure that trials are conducted properly, which is essential for the credibility of the investigational drugs.
What is an Investigational New Drug (IND) application?
An IND application is a request made to the FDA that allows Medtech and Biopharma firms to initiate human clinical studies for new medications. It includes data from preclinical studies, manufacturing details, and proposed clinical study protocols.
What is the success rate for IND submissions in the Medtech sector?
The success rate for IND submissions in the Medtech sector is approximately 12%, meaning that only about 12% of drugs entering clinical trials ultimately secure FDA approval.
What are the three primary categories of IND applications?
The three primary categories of IND applications are Commercial INDs, Research INDs, and Expanded Access INDs. Commercial INDs are for drugs intended for sale, Research INDs are for non-commercial investigational studies, and Expanded Access INDs allow patients access to investigational drugs outside of clinical trials.
What percentage of IND submissions were Commercial INDs in 2025?
In 2025, approximately 70% of IND submissions were Commercial INDs, reflecting the industry's focus on introducing new therapies to the market.
What is the purpose of Expanded Access INDs?
Expanded Access INDs allow patients with serious or life-threatening conditions to access investigational drugs outside of clinical trials, typically when they have exhausted other treatment options.
How does the IND process contribute to public health standards?
The IND process ensures that investigational drugs undergo thorough safety assessments prior to human use, which is vital for upholding public health standards and ensuring that new therapies are rigorously tested and monitored.