


The primary objective of the article "10 Key Strategies for Effective Medical Device Marketing" is to delineate crucial strategies that can significantly bolster the marketing endeavors of medical device companies. It underscores the necessity of comprehending target audiences, harnessing digital marketing, and employing data analytics, among other pivotal strategies, to formulate impactful promotional campaigns that resonate with healthcare professionals and enhance market success.
In the competitive realm of medical device marketing, the stakes are higher than ever. Companies strive to launch innovative products while navigating complex regulatory landscapes. This article delves into ten key strategies that can empower marketers to enhance their effectiveness and drive successful product launches. However, with rapid advancements in technology and evolving consumer expectations, how can organizations ensure their marketing approaches remain relevant and impactful? Exploring these strategies not only reveals best practices but also addresses the pressing challenges that medical device firms face in today's dynamic environment.
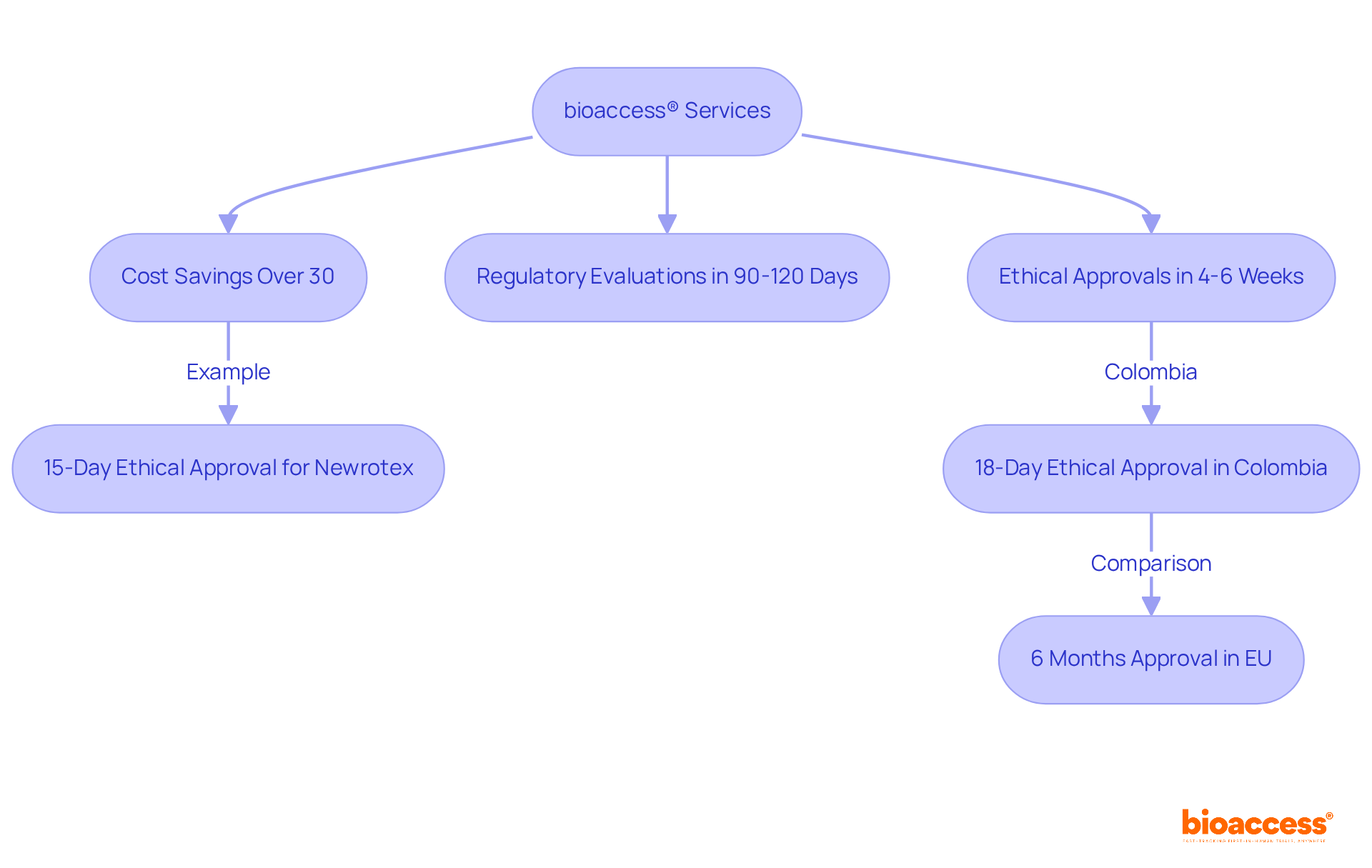
bioaccess® delivers exceptional clinical research services that empower medical device marketing firms to significantly expedite their launch schedules. By capitalizing on Colombia's competitive advantages—such as cost savings exceeding 30% compared to North America and Western Europe, regulatory evaluations completed in just 90-120 days, and a high-quality medical system recognized among the best globally—bioaccess® achieves ethical approvals in an impressive 4 to 6 weeks. This swift process not only allows companies to hasten their product launches in medical device marketing but also enhances their competitive advantage, enabling timely promotional campaigns that effectively engage healthcare professionals and stakeholders.
For example, a recent partnership with Newrotex resulted in an extraordinary 15-day ethical approval for a nerve regeneration technology trial in Panama, demonstrating how rapid approvals can facilitate quicker market entry. Furthermore, Colombia has attained an 18-day ethical approval for trials, in stark contrast to the six months typically required in the EU, underscoring the regulatory efficiency of the region.
With over 159 regulatory submissions completed for more than 75 medical equipment trials, bioaccess® has established itself as a leader in accelerating clinical research and advancing medical device marketing strategies for its clients. Industry leaders assert that rapid clinical research is vital for maintaining relevance in a swiftly evolving market. As Adrian Ebner, a pioneer in MedTech clinical research, articulates, 'The speed of clinical trials can make or break a product's success in the market.'
To leverage these advantages, medical equipment firms should consider partnering with bioaccess® to navigate the complexities of clinical research and enhance their medical device marketing efforts.
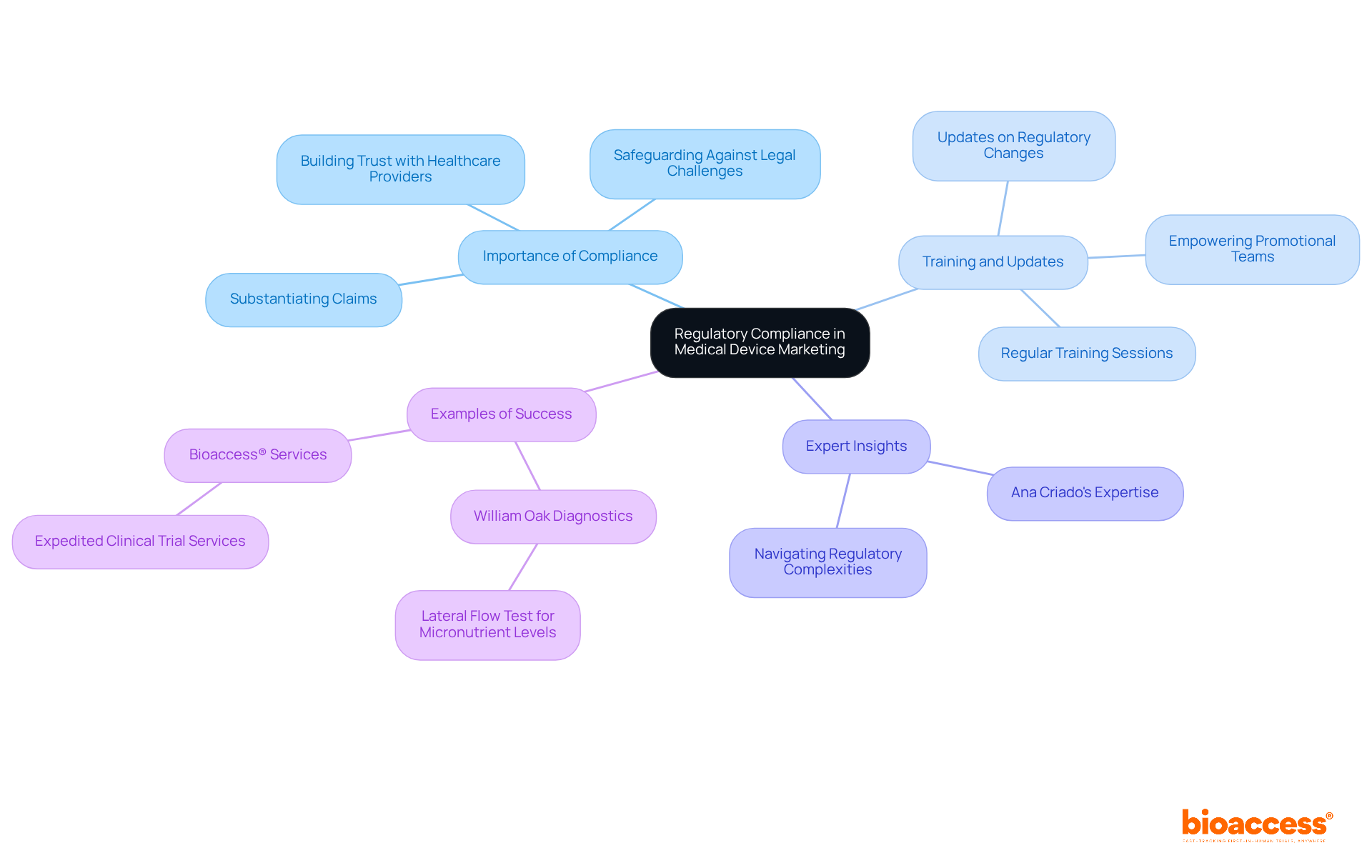
Navigating the intricate landscape of regulatory compliance is essential for successful medical device marketing. Marketers in medical device marketing must be well-versed in the specific regulations governing their products, ensuring that all claims are substantiated and adhere to compliance standards. This understanding safeguards the company against legal challenges and fosters trust among healthcare providers and patients.
Regular training sessions and updates on regulatory changes empower promotional teams in medical device marketing to create compliant messaging that resonates effectively with their target audience. Timeliness is critical for life sciences companies providing life-saving products, making compliance even more urgent.
Experts like Ana Criado, Director of Regulatory Affairs at bioaccess, offer invaluable insights with her extensive background in biomedical engineering and regulatory affairs, ensuring that companies can navigate these complexities effectively.
Bioaccess® provides expedited clinical trial services, linking cutting-edge Medtech, Biopharma, and Radiopharma startups with highly regarded clinical research locations, greatly enhancing success in the Medtech field.
Firms that have effectively managed these challenges, such as William Oak Diagnostics with their lateral flow test for micronutrient levels, demonstrate that a dedication to compliance in medical device marketing can significantly enhance promotional success.
By adopting a comprehensive approach to quality management and dismantling barriers, organizations can enhance their promotional methods while ensuring compliance with regulatory standards.
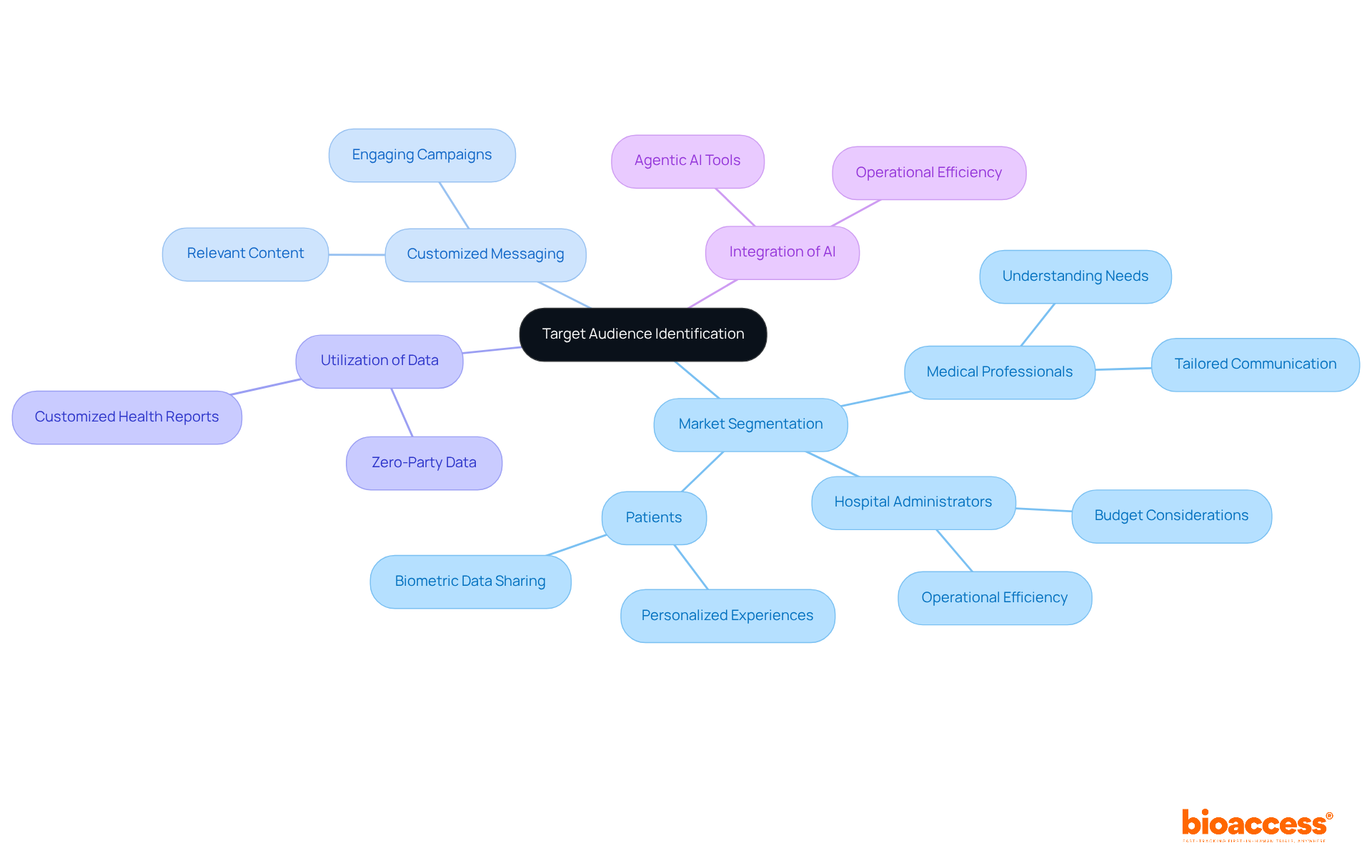
To enhance the effectiveness of promotional strategies in medical device marketing, it is essential to identify and understand target audiences within the medical field. This process involves segmenting the market based on demographics, needs, and behaviors. By customizing marketing messages for specific groups—such as medical professionals, hospital administrators, or patients—companies can create more relevant and engaging campaigns in medical device marketing.
For instance, with over half of Americans owning wearable health devices, there is a growing expectation for personalized experiences driven by biometric data. Consumers are willing to share their metrics and can be motivated by an immediate benefit in return, allowing brands in the wellness sector to leverage zero-party data. This enables them to provide customized health reports that enhance the doctor-patient relationship and facilitate earlier diagnoses.
Employing tools such as surveys and focus groups can generate valuable insights into audience preferences, enabling marketers to enhance their approaches efficiently. Moreover, the integration of agentic AI is becoming increasingly vital, as it enables brands to autonomously complete tasks such as targeting and delivering content, thereby optimizing operational efficiency.
As noted by Maya Avrasin, Group Director of Engagement Strategy at AREA 23, "Integrating and using AI is no longer optional." This underscores the necessity of AI for enhancing promotional efforts in medical device marketing. By adopting these customized promotional strategies, organizations can foster stronger connections with their audiences and enhance overall engagement.
In today's digital era, effectively connecting with medical professionals through online platforms is not just beneficial but essential. Statistics reveal that 73% of Americans obtain health information from the internet, underscoring the critical need for a robust online presence. Moreover, 92% of healthcare marketers in the U.S. utilize Instagram, recognizing its potential for high engagement, especially when leveraging educational content. By creating informative resources—such as webinars and blogs—companies can establish themselves as thought leaders in the industry, fostering both trust and credibility.
Furthermore, optimizing websites for search engines is crucial; search engines attract three times more visitors to hospital sites compared to non-search methods, ensuring that prospective clients can easily find relevant information about medical products. The typical open rate for healthcare-related email campaigns stands at 41%, highlighting the effectiveness of email promotions in this context. By implementing these strategies, companies can significantly enhance their outreach and influence within the competitive medical equipment market.

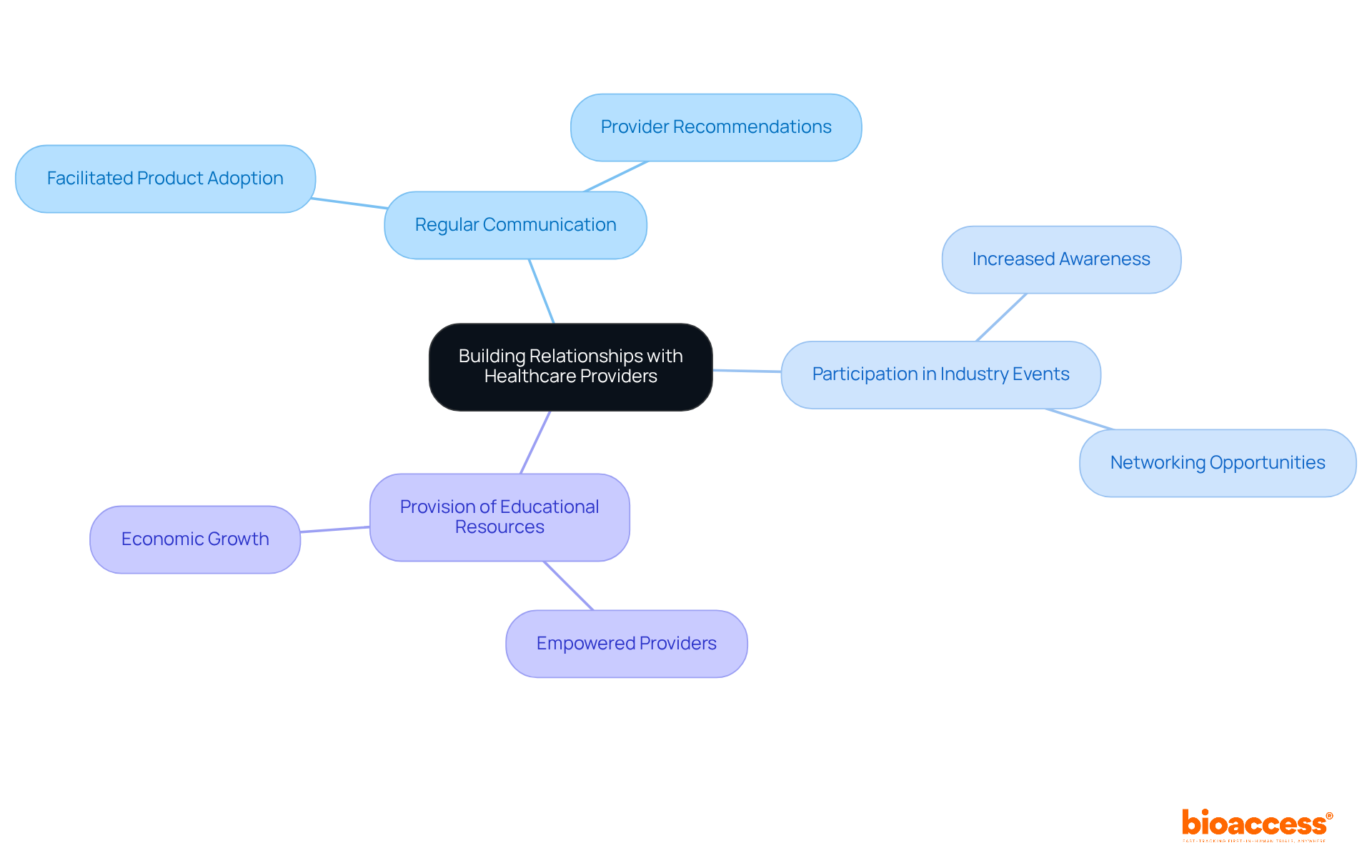
Building strong connections with medical professionals is essential for effective medical device marketing of medical equipment. Engaging clinicians and decision-makers allows companies to gather crucial insights into their needs and preferences. Regular communication, participation in industry events, and the provision of educational resources are key strategies for fostering trust and collaboration.
These relationships not only facilitate the adoption of products but also empower medical providers to promote the devices within their professional networks. Effective partnerships, particularly in the aesthetic and surgical sectors, illustrate how trust can significantly influence provider recommendations.
Industry leaders emphasize that trust is fundamental in medical device marketing, as it directly impacts the willingness of providers to support and utilize new medical technologies. Furthermore, by participating in clinical studies, companies like bioaccess® not only drive innovation and regulatory excellence but also contribute to job creation and economic growth within local economies.
This global partnership enhances the overall medical landscape, showcasing the transformative impact of advanced Medtech in improving health outcomes.
Effective communication techniques are essential for clearly expressing the advantages of medical device marketing. Marketers must prioritize crafting clear and concise messages that emphasize the unique value propositions of their products. Storytelling techniques significantly enhance relatability and memorability, making the benefits resonate with healthcare professionals. For instance, stories that demonstrate real-world uses of the tool can create emotional connections and build trust.
Incorporating data and testimonials further strengthens the message, providing tangible evidence of efficacy and safety. Statistics indicate that content with visuals can boost readership rates by 80%, making it crucial to include compelling graphics or infographics that highlight key benefits. Furthermore, utilizing quotes from communication experts reinforces the significance of storytelling in medical marketing, as they frequently assert that captivating narratives can convert complicated information into understandable insights.
When explaining the advantages of medical equipment to healthcare professionals, it is essential to consider their specific needs and concerns. Customizing messages to illustrate how the tool enhances patient outcomes or boosts operational efficiency can greatly influence decision-making. By merging clear communication with effective storytelling in medical device marketing, marketers can create a persuasive narrative that not only informs but also inspires confidence in their medical devices.
Employing data analytics is essential for making informed promotional decisions. By analyzing market trends, customer behavior, and campaign performance, companies can pinpoint what works and what doesn't. This data-oriented approach not only enhances promotional strategies but also ensures efficient allocation of resources. Furthermore, implementing tools to monitor key performance indicators (KPIs) provides ongoing insights, empowering marketers to adapt their strategies in real-time.
Investing in ongoing education for promotional teams is crucial for maintaining a competitive edge in medical device marketing. Regular training sessions, workshops, and access to industry publications empower teams to stay abreast of the latest trends, technologies, and regulatory changes, including the oversight provided by INVIMA, Colombia's National Food and Drug Surveillance Institute. Understanding INVIMA's function in examining and overseeing health products, along with its designation as a Level 4 health authority by PAHO/WHO, is essential for effective promotional approaches.
Furthermore, acknowledging the influence of Medtech clinical studies on local economies—such as job creation and healthcare enhancement—further improves promotional effectiveness. This proactive approach not only enables swift adaptation of strategies in a rapidly evolving landscape but also fosters improved collaboration and innovation within teams. Industry leaders stress that a knowledgeable promotional team can significantly affect product positioning and market penetration, ultimately driving growth and success in medical device marketing within the Medtech sector.
As the medical field continues to evolve with advancements in AI and digital solutions, the need for ongoing education becomes even more critical. This ensures that professionals in promotion can effectively navigate these changes and leverage new opportunities.
Establishing robust feedback systems is crucial for enhancing promotional plans through stakeholder contributions. Actively seeking input from healthcare providers, patients, and internal teams yields valuable insights that can significantly elevate the effectiveness of promotional efforts. For instance, businesses that actively involve stakeholders are 50% more likely to achieve their objectives, while those that engage stakeholders see a 30% greater success rate with new products. This underscores the importance of integrating diverse perspectives into planning.
Feedback not only informs adjustments to messaging and targeting in medical device marketing but also shapes the overall promotional strategy, ensuring that medical device marketing campaigns resonate with the intended audience. A biotech firm that incorporated stakeholder feedback into its product development experienced a 30% higher adoption rate, illustrating the tangible benefits of this practice.
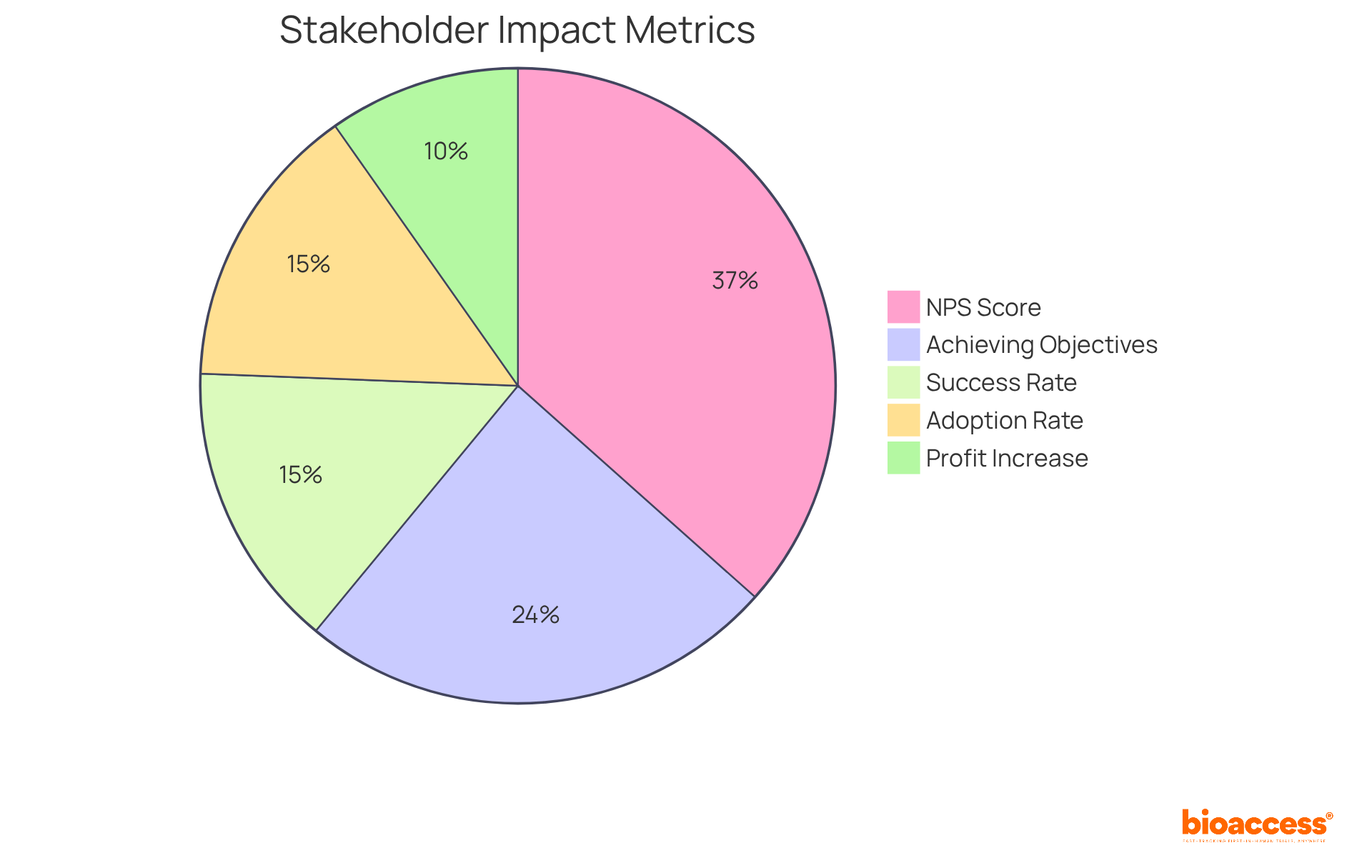
Moreover, organizations that prioritize stakeholder involvement witness a 20% increase in profits, highlighting the economic advantages of refining approaches based on stakeholder insights. The average Net Promoter Score (NPS) for engaged stakeholders stands at 75, reflecting high satisfaction and loyalty. By fostering an environment where feedback is valued, companies can enhance their promotional effectiveness and achieve desired outcomes, ultimately leading to greater stakeholder satisfaction and loyalty. As industry experts note, "Stakeholder satisfaction and feedback are key to adding value to a company." Additionally, understanding stakeholder dynamics, as detailed in the case study "Influence Impact: Evaluating Stakeholder Power and Interest," can further refine promotional strategies.
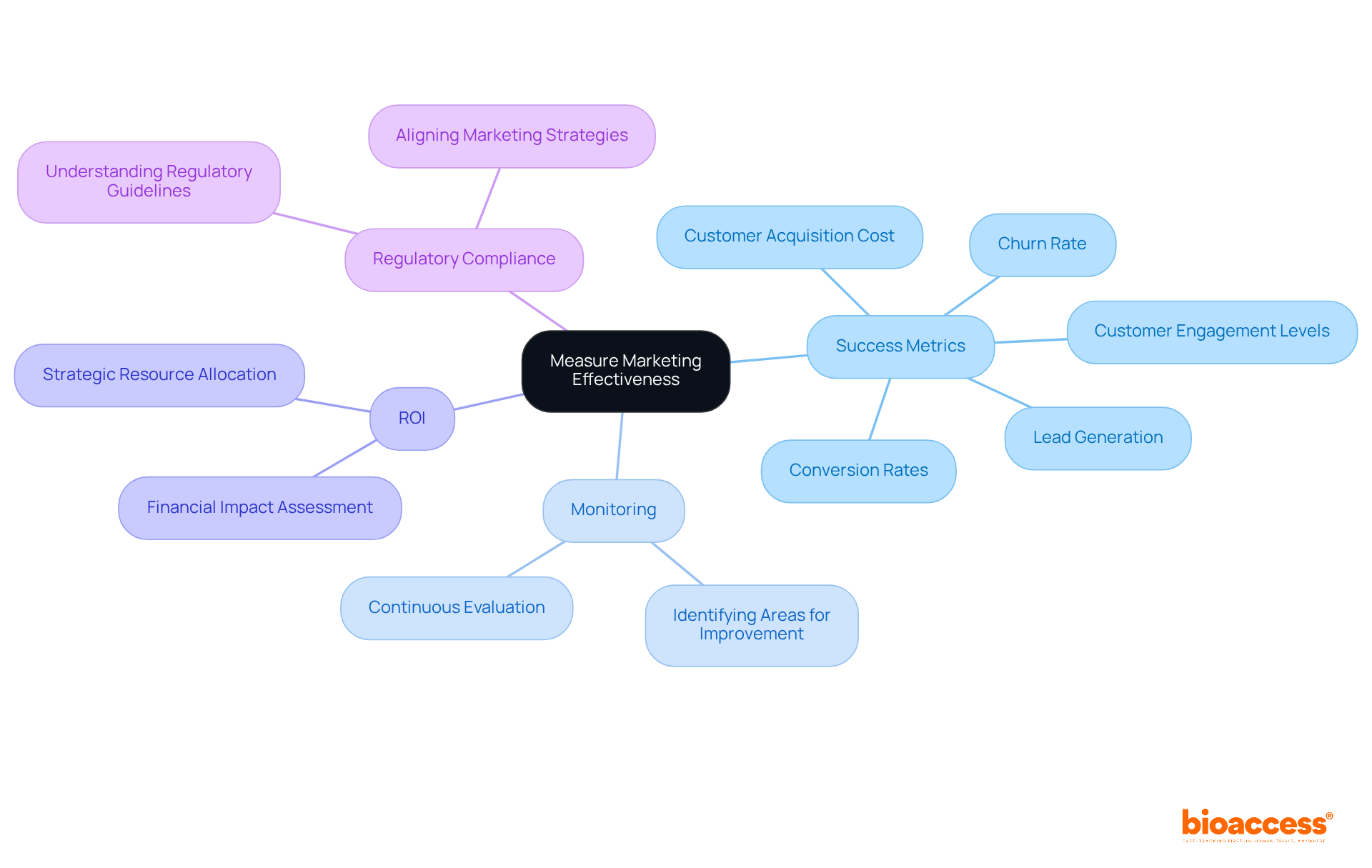
Frequent assessment of promotional effectiveness is essential for understanding the impact of strategies and facilitating informed adjustments. Companies must establish clear success metrics, such as lead generation, conversion rates, and return on investment (ROI). By efficiently monitoring these metrics, organizations can identify areas for improvement, leading to enhanced promotional strategies. This continuous evaluation not only ensures alignment with business objectives but also bolsters overall success. Focusing on ROI allows companies to gauge the financial impact of their promotional endeavors, enabling strategic allocation of resources to the most effective channels.
Effective metrics for evaluating medical device marketing strategies encompass customer engagement levels and churn rates, which provide insights into market responsiveness and customer retention. Notably, customer acquisition cost is calculated by dividing total promotional expenditure by the number of customers, offering a clear measure of ROI. Ultimately, a robust framework for assessing promotional effectiveness empowers organizations to adapt and excel in a competitive landscape. Additionally, aligning medical device marketing strategies with regulatory compliance is vital for navigating the complexities of the medical device market.
Effective medical device marketing relies on a multifaceted approach that integrates swift clinical research, regulatory compliance, targeted audience identification, and robust digital strategies. By embracing these key strategies, companies can enhance their product launches and build trust and credibility within the healthcare community. The insights shared throughout the article underscore the importance of adapting to market dynamics and leveraging innovative tools to remain competitive.
Key arguments emphasize the necessity of understanding regulatory frameworks, the power of data analytics, and the value of continuous education for marketing teams. By fostering relationships with healthcare providers and implementing feedback mechanisms, organizations can refine their strategies and ensure alignment with stakeholder needs. This comprehensive approach drives marketing effectiveness and propels growth within the medical device sector.
Ultimately, the evolving landscape of medical device marketing demands agility and a commitment to excellence. Companies are encouraged to take proactive steps in adopting these strategies, ensuring they remain at the forefront of innovation and responsiveness in a rapidly changing environment. By investing in these core areas, organizations can significantly enhance their market presence and deliver impactful solutions that improve patient outcomes and drive industry success.
What services does bioaccess® provide for medical device marketing?
bioaccess® delivers exceptional clinical research services that help medical device marketing firms expedite their launch schedules by facilitating rapid regulatory evaluations and ethical approvals.
How does bioaccess® leverage Colombia's advantages for clinical research?
Colombia offers cost savings exceeding 30% compared to North America and Western Europe, regulatory evaluations completed in 90-120 days, and high-quality medical systems, allowing bioaccess® to achieve ethical approvals in just 4 to 6 weeks.
Can you provide an example of bioaccess®'s rapid approval process?
A partnership with Newrotex resulted in a remarkable 15-day ethical approval for a nerve regeneration technology trial in Panama, showcasing the efficiency of bioaccess®'s services.
How does Colombia's ethical approval timeline compare to the EU?
Colombia has achieved ethical approvals in as little as 18 days, while the EU typically requires around six months for the same process.
Why is regulatory compliance important in medical device marketing?
Regulatory compliance ensures that all product claims are substantiated and adhere to standards, safeguarding companies against legal challenges and building trust among healthcare providers and patients.
What role does training play in regulatory compliance for medical device marketers?
Regular training sessions and updates on regulatory changes empower promotional teams to create compliant messaging that effectively resonates with their target audience.
How can medical device firms enhance their promotional strategies?
By identifying and understanding their target audiences—such as medical professionals, hospital administrators, or patients—firms can tailor their marketing messages for maximum impact.
What tools can be used to gain insights into target audience preferences?
Tools such as surveys and focus groups can provide valuable insights into audience preferences, allowing marketers to enhance their strategies.
How is AI integrated into medical device marketing?
The integration of agentic AI enables brands to autonomously complete tasks such as targeting and delivering content, optimizing operational efficiency in their promotional efforts.
What is the significance of adopting customized promotional strategies?
Customized promotional strategies foster stronger connections with target audiences and enhance overall engagement, making marketing efforts more effective.
Best Practices
Case Studies (https://ors.od.nih.gov/OD/OQM/benchmarking/bestpractice/Pages/case_studies.aspx)
30 Quotes About the Future of Healthcare: Expert Takes (https://deliberatedirections.com/quotes-future-of-healthcare)
Medical Device Marketing Strategies That Actually Work (https://co-labb.co.uk/post/medical-device-marketing-strategies)