


The article titled "10 Key Strategies for Effective Chemistry Manufacturing and Controls" presents essential strategies aimed at enhancing practices in chemistry manufacturing and controls (CMC). It emphasizes the implementation of robust quality control measures, thorough risk assessments, and the adoption of innovative technologies. Collectively, these approaches are designed to improve compliance, product integrity, and operational efficiency within the CMC sector, highlighting their critical role in advancing industry standards.
In an industry where precision and compliance are paramount, the landscape of chemistry manufacturing and controls is in a state of constant evolution. Organizations confront the dual challenge of adhering to stringent regulations while pursuing operational excellence. This article explores ten key strategies that not only enhance compliance but also optimize processes, ensuring that companies remain competitive and innovative. As the pharmaceutical sector adapts to new technologies and methodologies, one critical question emerges: how can stakeholders effectively navigate these complexities to achieve superior outcomes in their manufacturing practices?
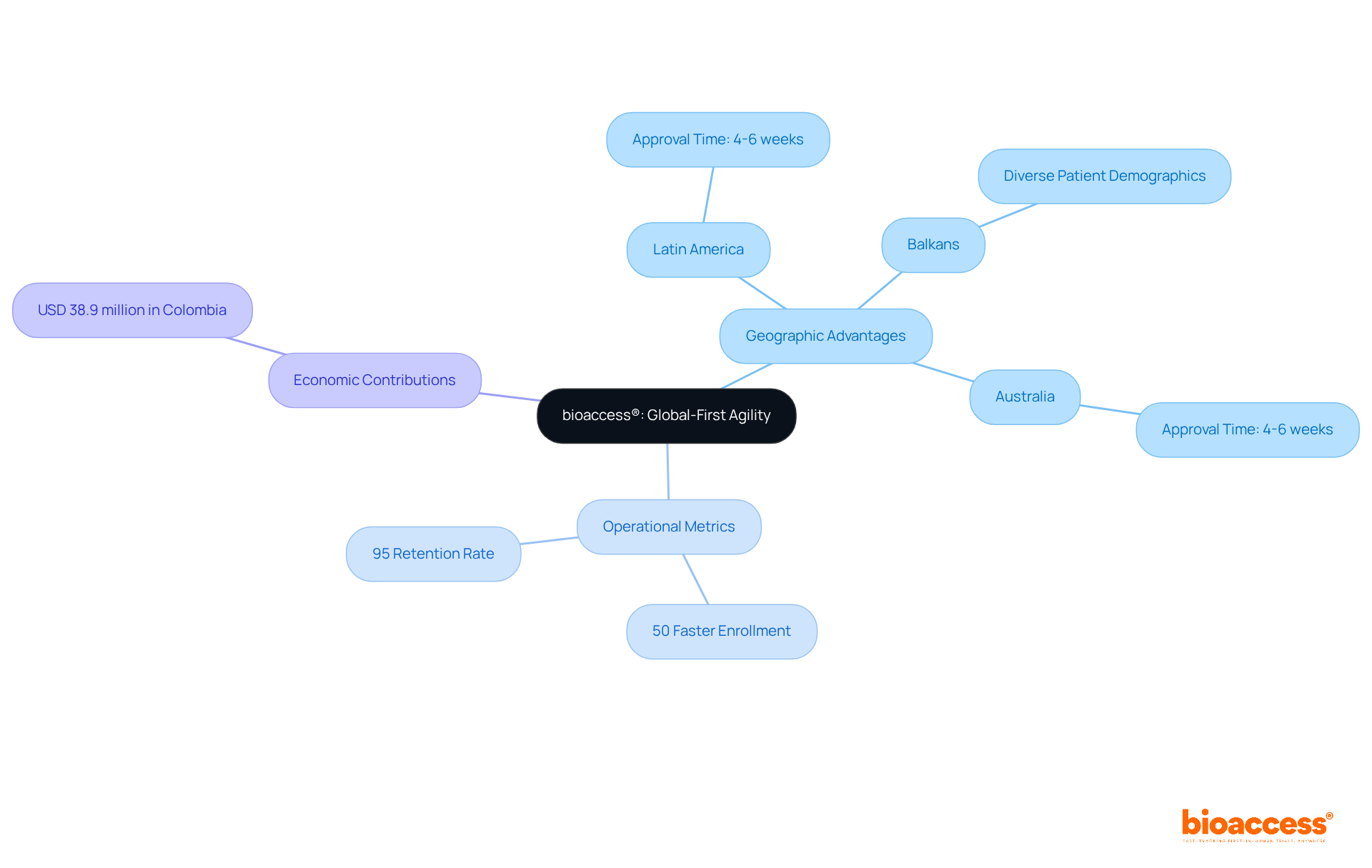
bioaccess® harnesses the regulatory speed of Latin America, the diverse patient demographics of the Balkans, and the streamlined pathways in Australia to secure ethical approvals in an impressive 4 to 6 weeks. This global-first agility empowers Medtech, Biopharma, and Radiopharma innovators to significantly accelerate their clinical research processes, achieving enrollment rates that are 50% faster than traditional markets.
Avantec Vascular's first-in-human study in Latin America exemplifies this, demonstrating a notable increase in patient enrollment for subsequent phases. This underscores the effectiveness of bioaccess®'s approach. By leveraging these strategic advantages, bioaccess® positions itself as a leader in early-phase clinical research, ensuring that groundbreaking medical solutions reach the market swiftly and efficiently, ultimately enhancing patient care and outcomes.
The entity also boasts a 95% retention rate in clinical studies, further demonstrating its commitment to participant engagement. Notably, clinical research in Colombia contributed USD 38.9 million to the economy in 2023, highlighting the significance of bioaccess®'s operations.
To achieve compliance with the latest guidelines in chemistry manufacturing and controls, entities must remain vigilant regarding updates from governing bodies such as the FDA. Conducting thorough gap analyses is essential for identifying discrepancies between existing practices and regulatory standards. Studies indicate that effective gap analysis can significantly enhance compliance outcomes; entities that engage in rigorous gap analyses report a 50% improvement in compliance rates.
Furthermore, implementing a robust Quality Management System (QMS) that encompasses all aspects of manufacturing—from raw material sourcing to final product testing—is critical for upholding high standards. Best practices include:
These practices not only reinforce compliance but also foster a culture of accountability and continuous improvement within the organization. As noted by Bermingham, Castleman & Pierce Inc., 'Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction, and skillful execution.'
This holistic approach ensures that quality management transcends mere checklists, becoming a fundamental aspect of the organizational culture, ultimately leading to enhanced compliance and superior product quality.

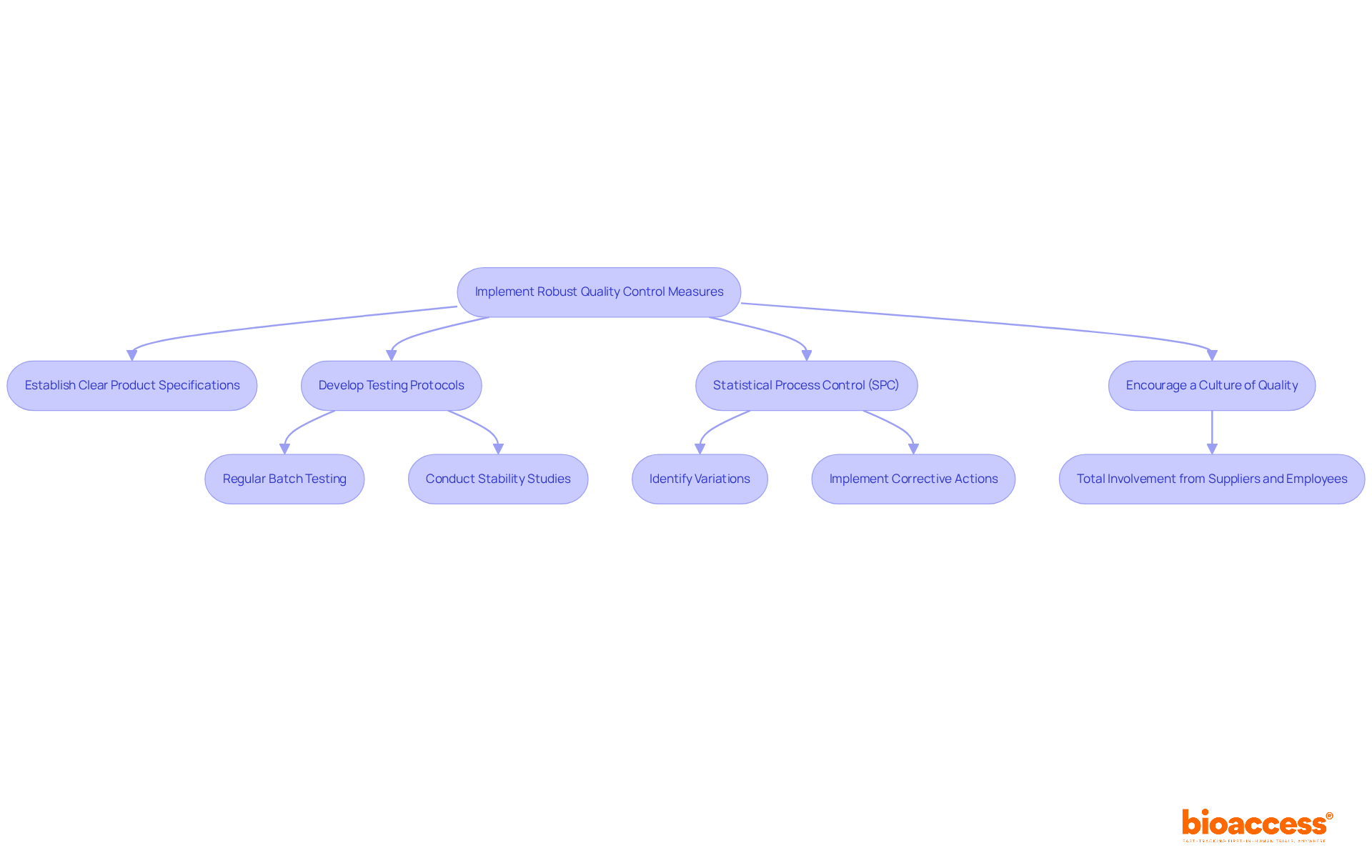
Implementing robust quality control measures is essential for maintaining product integrity in chemistry manufacturing and controls. Establishing clear product specifications and testing protocols serves as a foundation. Regular batch testing and stability studies are critical components of a comprehensive quality control strategy, ensuring that products meet regulatory standards and consumer expectations.
Furthermore, statistical process control (SPC) techniques play a vital role by identifying variations in manufacturing, enabling timely corrective actions to prevent defects. For instance, entities that have adopted SPC have reported a 37% reduction in defect rates within six months of implementation in an automotive plant, showcasing the effectiveness of these techniques. Additionally, a packaging industry client reported annual savings of $1.2 million attributed to their SPC program, highlighting the financial benefits of quality control measures.
Encouraging a culture of quality within the establishment is equally important; as Dossenbach emphasizes, 'Get total involvement and cooperation from suppliers and employees.' When all employees understand their role in maintaining product integrity, it enhances compliance and operational efficiency. This approach not only protects product integrity but also adheres to the changing compliance landscape in chemistry manufacturing and controls, ensuring that companies remain competitive in the global market.
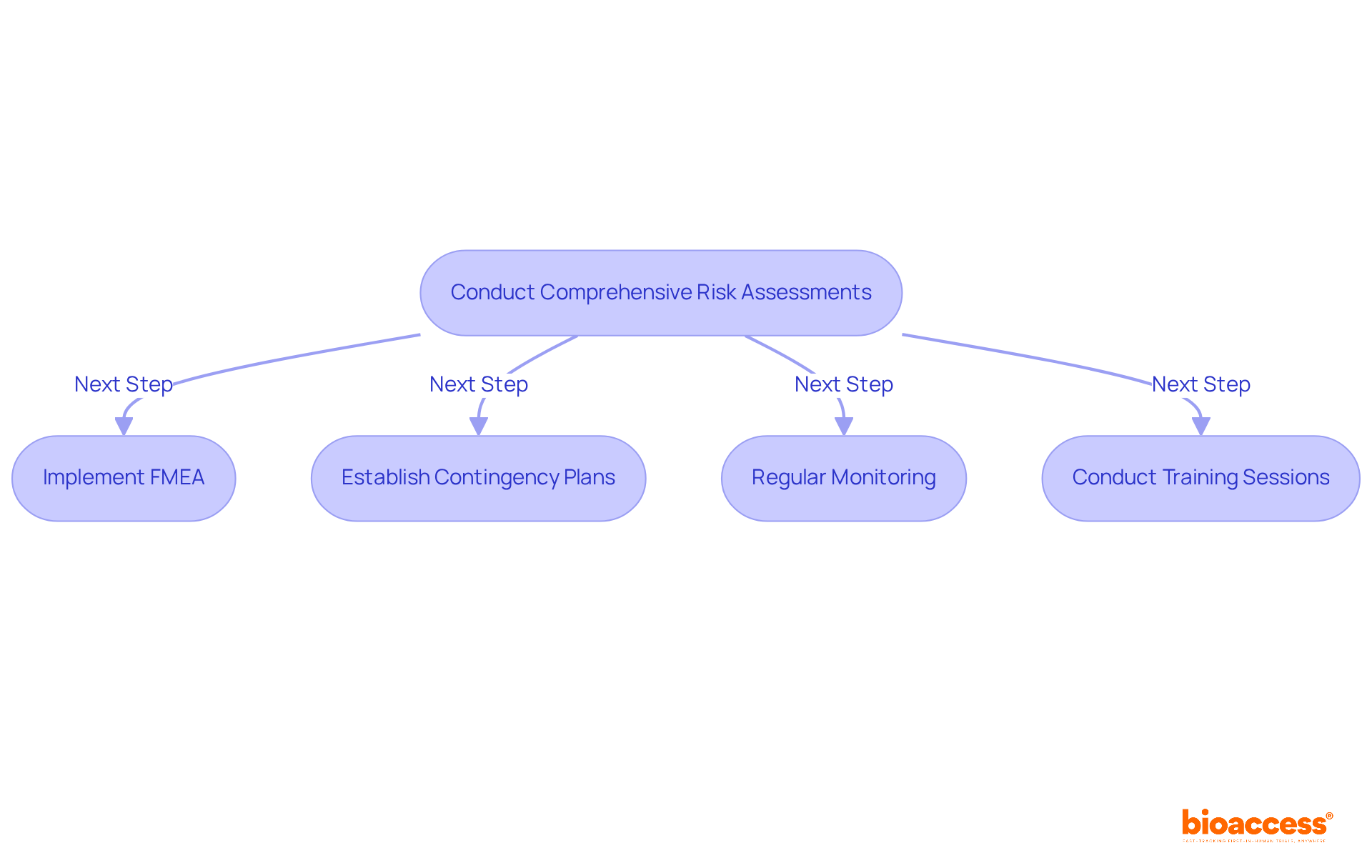
To formulate effective risk management strategies in chemistry manufacturing and controls, entities must conduct comprehensive risk assessments to identify potential hazards throughout the manufacturing process. Implementing Failure Mode and Effects Analysis (FMEA) is a critical tool in this endeavor, enabling teams to prioritize risks based on their potential impact and likelihood of occurrence. As Allison Dunn aptly states, "the biggest risks in business aren’t the ones you see—they’re the ones you overlook," highlighting the necessity of proactive risk management.
For instance, a recent case study illustrates how a pharmaceutical manufacturer employed FMEA to pinpoint critical failure points in their production line, resulting in a 30% reduction in defects and enhanced compliance with regulatory standards. Furthermore, a regional manufacturing firm achieved a 12% restoration in profitability after addressing outdated practices, showcasing the tangible benefits of effective risk management.
Establishing contingency plans is equally vital. By preparing for various risk scenarios, companies can respond swiftly to emerging issues, thereby minimizing disruptions. Regular monitoring of risk factors ensures that any changes in the manufacturing environment are promptly addressed. Expert opinions underscore that integrating FMEA into the chemistry manufacturing and controls framework not only enhances the effectiveness of risk assessments but also promotes a culture of continuous improvement. This proactive approach is essential for maintaining compliance and ensuring the safety and efficacy of products within the highly regulated biopharma landscape.
To implement FMEA effectively, organizations should consider conducting regular training sessions for their teams, ensuring that everyone comprehends the methodology and its significance in risk management.
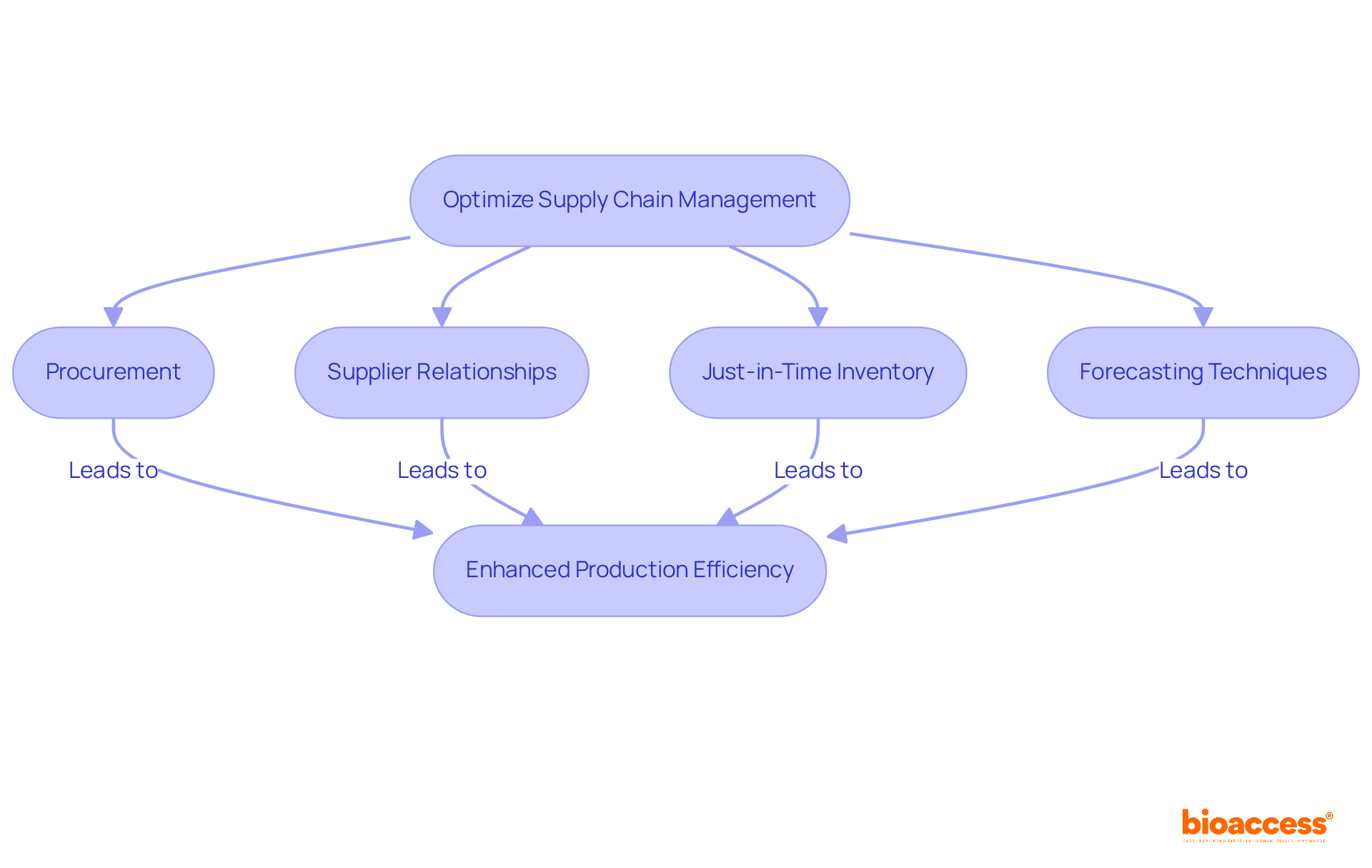
Efficient supply chain management is paramount for enhancing operations from procurement to production. Establishing robust relationships with suppliers and adopting just-in-time (JIT) inventory practices are crucial strategies that significantly reduce lead times and minimize excess stock.
For instance, in pharmaceutical production, JIT practices ensure that materials arrive precisely when needed, thereby enhancing workflow and reducing storage costs.
Experts emphasize the integration of advanced forecasting techniques and data analytics into demand planning, further aligning production schedules with market needs and improving responsiveness.
Regular reviews and adjustments of supply chain strategies based on key performance metrics are essential for maintaining efficiency and adapting to fluctuations in demand.
This proactive approach not only streamlines operations but also enhances overall production efficiency, positioning companies to meet customer expectations effectively.
Embracing ongoing improvement is essential for enhancing operational efficiency in manufacturing. Techniques such as Lean Manufacturing and Six Sigma play a pivotal role in identifying and eliminating waste and variability in production processes. Waste, as noted, is worse than loss; it signifies a squandering of resources that could have been utilized effectively.
By fostering a culture that encourages employee feedback, companies can gain valuable insights that drive meaningful enhancements. Continuous improvement can lead to being twice as good in just 70 days by improving 1% every day. A structured approach to continuous improvement not only ensures agility in responding to industry changes but also propels organizations toward operational excellence.
Companies that implement these methodologies can achieve significant reductions in processing times and costs. For instance, if batch sizes are reduced by half, the time to process a batch is also reduced by half, ultimately leading to faster turnaround and improved product quality.
As W. Edwards Deming stated, 'It is not enough to do your best; you must know what to do and then do your best.
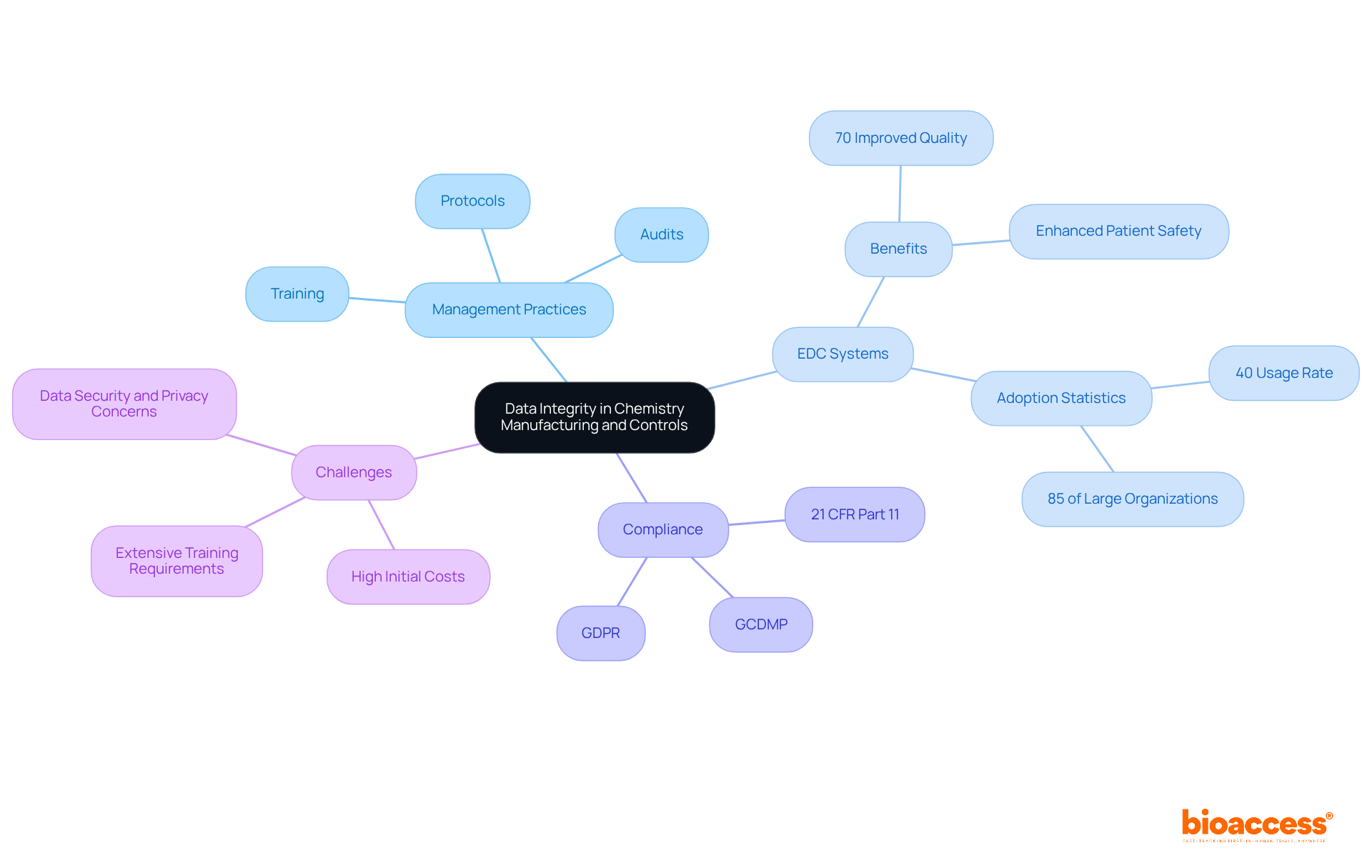
Maintaining information integrity in chemistry manufacturing and controls is paramount and necessitates the adoption of stringent management practices that ensure the accuracy and reliability of details. Establishing clear protocols for information entry, storage, and retrieval is essential. Additionally, conducting regular audits to identify and rectify discrepancies is crucial.
Electronic Data Capture (EDC) systems play a pivotal role in enhancing information accuracy; studies indicate that 70% of EDC users report improved quality compared to traditional methods. Furthermore, more than 40% of research institutions in the U.S. are utilizing EDC systems for data management, underscoring their widespread adoption and relevance in the current landscape. These systems facilitate adherence to legal requirements, such as 21 CFR Part 11, which governs electronic records and signatures.
However, EDC software providers face substantial challenges in meeting these compliance requirements, highlighting the complexity of the oversight landscape. Training personnel on the importance of information integrity and the consequences of non-compliance fosters a culture of responsibility and diligence in information management.
As the pharmaceutical landscape evolves, particularly in 2025, the significance of robust information management practices in chemistry manufacturing and controls will only intensify, emphasizing the necessity for entities to implement advanced EDC solutions to optimize their processes and ensure regulatory compliance.
As noted by Abu Saleh Mohammad Mosa, 'the EDC system allows the CTR researchers to collect, store, and manage research data electronically in a secure and HIPAA-compliant central research data repository.' Furthermore, entities must consider potential challenges associated with EDC systems, such as high initial costs and the requirement for extensive training, to ensure successful implementation.
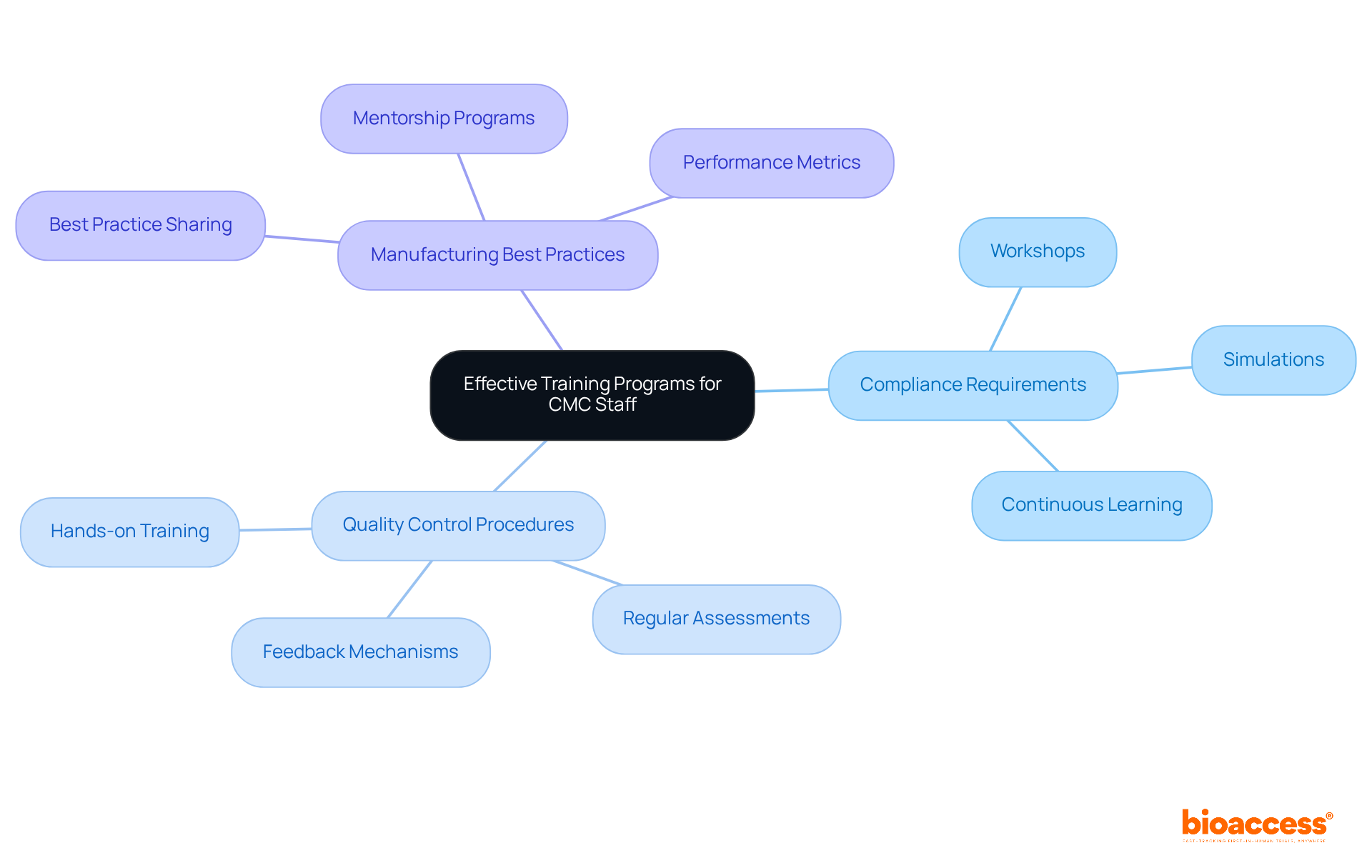
To execute effective training programs for chemistry manufacturing and controls personnel, organizations must develop comprehensive curricula that encompass:
Regular training sessions, workshops, and simulations are vital for enhancing staff competency and equipping them for real-world challenges. For example, incorporating hands-on simulations provides practical experience, enabling employees to apply theoretical knowledge in a controlled environment.
Furthermore, fostering a culture of continuous learning and professional development is essential. This approach ensures that employees stay updated on industry trends and regulatory changes in chemistry manufacturing and controls, which is crucial for maintaining compliance and achieving operational excellence.
By prioritizing these training strategies, organizations can significantly enhance their workforce's effectiveness and adaptability in the ever-evolving pharmaceutical landscape.
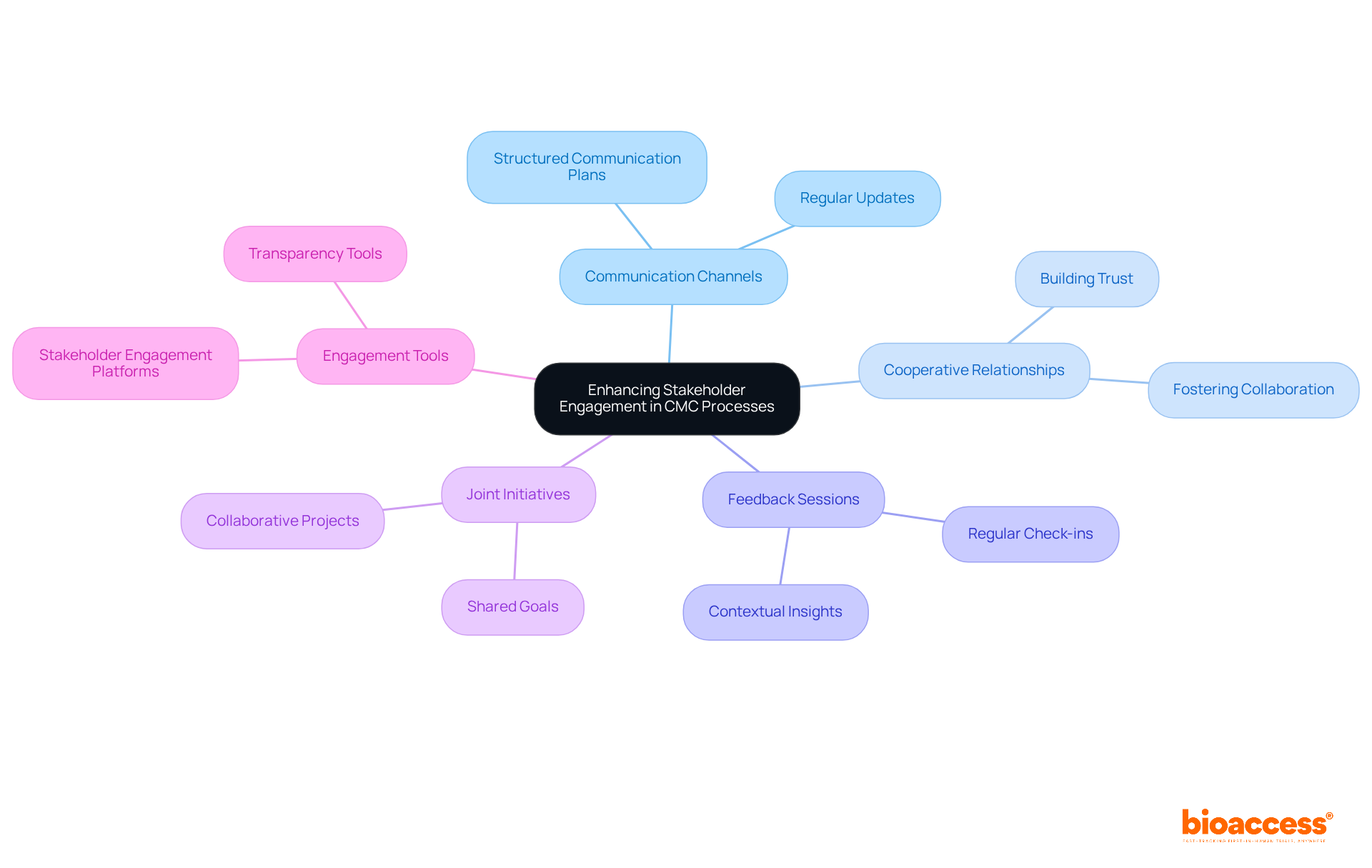
Enhancing stakeholder involvement in chemistry manufacturing and controls activities is imperative. This necessitates the establishment of clear communication channels and the cultivation of cooperative relationships among all parties, including suppliers, regulatory bodies, and internal teams. Regular updates and feedback sessions play a critical role in reinforcing partnerships and ensuring alignment with project objectives.
Furthermore, joint initiatives can significantly strengthen these relationships, fostering a culture of collaboration that is essential for success. Employing stakeholder engagement tools and platforms not only boosts transparency but also simplifies decision-making processes, ultimately leading to improved outcomes in chemistry manufacturing and controls.
For example, the implementation of structured communication plans has demonstrated a marked enhancement in project execution and stakeholder alignment, as evidenced by successful case studies within the pharmaceutical sector. By prioritizing effective communication and collaboration, organizations can navigate the complexities of chemistry manufacturing and controls with greater efficiency, ensuring that all stakeholders remain informed and engaged throughout the project lifecycle.
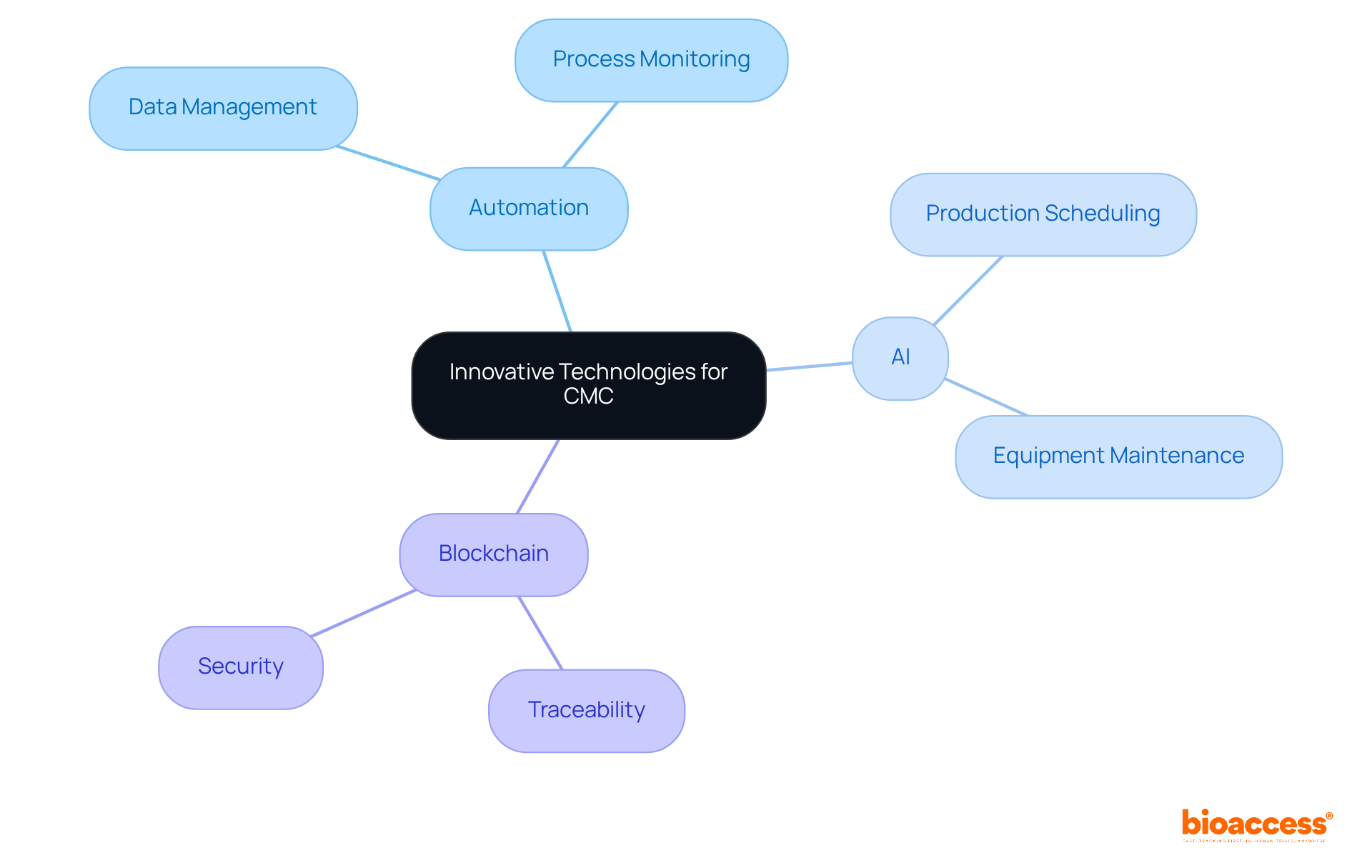
Adopting innovative technologies is essential for optimizing chemistry manufacturing and controls operations. Automation and artificial intelligence (AI) are at the forefront of this transformation, enabling organizations to enhance data management, improve process monitoring, and facilitate real-time decision-making.
For instance, AI-driven analytics can optimize production schedules and predict equipment maintenance needs, which significantly reduces downtime and operational costs. Furthermore, the integration of blockchain technology enhances traceability and security within the supply chain, ensuring compliance with regulatory standards.
By embracing these advancements, organizations can not only improve operational efficiency but also drive superior outcomes in chemistry manufacturing and controls (CMC), thereby positioning themselves competitively in the pharmaceutical manufacturing landscape.
The landscape of chemistry manufacturing and controls is rapidly evolving, making the implementation of effective strategies essential for success. By concentrating on critical areas such as:
organizations can significantly enhance their operational efficiency and sustain a competitive edge in the market.
Throughout this article, various strategies have been underscored, including the necessity of thorough gap analyses to ensure compliance with regulatory standards, the establishment of robust quality control measures to maintain product integrity, and the adoption of effective risk management practices to mitigate potential hazards. Furthermore, embracing advanced technologies and cultivating a culture of continuous improvement can greatly streamline processes and elevate overall productivity.
As the industry continues to navigate challenges and transformations, it is imperative for organizations to proactively adopt these strategies. By prioritizing compliance, quality, and innovation, stakeholders can ensure that their manufacturing processes not only meet current standards but also adapt to future demands. Embracing these best practices will ultimately lead to improved patient outcomes and a more efficient pharmaceutical landscape.
What is bioaccess® and how does it benefit clinical research?
bioaccess® is a platform that accelerates clinical research by leveraging regulatory speed in Latin America, diverse patient demographics in the Balkans, and streamlined pathways in Australia. It enables ethical approvals in 4 to 6 weeks, allowing Medtech, Biopharma, and Radiopharma innovators to achieve enrollment rates that are 50% faster than traditional markets.
Can you provide an example of bioaccess®'s effectiveness?
Avantec Vascular's first-in-human study in Latin America is an example of bioaccess®'s effectiveness, as it demonstrated a significant increase in patient enrollment for subsequent phases of the study.
What is the retention rate for clinical studies conducted through bioaccess®?
bioaccess® boasts a 95% retention rate in clinical studies, showcasing its commitment to participant engagement.
How did clinical research in Colombia impact the economy?
Clinical research in Colombia contributed USD 38.9 million to the economy in 2023, highlighting the significance of bioaccess®'s operations in the region.
What is essential for achieving compliance in chemistry manufacturing and controls?
To achieve compliance, entities must stay updated with guidelines from governing bodies like the FDA and conduct thorough gap analyses to identify discrepancies between existing practices and regulatory standards.
What are the benefits of conducting gap analyses?
Engaging in rigorous gap analyses can significantly enhance compliance outcomes, with studies indicating a 50% improvement in compliance rates for entities that implement them.
What practices are recommended for maintaining a robust Quality Management System (QMS)?
Best practices for a robust QMS include regular audits and comprehensive training sessions for staff, which help reinforce compliance and foster a culture of accountability and continuous improvement.
Why is it important to implement robust quality control measures in chemistry manufacturing?
Robust quality control measures are essential for maintaining product integrity, ensuring that products meet regulatory standards and consumer expectations.
What role do statistical process control (SPC) techniques play in quality control?
SPC techniques help identify variations in manufacturing, enabling timely corrective actions to prevent defects. Entities that adopt SPC have reported significant reductions in defect rates and financial savings.
How can companies foster a culture of quality within their organization?
Encouraging total involvement and cooperation from suppliers and employees is crucial. When all employees understand their role in maintaining product integrity, it enhances compliance and operational efficiency.