


The article "10 Key Strategies for GCP in Research Compliance" highlights the critical strategies necessary for enhancing Good Clinical Practice (GCP) adherence within clinical research. It underscores the necessity of:
These elements are essential for ensuring ethical conduct, maintaining data integrity, and achieving overall compliance. Consequently, this approach not only improves research quality but also safeguards participant safety.
The landscape of clinical research is increasingly complex, with Good Clinical Practice (GCP) serving as a critical framework for ensuring ethical and reliable studies. As organizations strive to navigate regulatory requirements and enhance research quality, the need for effective strategies becomes paramount.
This article delves into ten key strategies that not only bolster GCP compliance but also accelerate the research process, addressing the intricate challenges faced by researchers today.
How can stakeholders collaborate effectively to elevate GCP standards and drive innovation in clinical trials?
bioaccess® leverages its extensive experience across Latin America, the Balkans, and Australia to enhance GCP in research studies. By capitalizing on Colombia's competitive advantages—cost savings exceeding 30% compared to North America and Western Europe, a regulatory approval process of merely 90-120 days, and a high-quality healthcare system recognized globally—bioaccess® not only guarantees compliance with GCP in research but also accelerates the approval process, achieving ethical approvals in just 4-6 weeks. This dual emphasis on speed and adherence establishes bioaccess® as a leader in facilitating efficient medical studies.
Recent advancements in GCP in research, including the integration of electronic data capture (EDC) systems, have revolutionized data management, significantly enhancing accuracy and security. Furthermore, effective strategies implemented in Latin America, including comprehensive training programs for research personnel, foster a culture of continuous improvement and adherence to GCP in research standards. As a result, bioaccess® adeptly navigates complex regulatory environments, ensuring that research studies are conducted ethically and efficiently, ultimately contributing to positive health outcomes and societal benefits.
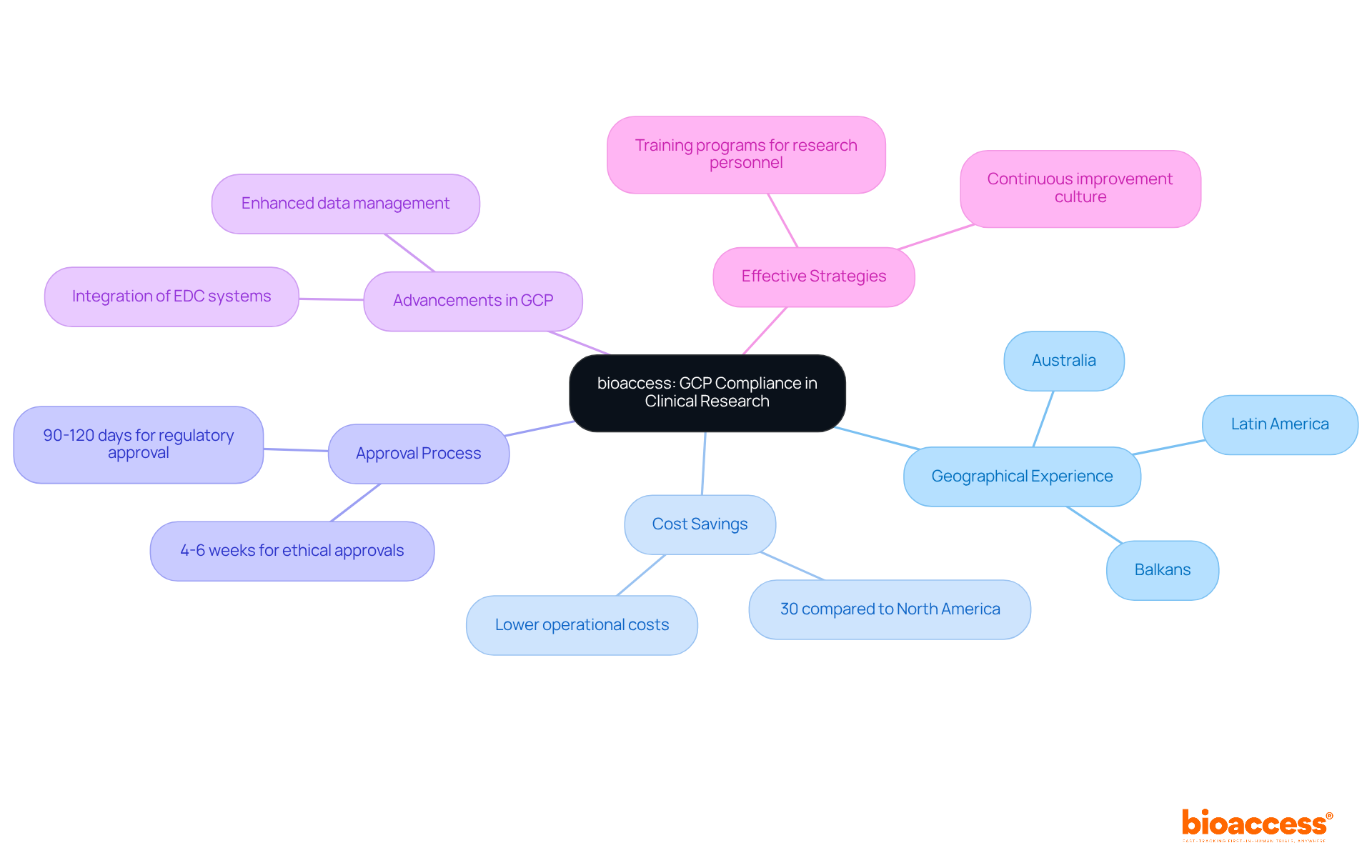
The ICH E6 guidelines establish the foundational principles of Good Clinical Practice (GCP) in research, prioritizing the protection of human subjects, data integrity, and ethical conduct. Acknowledged worldwide, these guidelines guarantee that studies involving human subjects are carefully planned, conducted, and documented to protect the rights and safety of participants. Compliance with ICH E6 is not merely a regulatory requirement; it signifies a profound dedication to GCP in research, which enhances the credibility and reliability of research outcomes.
Real-world examples underscore the importance of these guidelines. For instance, the implementation of a risk-based strategy in clinical studies has empowered sponsors to proactively identify and manage potential risks, thereby enhancing patient safety and data quality. This transition has been particularly evident in the rapid development of COVID-19 vaccines, where organizations streamlined processes and improved technological integration to meet urgent timelines while maintaining compliance.
Moreover, the emphasis on collaboration among study stakeholders fosters improved communication and alignment with regulatory requirements, ultimately leading to more efficient management. As one specialist remarked, the ICH E6 guidelines serve as a 'north star' for research, offering vital principles that direct ethical behavior and ensure the safety of individuals involved.
The substantial impact of ICH E6 guidelines is evident in GCP in research outcomes. By cultivating a culture of quality and integrity, these guidelines not only safeguard the rights of individuals but also enhance the overall reliability of research data, paving the way for successful product registrations and market entry. Adherence to ICH E6 guidelines is, therefore, crucial for advancing medical innovations while ensuring that GCP in research upholds ethical standards throughout the process.
To maximize the benefits of ICH E6 compliance, consider leveraging comprehensive research management services, which include:
These services are essential for overcoming patient recruitment challenges and driving global health improvement through international collaboration and innovation in Medtech.
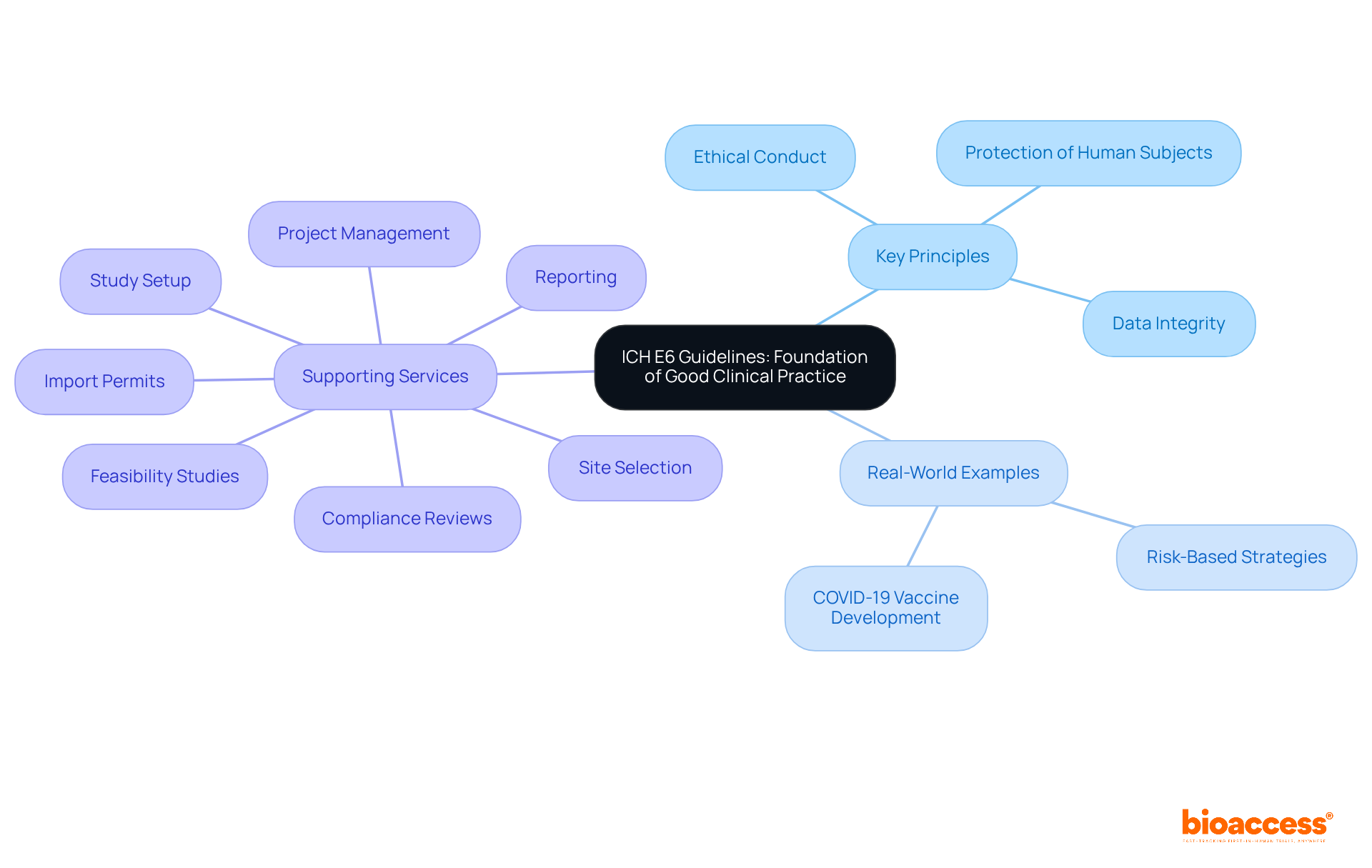
Upholding ethical standards in clinical trials is paramount, particularly in ensuring informed consent, safeguarding the confidentiality of individuals, and minimizing risks. A successful informed consent process is not merely a formality; it is a crucial element that empowers individuals by providing clear, comprehensive information about the study. Research indicates that only 75.8% of individuals understood their right to withdraw at any time, underscoring the need for improved communication strategies.
Furthermore, ethical standards significantly influence the retention of subjects, as confidence in the research process is established through openness and respect for individual autonomy. By prioritizing the welfare of subjects and adhering to ethical guidelines, researchers not only enhance the integrity of their studies but also improve the overall quality and reliability of research outcomes. This unwavering commitment to ethical practices is essential for fostering a collaborative environment where participants feel valued and informed.
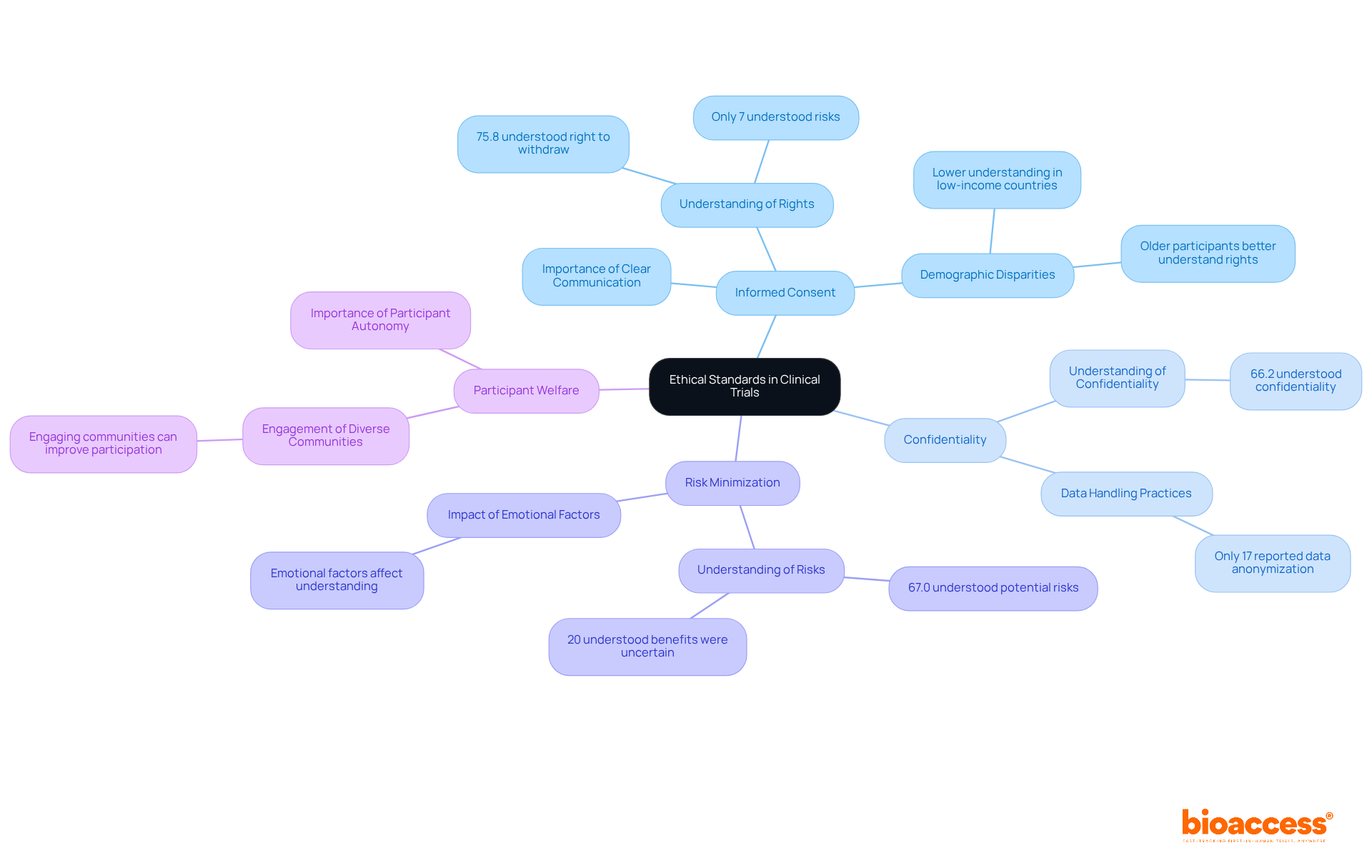
Regular training on GCP in research guidelines is essential for all personnel involved in clinical trials. This training covers regulatory requirements, ethical considerations, and best practices for data management. Comprehensive training programs not only equip teams with crucial knowledge but also significantly enhance adherence to GCP in research.
For example, organizations that implement structured GCP training experience a notable increase in adherence, which minimizes the risk of audit findings and safeguards data integrity. Expert opinions underscore that tailored training, focused on specific roles within research teams, is critical for fostering a culture of adherence. By investing in effective GCP in research training, organizations can enhance the quality of their research and protect participant rights, ultimately leading to more successful trial outcomes.
Moreover, bioaccess offers a wide range of extensive research management services, including:
These services, overseen by specialists such as Katherine Ruiz, ensure that all aspects of research studies are meticulously managed, further reinforcing GCP in research compliance and enhancing the overall integrity of the research process.
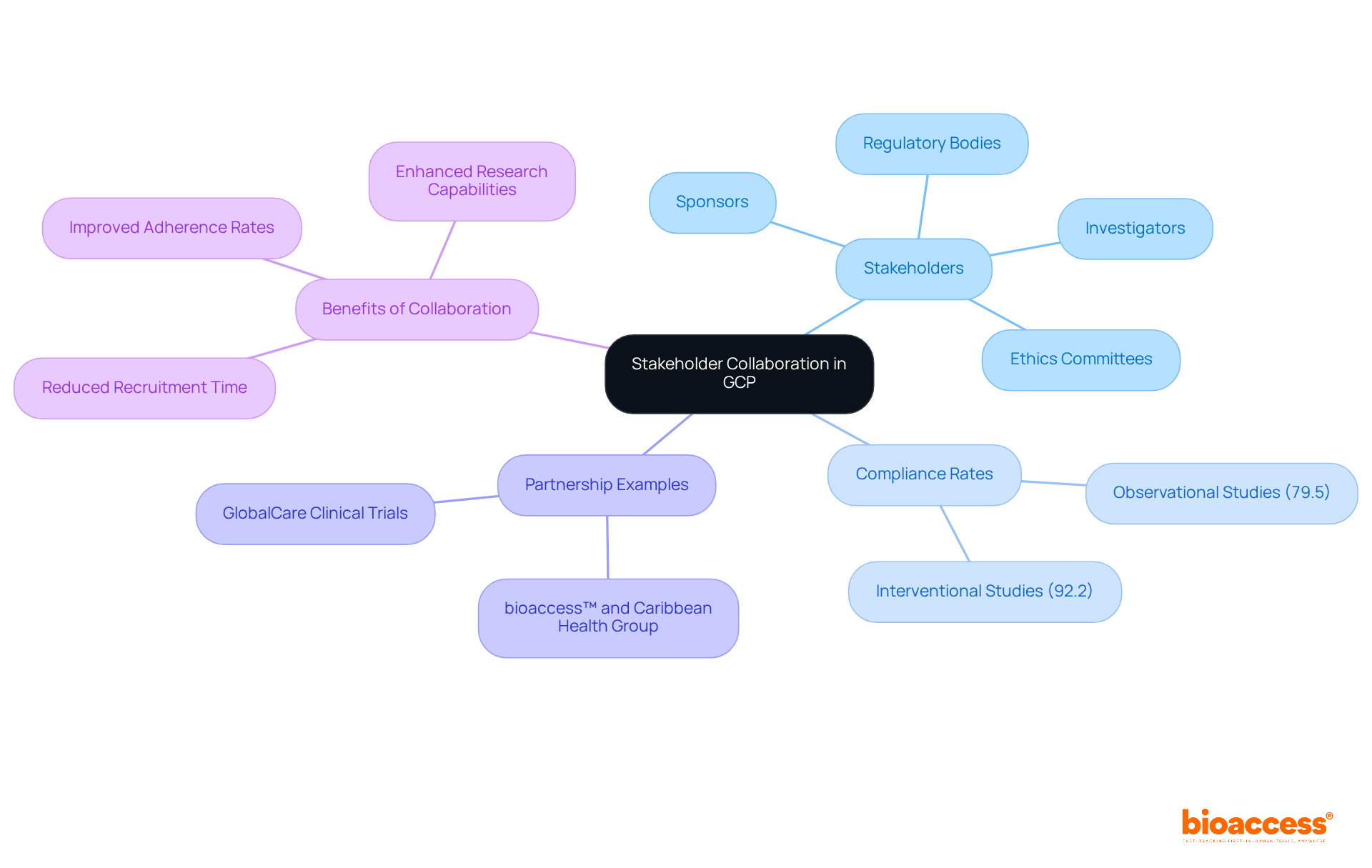
Effective documentation practices are essential for achieving adherence to GCP in research standards during clinical trials. Maintaining accurate records of all trial-related activities ensures that data is traceable and verifiable, which is crucial for regulatory oversight. A study revealed that GCP adherence for interventional studies averaged 92.2%, while observational studies lagged at 79.5%. This underscores the need for meticulous documentation to enhance adherence rates.
Proper documentation not only supports regulatory compliance but also facilitates audits and inspections, providing concrete evidence of adherence to GCP in research standards. For instance, the quality of informed consent forms (ICFs) significantly affects the understanding and rights of individuals involved. A review of ICFs indicated that none met standard readability criteria, with 100% of observational study ICFs categorized as 'difficult' to read. This highlights the necessity for clear and accessible documentation to protect participant rights and ensure ethical standards are met.
Furthermore, the significance of documentation extends beyond adherence; it plays a crucial role in the integrity of research data. Protocol deviations can compromise data integrity and patient safety, making effective management of documentation critical. Creating a strong protocol adherence framework and educating staff on following it can reduce regulatory risks and guarantee study integrity. As highlighted by specialists, thorough documentation practices are not merely a regulatory requirement but a cornerstone of ethical medical research, particularly in relation to GCP in research.
Consistent observation and evaluation of clinical studies are essential for ensuring adherence to Good Clinical Practice (GCP). These processes not only help identify potential issues early but also facilitate timely corrective actions, thereby enhancing the overall quality and integrity of the trial.
By implementing effective monitoring strategies—such as establishing clear Standard Operating Procedures (SOPs) and leveraging advanced technology—organizations can significantly boost adherence rates. Regular audits act as a proactive measure to evaluate compliance with GCP standards; findings indicate that studies with frequent audits experience higher levels of compliance.
Furthermore, continuous training for monitoring teams is vital to adapt to evolving technologies and regulatory requirements, ensuring that organizations uphold high standards throughout the trial lifecycle. By prioritizing these practices, organizations can mitigate risks and enhance the credibility of their clinical research efforts.
Informed consent stands as a cornerstone of ethical research, ensuring that individuals are fully apprised of the study's purpose, risks, and benefits prior to consenting to participate. Researchers must deliver clear and comprehensive information, empowering individuals to make informed decisions regarding their involvement. This essential process safeguards the rights of participants and bolsters the ethical integrity of the research.
Implementing effective informed consent strategies—such as employing straightforward language and customized communication methods—can greatly enhance understanding and foster trust among individuals. Furthermore, the caliber of informed consent directly impacts participant confidence in the research endeavor, nurturing a more transparent and respectful relationship between researchers and participants.
By prioritizing informed consent, researchers not only uphold ethical standards but also cultivate a culture of trust and accountability within medical studies.
Implementing robust risk management strategies is essential for upholding GCP in research during clinical studies. This process begins with identifying potential risks, followed by a comprehensive evaluation of their impact on operational activities. Developing effective mitigation plans is crucial to proactively address these risks. By managing risks effectively, organizations enhance participant safety, uphold regulatory standards, and ensure the integrity of study data.
Effective risk mitigation strategies encompass:
As emphasized by the International Conference on Harmonisation, risk management is a shared responsibility among all stakeholders. Furthermore, statistics indicate that 80% of sites not using a risk management tool cite a lack of experience in risk analysis as a barrier, highlighting the need for improved training and resources.
At bioaccess, our comprehensive clinical study management services encompass:
These services are designed to assist with efficient risk management and ensure that studies comply with GCP in research while remaining effective. The Clinical Experiment Risk Tool, an AI-driven solution, can also enhance ongoing risk evaluation, ensuring that studies continue to meet compliance and effectiveness standards.
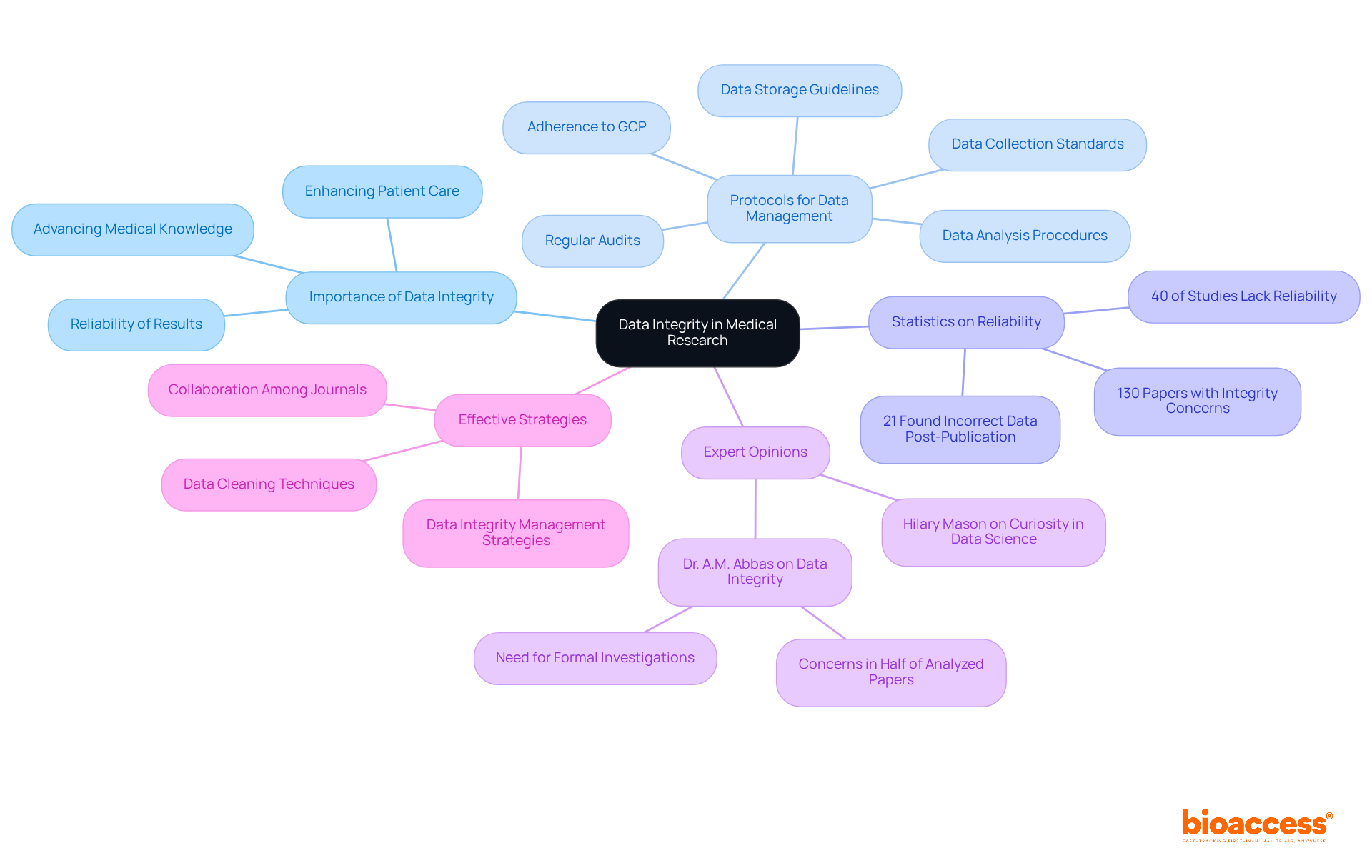
Data integrity stands as a cornerstone in medical research, underpinning the reliability and validity of results. Implementing rigorous protocols for data collection, storage, and analysis is essential to prevent errors and ensure accuracy. Research indicates that up to 40% of published studies may lack reliability, highlighting the critical need for maintaining high data integrity standards. By prioritizing data integrity, researchers not only bolster the credibility of their findings but also make significant contributions to advancing medical knowledge and enhancing patient care. Experts assert that strong data integrity standards can directly influence research outcomes, leading to more effective therapies and innovations in healthcare.
As Dr. A.M. Abbas pointed out, 'We find data integrity concerns in half of the analysed papers,' underscoring the urgency of this matter. Effective data management strategies, including regular audits and adherence to GCP in research guidelines, alongside necessary data cleaning prior to analysis, further fortify the integrity of research studies, ensuring that the data collected is both reliable and actionable.
Collaboration among stakeholders—including sponsors, investigators, regulatory bodies, and ethics committees—is essential for fortifying GCP in research standards. Effective communication and collaboration not only simplify research procedures but also greatly improve adherence rates. For instance, GCP compliance for interventional studies averages 92.2%, while observational studies lag at 79.5%, highlighting the need for improved collaboration in the latter.
A significant instance of this is the partnership between bioaccess™ and Caribbean Health Group, which seeks to establish Barranquilla as a premier location for medical trials in Latin America, backed by Colombia's Minister of Health. As Bill Andrews, Ph.D., President & CEO of Sierra Sciences, stated, 'The partnership with bioaccess has been instrumental in enhancing our research capabilities.'
By promoting a culture of collaboration and offering required training for investigators, stakeholders can jointly address challenges, exchange insights, and propel innovations in research. Additionally, GlobalCare Clinical Trials' partnership with bioaccess™ has led to an impressive over 50% reduction in recruitment time and 95% retention rates, showcasing the tangible benefits of such collaborations.
Utilizing online collaboration tools can further boost team productivity and engagement. This collaborative spirit is encapsulated in the notion that 'without collaboration, there is no growth; without growth, there is no success.' Ultimately, a united approach to GCP in research leads to better research outcomes and a more robust clinical research ecosystem.
The principles of Good Clinical Practice (GCP) are essential for upholding the integrity and ethical standards of clinical research. Effective strategies, including comprehensive training programs, meticulous documentation practices, and fostering collaboration among stakeholders, significantly enhance compliance with GCP guidelines. The focus on ethical considerations and informed consent further underscores the commitment to safeguarding participants' rights and improving research outcomes.
This article has explored key strategies for achieving GCP compliance. From the foundational ICH E6 guidelines that shape ethical conduct to the critical importance of risk management and data integrity, each element plays a vital role in the successful execution of clinical trials. Furthermore, the contributions of organizations like bioaccess® in streamlining processes and promoting adherence to GCP standards have been highlighted, demonstrating the advantages of efficient research management.
Ultimately, the commitment to GCP in research transcends regulatory requirements; it embodies a moral obligation that enhances the credibility of scientific findings and leads to improved health outcomes. By prioritizing these strategies and cultivating a culture of collaboration, researchers can ensure that their studies are conducted ethically, efficiently, and with the utmost respect for the participants involved. Embracing these practices today paves the way for more reliable and impactful medical advancements tomorrow.
What is bioaccess® and how does it enhance GCP compliance in clinical research?
bioaccess® leverages its experience across Latin America, the Balkans, and Australia to enhance Good Clinical Practice (GCP) in research studies by ensuring compliance and accelerating the approval process, achieving ethical approvals in just 4-6 weeks.
What competitive advantages does Colombia offer for clinical research?
Colombia offers cost savings exceeding 30% compared to North America and Western Europe, a regulatory approval process of 90-120 days, and a high-quality healthcare system recognized globally.
How has electronic data capture (EDC) systems impacted GCP in research?
The integration of EDC systems has revolutionized data management in clinical research, significantly enhancing accuracy and security.
What strategies does bioaccess® implement in Latin America to promote GCP compliance?
bioaccess® implements comprehensive training programs for research personnel, fostering a culture of continuous improvement and adherence to GCP standards.
What are the ICH E6 guidelines and why are they important?
The ICH E6 guidelines establish foundational principles of GCP in research, prioritizing the protection of human subjects, data integrity, and ethical conduct, thereby enhancing the credibility and reliability of research outcomes.
How do ICH E6 guidelines improve patient safety and data quality?
The implementation of a risk-based strategy in clinical studies allows sponsors to proactively identify and manage potential risks, enhancing patient safety and data quality.
What services can be leveraged to maximize the benefits of ICH E6 compliance?
Comprehensive research management services include feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting.
Why are ethical standards crucial in clinical trials?
Upholding ethical standards ensures informed consent, safeguards confidentiality, and minimizes risks, which enhances the integrity of studies and the overall quality and reliability of research outcomes.
What is the significance of informed consent in clinical trials?
Informed consent is crucial as it empowers individuals by providing clear, comprehensive information about the study, yet research shows that only 75.8% of individuals understood their right to withdraw at any time, indicating a need for improved communication.
How does prioritizing ethical standards affect participant retention in clinical trials?
By establishing confidence through openness and respect for individual autonomy, prioritizing ethical standards influences the retention of subjects, enhancing the integrity of the research process.