


The article emphasizes key strategies for ensuring compliance with FDA guidance on data integrity, highlighting the critical role of robust information management practices and comprehensive training. Organizations can significantly enhance their compliance and reliability by:
These collective efforts not only mitigate risks but also improve the quality of clinical research outcomes, reinforcing the necessity for organizations to prioritize these strategies in today's Medtech landscape.
In the intricate world of clinical research, the integrity of data stands as a cornerstone of scientific credibility, transcending mere regulatory requirements. As organizations navigate the evolving landscape of FDA guidelines, the stakes have never been higher; maintaining precise, comprehensive, and trustworthy information is essential for successful outcomes. This article delves into ten key strategies that empower research teams to comply with FDA data integrity standards while enhancing the overall reliability of their findings. What challenges await those who fail to adapt, and how can proactive measures safeguard against the looming consequences of non-compliance?
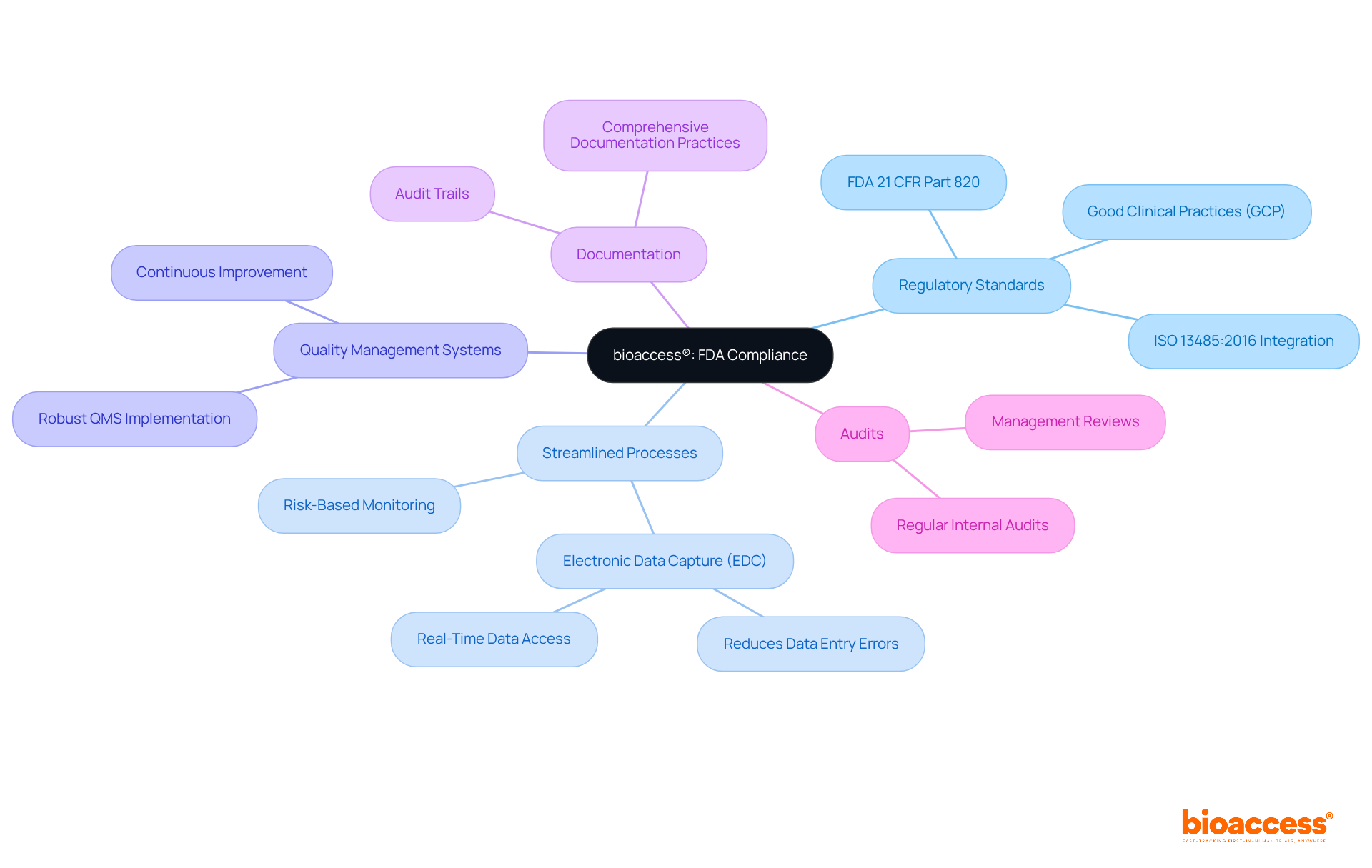
bioaccess® harnesses its extensive knowledge of regulatory standards to assist clients in effectively adhering to FDA information reliability guidelines. By implementing streamlined processes and leveraging local expertise, bioaccess® guarantees that clinical studies uphold the highest standards of information integrity. This dedication not only accelerates compliance but also enhances the credibility of findings, ultimately expediting approval times.
Organizations that adopt robust quality management systems, in fact, report improved operational efficiency and reduced time to market. Additionally, maintaining comprehensive documentation and conducting regular audits are essential strategies that bioaccess® employs to cultivate trust among stakeholders, including regulatory bodies and investors.
Recent updates to FDA guidelines underscore the necessity of precise information management, reinforcing the significance of bioaccess®'s approach in navigating the evolving regulatory landscape. As Katherine Ruiz, a Regulatory Affairs Expert at bioaccess®, emphasizes, knowledgeable professionals are vital in ensuring adherence to these guidelines, which ultimately enhances product safety and efficacy.
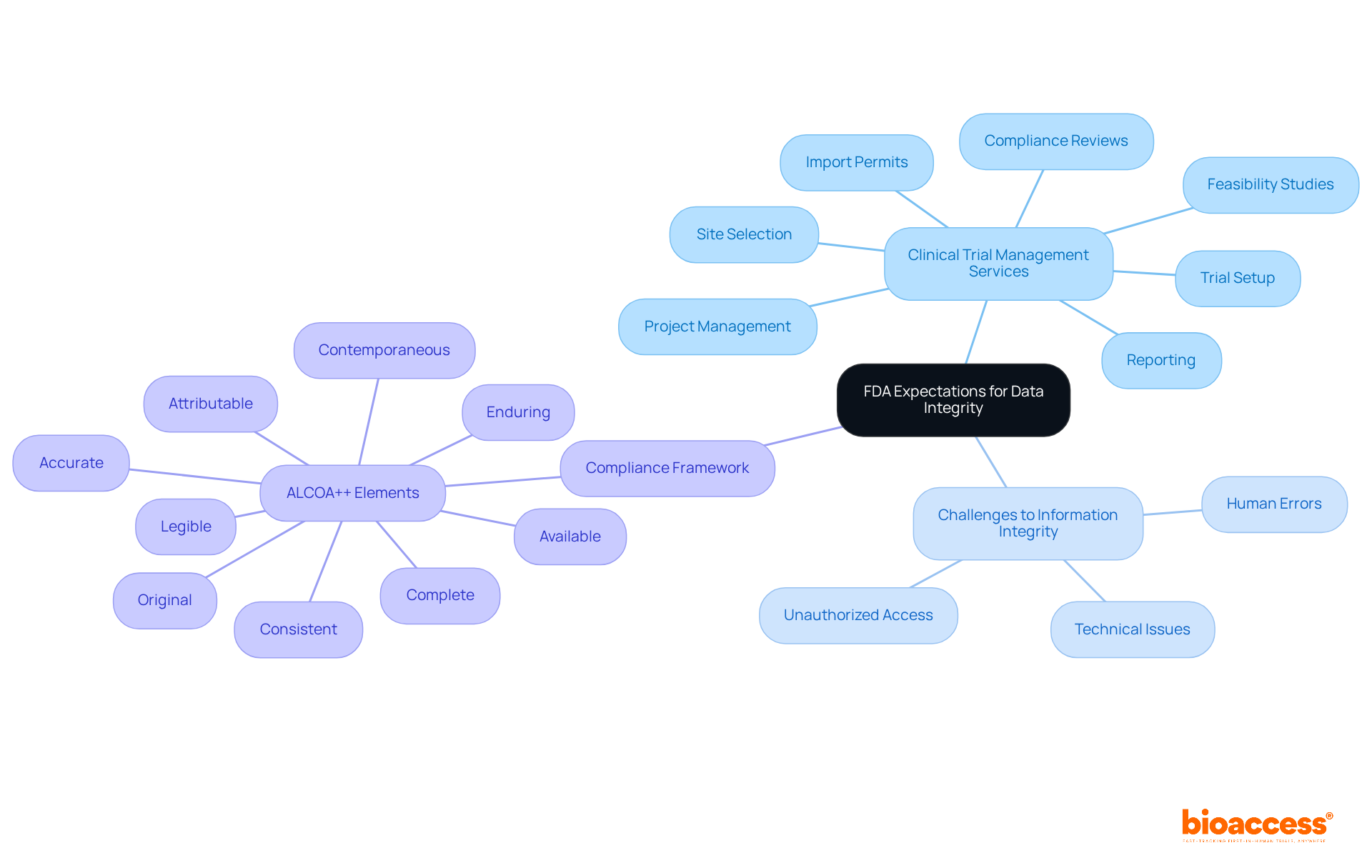
The FDA guidance data integrity emphasizes that maintaining information integrity is vital in clinical research, requiring details to be precise, comprehensive, and trustworthy. Researchers must guarantee that all information generated during studies is recorded and maintained in a manner that prevents unauthorized access or alterations. This includes the necessity for records to be enduring and accessible when needed, which is crucial for effective information management systems. An astonishing 60% to 80% of professionals' time is often consumed by searching for information, significantly impacting information integrity and compliance. Understanding these expectations is essential for adherence and helps establish a robust framework for information management throughout the research lifecycle.
At bioaccess, our extensive clinical trial management services encompass:
These services are designed to assist researchers in maintaining information integrity and ensuring compliance with FDA guidance data integrity. By implementing validated interfaces and calibrated devices, we ensure that information is readable and reviewable in its original context, further enhancing quality. Additionally, sustaining thorough audit trails is essential to preserve history without obscuring the original information. By addressing common challenges related to information accuracy, such as human errors and technical issues, researchers can mitigate risks and uphold the standards expected by regulatory bodies. This proactive approach not only fosters compliance but also enhances the overall reliability of medical study results. Incorporating the ALCOA++ framework—Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, and Available—provides a structured method to ensure compliance with FDA guidance data integrity throughout the research process. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, underscores the significance of these practices in achieving successful clinical outcomes.
Research directors encounter significant challenges, including input mistakes, inconsistent procedures, and inadequate training among staff. In 2025, over 63% of pharmaceutical firms reported grappling with the overwhelming amount of information generated by wearable health technology, exacerbating these issues. To effectively address these challenges, it is crucial to implement robust information management systems that enhance accuracy and streamline processes. For instance, leveraging Electronic Information Capture (EDC) systems can improve accuracy by more than 30%, reducing reliance on manual input and minimizing errors through integrated validation checks.
Establishing clear, standardized procedures is equally essential. Consistent Standard Operating Procedures (SOPs) and information dictionaries can significantly reduce site-to-site variability in data entry practices, leading to more reliable information collection and analysis. Furthermore, ongoing training initiatives for trial personnel are vital to ensure a thorough understanding of protocols and EDC tools, which can decrease entry errors by as much as 40%.
Cultivating a culture of accountability among team members is also paramount. Regular monitoring and audits can facilitate the early identification of discrepancies, ensuring adherence to FDA guidance data integrity. By proactively addressing these challenges, project leaders can enhance information reliability, mitigate risks, and ultimately improve the quality of trial outcomes.

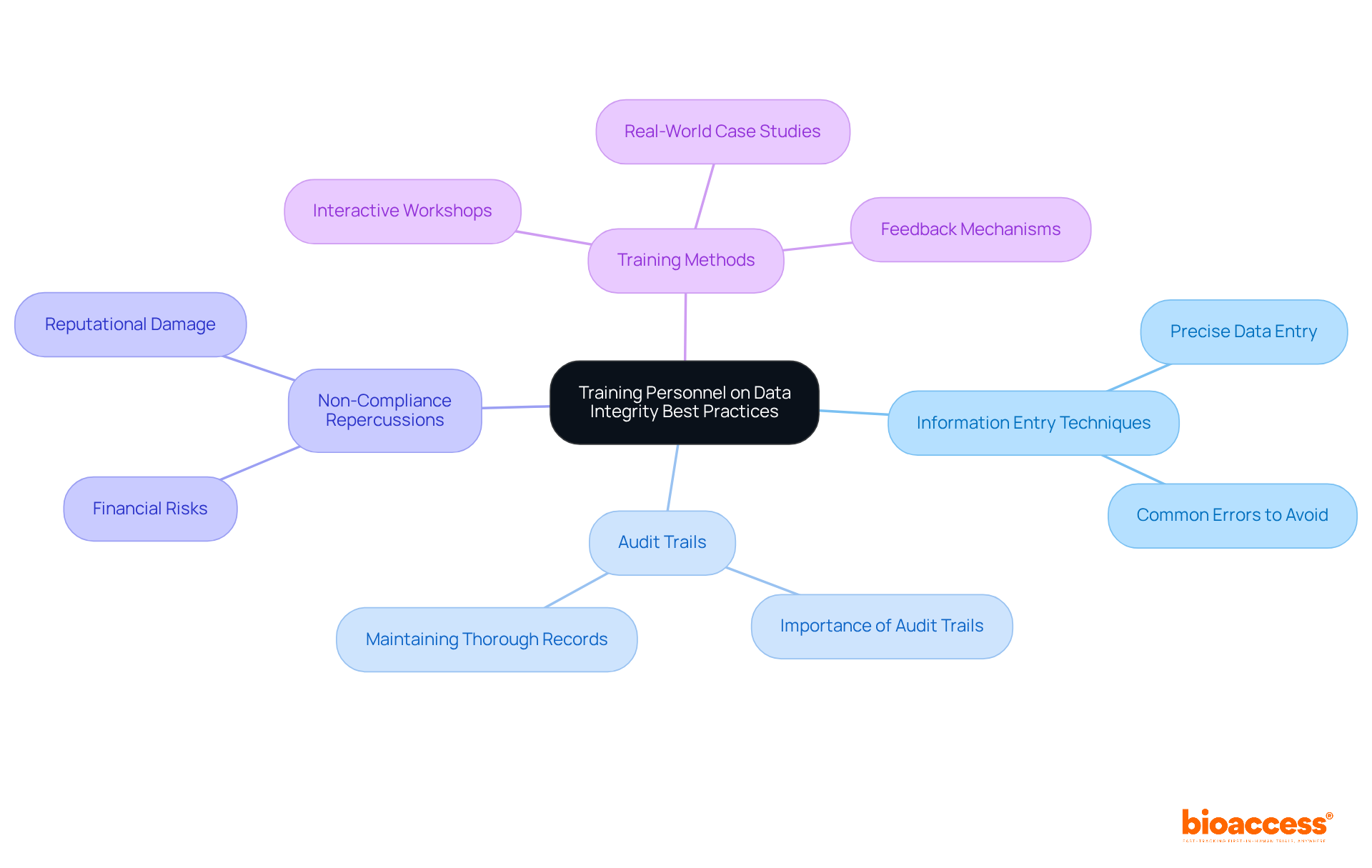
Frequent training sessions on information accuracy best practices are vital for all staff involved in clinical studies. These sessions must encompass critical topics, such as:
By cultivating an environment of continuous education, organizations empower their teams to uphold the highest standards of information accuracy throughout the investigative process. Effective training methods, including interactive workshops and real-world case studies, significantly enhance both understanding and retention of these practices.
Additionally, implementing feedback mechanisms allows organizations to evaluate training effectiveness and make necessary adjustments, ultimately resulting in improved compliance rates. Such training initiatives not only deepen comprehension of information accuracy but also instill a sense of responsibility among research groups, ensuring that information accuracy remains a primary focus in all research endeavors.
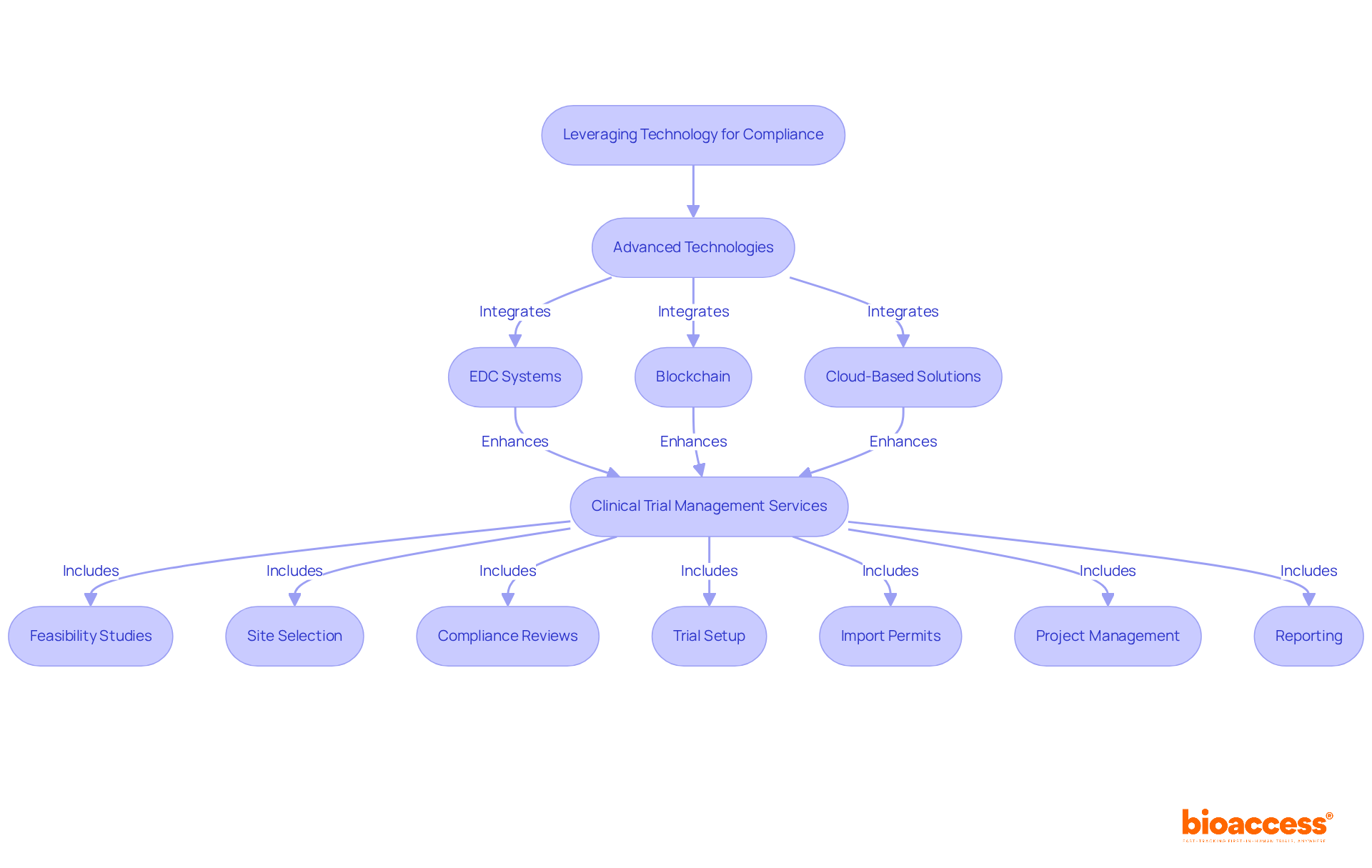
Integrating advanced technologies such as electronic information capture (EDC) systems, blockchain, and cloud-based solutions significantly enhances integrity compliance within research trials. These tools provide secure, real-time access to information and facilitate automated audit trails, effectively minimizing the risk of human error.
Moreover, bioaccess delivers comprehensive clinical trial management services, encompassing:
By merging these services with cutting-edge technology in their information management practices, organizations can ensure compliance with FDA guidance data integrity and prepare thoroughly for regulatory scrutiny.
Non-compliance with FDA guidance data integrity poses significant risks, including:
Notably, the FDA guidance data integrity has been underscored in numerous warning letters, with 21 out of 28 letters from a recent period highlighting such deficiencies. These lapses can lead to:
This is evidenced by cases where organizations faced penalties due to inadequate information management practices. The repercussions extend beyond immediate legal ramifications; they can disrupt the entire clinical study process, ultimately delaying the market introduction of potentially life-saving products. This underscores the critical need for stringent information reliability practices throughout the study lifecycle, especially in light of FDA guidance data integrity, given the exceptionally high stakes for both patient safety and organizational trust.

Audit trails are essential for effective information management, as they provide a chronological record of all modifications and access. This capability empowers organizations to trace alterations back to their origins, ensuring both accountability and transparency.
For instance, the I-SPY 2 study, which manages over 20 million information values, utilizes comprehensive audit trails to uphold information accuracy and adherence, thereby enhancing transparency within a complex investigative environment.
Furthermore, robust audit trail systems not only adhere to FDA guidance data integrity—emphasizing the importance of maintaining precise and comprehensive records—but also significantly improve the quality of medical studies by fostering trust among stakeholders.
Statistics reveal that many organizations conduct audits multiple times a year, underscoring the vital role that regular reviews of audit trails play in identifying anomalies and ensuring compliance with regulatory standards.
Expert insights further illustrate that establishing efficient audit trails bolsters accountability in medical studies. As Ben Baumann states, "An audit trail in medical studies is a chronological record of all actions taken on trial information, ensuring information accuracy and regulatory adherence."
This level of detail is crucial for adhering to Good Manufacturing Practices (GMP) and other regulatory standards, ultimately reinforcing the trustworthiness and reliability of research findings.
To enhance compliance further, organizations should routinely review and update their audit trail systems to adapt to evolving regulations.

Efficient records management is essential for preserving information integrity in clinical research. Organizations must establish clear protocols for information collection, storage, and retrieval, ensuring all processes are standardized.
Utilizing templates for information entry reduces mistakes and enhances consistency. Secure storage options are crucial for protecting sensitive information, while regular backups safeguard against potential loss. Notably, studies indicate that individuals spend 60% to 80% of their time attempting to locate information, underscoring the significance of efficient records management.
By prioritizing these practices, study teams can uphold the integrity of their information, ensuring compliance with FDA guidance data integrity. For instance, organizations that have implemented robust information management systems, as highlighted in the case study on 'Data-Driven Decision Making in Organizations,' have streamlined their operations and improved information reliability.
This proactive approach not only meets regulatory requirements but also fosters trust in research outcomes. As Thomas Redman emphasizes, inadequate information management often signals broader organizational issues, highlighting the necessity for a structured method to information oversight.
To further enhance information accuracy, organizations should consider adopting specific protocols, such as routine evaluations of management practices.

Implementing corrective and preventive actions (CAPA) is essential for effectively addressing integrity issues in clinical research. Organizations must adopt a systematic strategy that involves identifying, investigating, and resolving information discrepancies. This process commences with a comprehensive root cause analysis, which is crucial for understanding the underlying factors contributing to information issues. By documenting these actions and analyzing trends, research teams can not only rectify current problems but also establish preventive measures to avert future occurrences.
For instance, organizations that have successfully integrated CAPA processes have reported significant improvements in their information resolution rates. A structured CAPA plan empowers teams to evaluate the effectiveness of their actions, ensuring compliance with regulatory standards and enhancing overall information quality. As noted by industry experts, the effectiveness of CAPA documentation exemplifies a robust quality system, allowing manufacturers to swiftly identify problems and implement effective corrective actions.
Moreover, the CAPA process is not merely reactive; it cultivates a culture of continuous improvement within organizations. By consistently assessing and refining CAPA procedures, teams can adapt to evolving regulatory demands and technological advancements, ultimately safeguarding the reliability of clinical research information. This proactive approach is vital, especially in an era where information privacy and security are paramount, as organizations face increasing scrutiny from regulatory bodies and stakeholders alike.
To ensure adherence to FDA information reliability guidelines, organizations must proactively stay updated on regulatory changes and best practices. Regular training sessions, participation in industry conferences, and subscriptions to relevant publications are essential strategies in this endeavor. By fostering a culture of ongoing education, research teams can adeptly adjust to evolving standards, thereby enhancing information reliability.
Organizations that prioritize continuous learning have observed significant improvements in compliance rates, underscoring the direct correlation between knowledge and adherence to FDA regulations. Cultivating an environment where team members are encouraged to learn and exchange insights is vital for navigating the complexities of regulatory compliance. This commitment to education not only facilitates compliance with current standards but also equips teams for forthcoming changes in FDA guidance, ensuring that data integrity remains a paramount concern.
Maintaining compliance with FDA data integrity guidelines is crucial for organizations engaged in clinical research. Implementing effective strategies—such as robust quality management systems, comprehensive documentation, and regular audits—ensures the accuracy and reliability of data. This commitment not only facilitates regulatory compliance but also enhances the credibility of research findings, ultimately expediting the approval process for life-saving products.
The article emphasizes several key strategies for achieving data integrity compliance, including:
By utilizing advanced tools like electronic data capture systems and establishing clear protocols for records management, organizations can significantly mitigate risks associated with data inaccuracies. Furthermore, fostering a culture of continuous learning and accountability among staff members is essential for maintaining high standards of information integrity.
Given the evolving regulatory landscape, organizations must prioritize staying updated with changes in FDA guidance. By committing to ongoing education and adopting proactive measures, research teams can effectively navigate the complexities of compliance. Ultimately, the significance of data integrity extends beyond regulatory adherence; it plays a fundamental role in ensuring patient safety and fostering trust in the medical research process.
What is bioaccess® and how does it assist clients with FDA compliance?
bioaccess® utilizes its extensive knowledge of regulatory standards to help clients comply with FDA information reliability guidelines by implementing streamlined processes and leveraging local expertise. This enhances the credibility of clinical study findings and accelerates approval times.
What benefits do organizations experience when adopting robust quality management systems?
Organizations that implement robust quality management systems report improved operational efficiency and reduced time to market for their products.
What strategies does bioaccess® employ to build trust among stakeholders?
bioaccess® maintains comprehensive documentation and conducts regular audits, which are essential strategies to cultivate trust among stakeholders, including regulatory bodies and investors.
Why is precise information management important according to recent FDA guidelines?
Recent updates to FDA guidelines highlight the necessity of precise information management, emphasizing its significance in navigating the evolving regulatory landscape and ensuring adherence to safety and efficacy standards.
What does the FDA guidance on data integrity emphasize for clinical research?
The FDA guidance emphasizes that maintaining information integrity is vital, requiring details to be precise, comprehensive, and trustworthy, with all information generated during studies recorded and maintained securely.
What services does bioaccess offer to support researchers in maintaining information integrity?
bioaccess offers a range of clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
How does bioaccess ensure that information is readable and reviewable?
bioaccess implements validated interfaces and calibrated devices to ensure that information is readable and reviewable in its original context, enhancing quality and maintaining thorough audit trails.
What is the ALCOA++ framework and how does it relate to FDA compliance?
The ALCOA++ framework stands for Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, and Available. It provides a structured method to ensure compliance with FDA guidance on data integrity throughout the research process.
What challenges do research directors face regarding data integrity?
Research directors face challenges such as input mistakes, inconsistent procedures, and inadequate training among staff, particularly exacerbated by the overwhelming amount of information generated by wearable health technology.
What solutions can improve data accuracy in research?
Implementing Electronic Information Capture (EDC) systems can improve accuracy by over 30%, while establishing standardized procedures and ongoing training initiatives can significantly reduce entry errors and variability.
How can a culture of accountability improve trial outcomes?
Cultivating a culture of accountability through regular monitoring and audits helps identify discrepancies early, ensuring adherence to FDA guidance and ultimately improving the quality of trial outcomes.