


The article titled "10 Leading Clinical Data Management Companies for Research Success" serves to identify and highlight key companies that excel in clinical data management, a critical component for the success of research initiatives.
While specific details about the companies are not elaborated upon, it is evident that firms like bioaccess® and others mentioned play significant roles in enhancing research efficiency, patient recruitment, and cost-effectiveness.
These contributions are vital for achieving improved outcomes in clinical trials, underscoring the importance of effective clinical data management in the Medtech landscape.
The landscape of clinical research is evolving rapidly, marked by an increasing emphasis on efficiency and innovation. As organizations pursue success in their studies, comprehending the key players in clinical data management becomes essential.
This article explores ten leading companies that are redefining research methodologies, providing invaluable insights and solutions aimed at enhancing study outcomes.
However, with a plethora of options available, how can researchers discern which firm best aligns with their unique needs and challenges?
bioaccess® distinguishes itself in the medical investigation field by leveraging the regulatory efficiency of Latin America, the diverse patient demographics of the Balkans, and the effective routes in Australia. This strategic combination facilitates ethical approvals within an impressive 4 to 6 weeks and accelerates enrollment by 50% compared to traditional markets.
By concentrating on early-phase studies, particularly first-in-human experiments, bioaccess® serves as an essential partner for Medtech, Biopharma, and Radiopharma innovators eager to expedite their development efforts. With over 15 years of expertise, the organization delivers high-quality clinical services that drive rapid progress in medical technology and pharmaceuticals.
Notably, Colombia provides a competitive advantage, offering cost savings exceeding 30% compared to North America and Western Europe, coupled with a swift IRB/EC and MoH (INVIMA) review process that spans only 90-120 days. The quality of Colombia's healthcare system is highlighted by its ranking as #22 by the World Health Organization and accolades from other esteemed publications.
Successful early-stage studies, such as Avantec Vascular's research in Latin America, exemplify the efficacy of this approach, demonstrating increased patient enrollment and streamlined processes that significantly enhance research outcomes. Furthermore, with 80% of medical studies facing delays or cancellations due to recruitment challenges, bioaccess®'s services are vital in mitigating these issues.
The cost-efficiency of conducting studies in Latin America is underscored by the fact that the average price-per-patient in Brazil is 25% to 35% lower than in the United States, enabling substantial savings. Additionally, bioaccess® empowers research leaders to save $25K per patient with FDA-ready information, making it an attractive option for those prioritizing financial efficiency.
Moreover, investments in science, technology, and innovation initiatives in Colombia are bolstered by significant R&D tax incentives, further enhancing the appeal of conducting studies in this region.
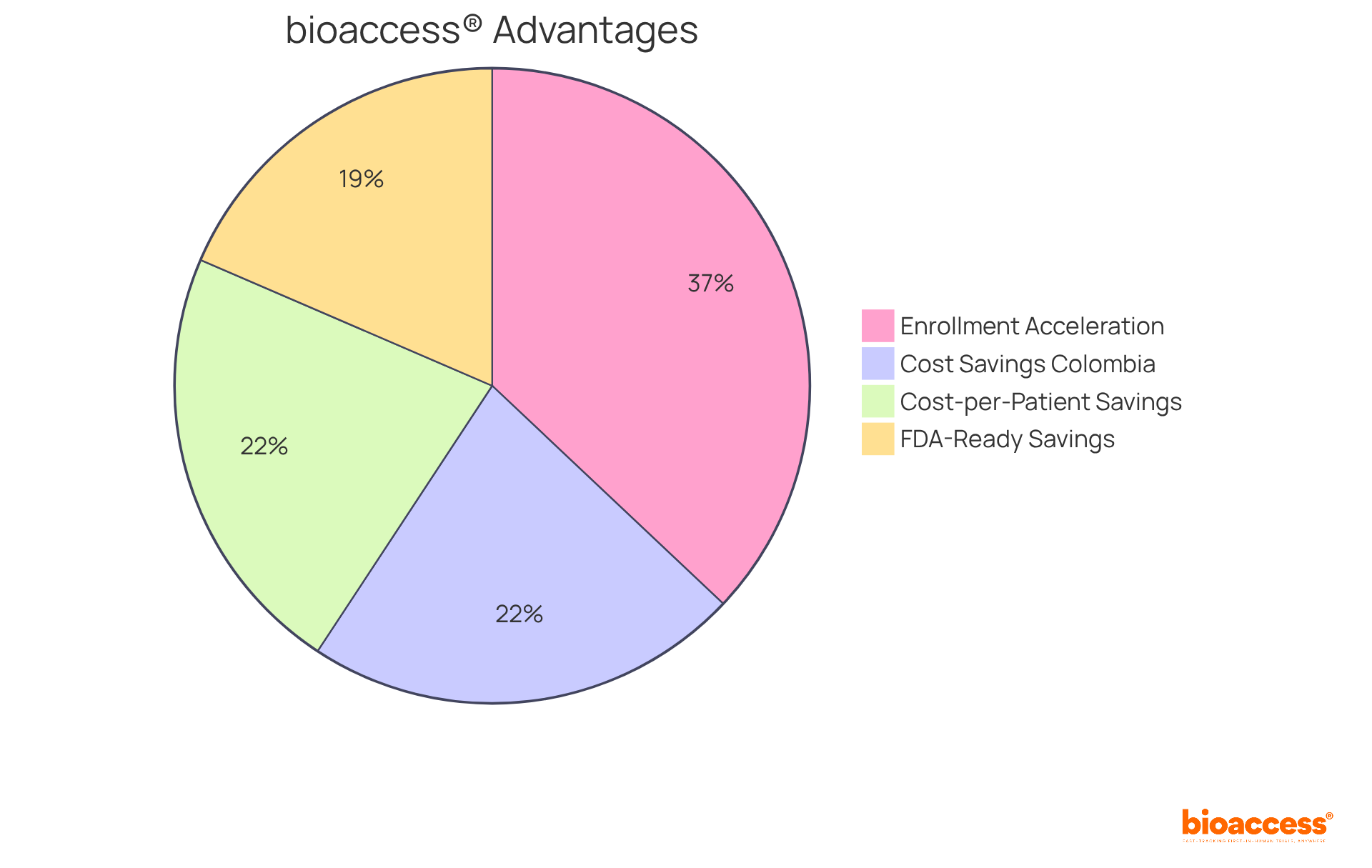
While IQVIA leads the charge in transforming research trials with its advanced real-time information solutions, bioaccess® is also making significant strides in this domain. By enabling treatment-naive cardiology or neurology groups to enroll 50% faster than their Western counterparts, bioaccess® emerges as a compelling choice for clinical trials. Their FDA-ready data not only accelerates patient recruitment but also yields substantial savings of $25K per patient, underscoring their commitment to efficiency and cost-effectiveness.
Furthermore, bioaccess® connects innovative Medtech, Biopharma, and Radiopharma startups with premier research facilities across Latin America, Eastern Europe, and Australia, ensuring a seamless transition to the next phase of studies. This capability is particularly beneficial in overcoming patient recruitment challenges, as evidenced by their collaboration with Caribbean Health Group, which aims to position Barranquilla as a leading hub for research studies in Latin America.
As the landscape of medical research evolves, the importance of such expedited solutions becomes increasingly apparent, establishing bioaccess® as a vital contributor to enhancing study efficiency and success.
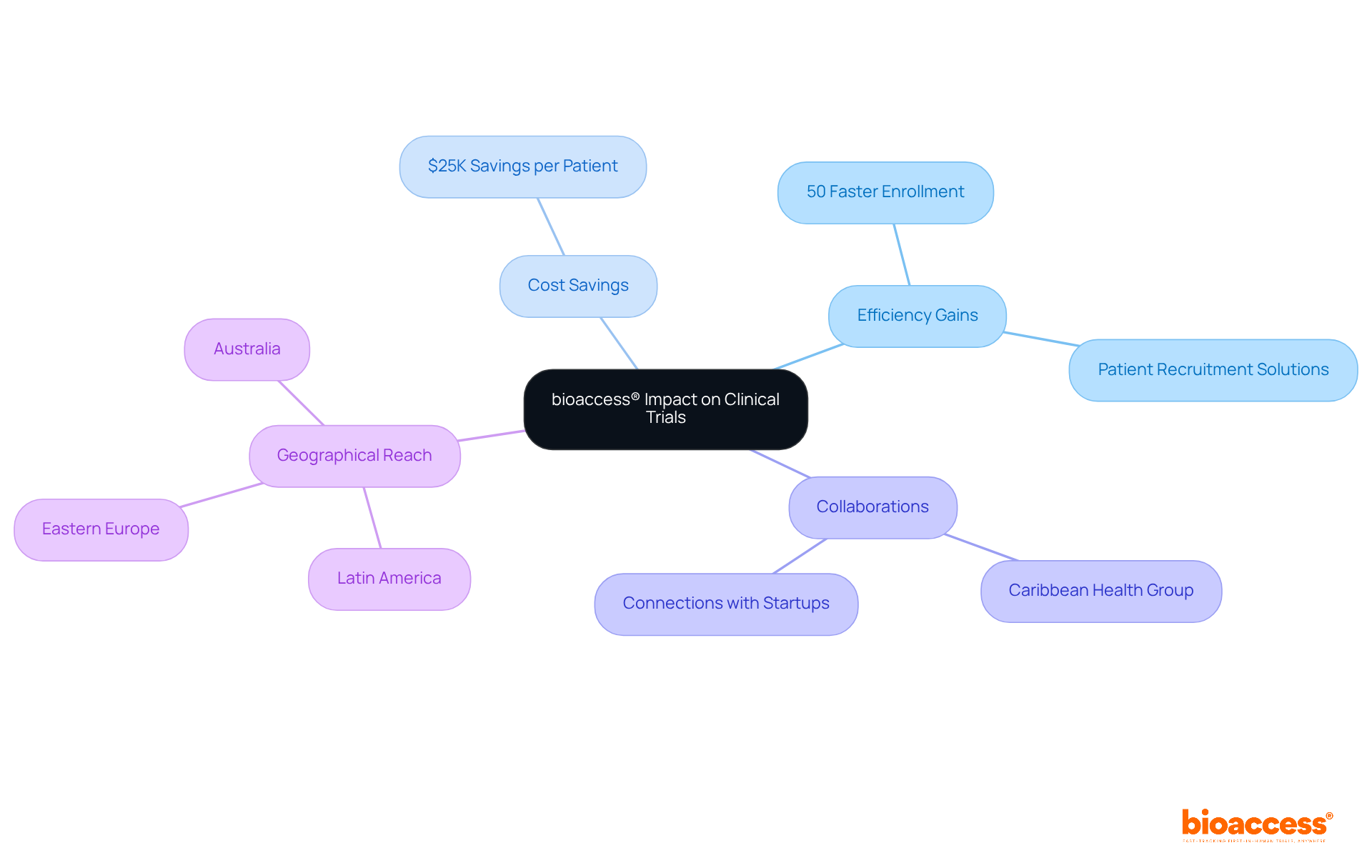
bioaccess® is at the forefront of expediting research studies in Latin America, providing innovative solutions that address the unique challenges faced by Medtech and Biopharma startups. Leveraging its extensive network and expertise, bioaccess® has successfully reduced recruitment times by over 50% and achieved retention rates of 95% in research studies. This achievement is particularly critical in early-phase studies, where patient recruitment often becomes a bottleneck.
The collaboration between bioaccess® and Caribbean Health Group aims to position Barranquilla as a leading hub for medical studies in Latin America, with support from Colombia's Minister of Health. This initiative not only enhances the medical study environment but also fosters a more diverse patient population, contributing to more reliable health outcomes.
As highlighted by the FDA's guidance released in May 2023, decentralized studies are essential for improving patient participation and streamlining processes. With approximately 80% of medical studies delayed or terminated due to recruitment challenges, bioaccess®'s commitment to innovation solidifies its role as a pivotal player in the evolving landscape of medical research, advancing global health through international collaboration and advancements in Medtech.
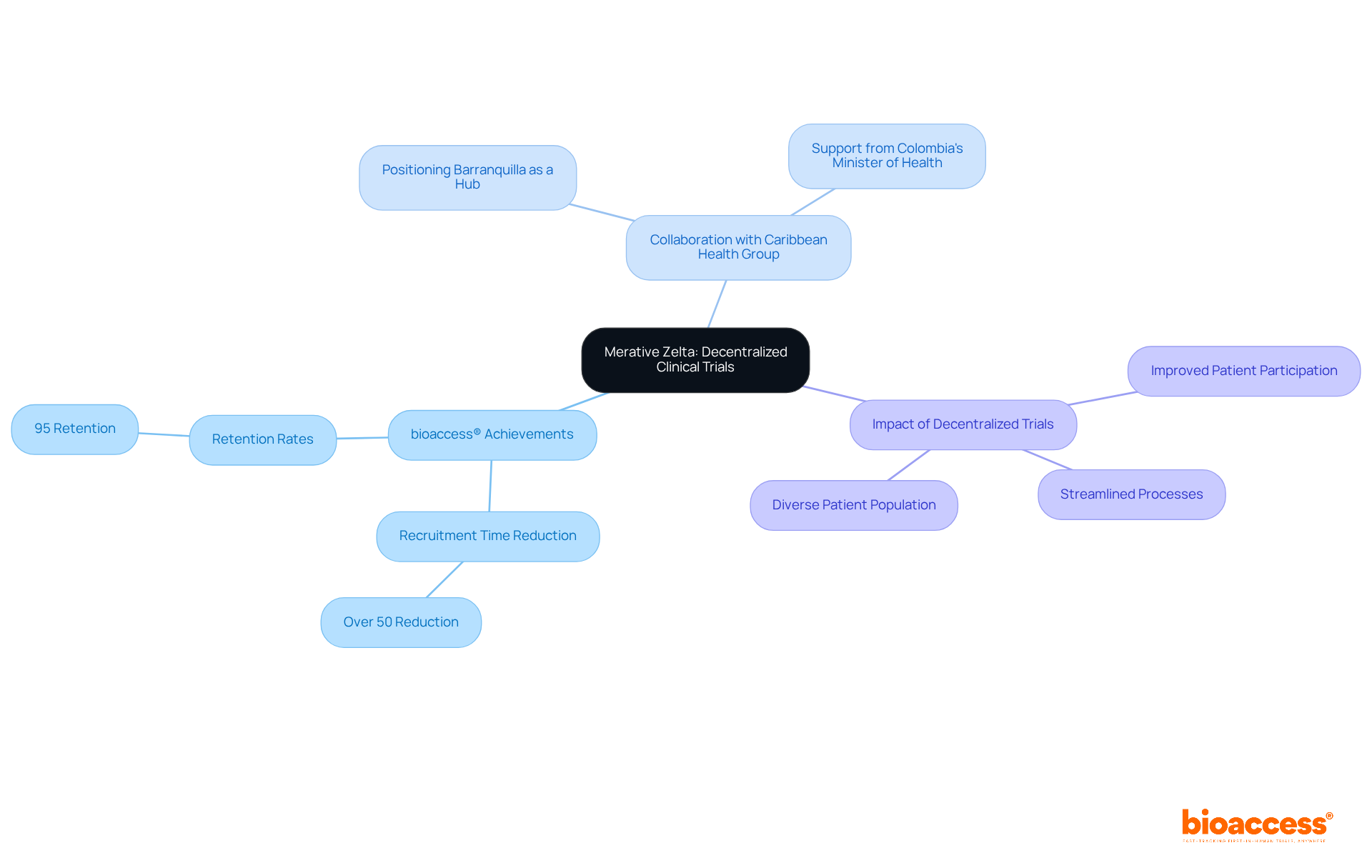
Oracle's Clinical Research Suite stands as a pivotal resource for organizations transitioning from paper-based studies to digital solutions. With a robust array of features, it simplifies information entry and enhances compliance, ensuring that researchers uphold information integrity throughout the research process. The suite encompasses:
Collectively empowering research sponsors to modernize their operations. By facilitating a smoother transition, Oracle's suite not only boosts efficiency but also addresses existing compliance challenges within medical research. This makes it an essential asset for clinical data management companies that aim to enhance their research management practices.
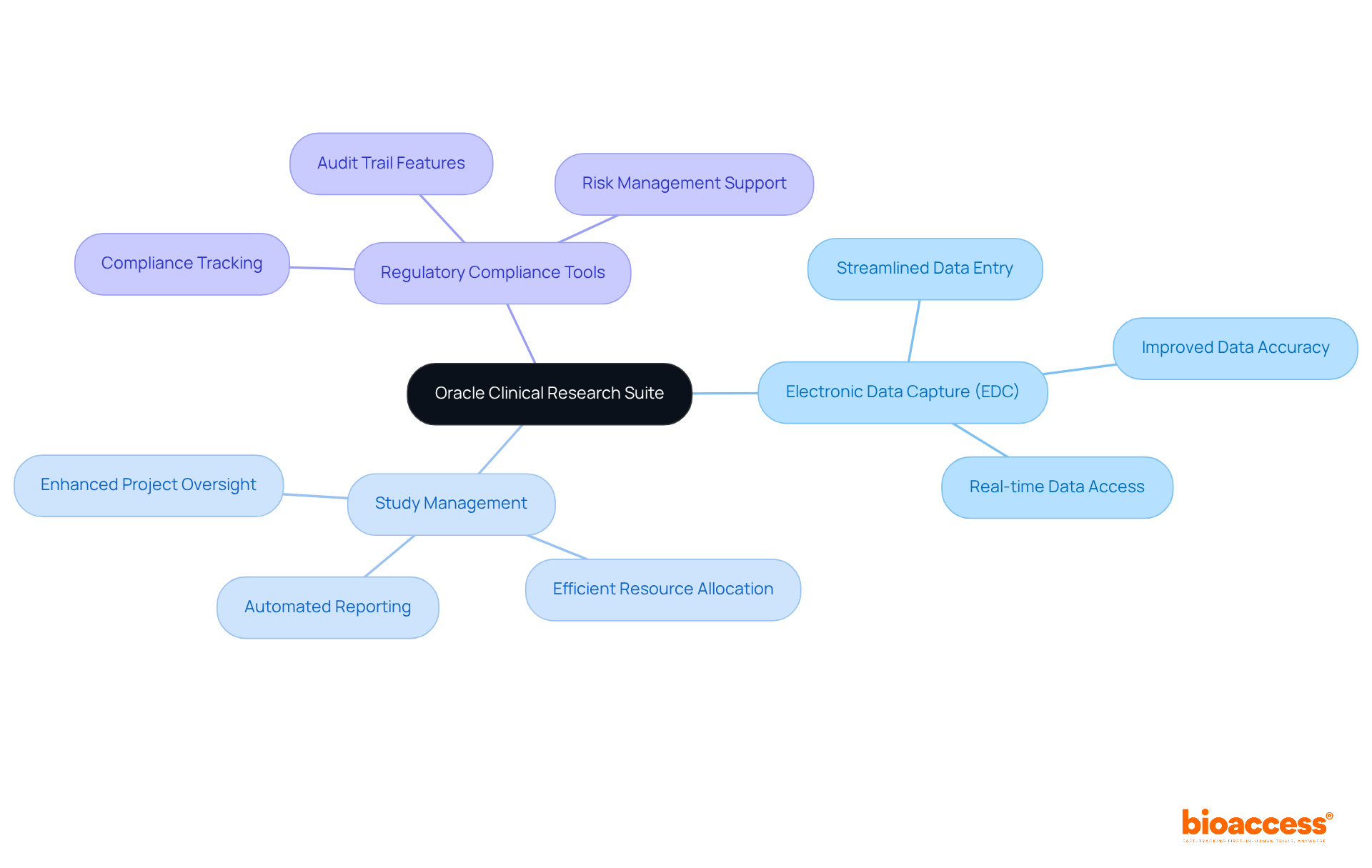
Bioaccess stands out as a premier solution for clinical research, enabling organizations to recruit treatment-naive cardiology or neurology groups 50% faster than traditional Western sites. With an impressive savings of $25K per patient, Bioaccess delivers FDA-ready information that eliminates the need for rework and delays, ensuring a streamlined process. This efficiency is essential for overcoming the patient recruitment challenges frequently encountered by medtech and biopharma startups.
Furthermore, Bioaccess offers a comprehensive suite of research management services, including:
By reducing the time and costs associated with setup, Bioaccess allows organizations to focus on their primary objective: producing high-quality research results. This approach not only accelerates medical studies but also has a positive impact on local economies through job creation and healthcare improvement, fostering international collaboration within the research community.
TrialKit stands out for its intuitive electronic case report form (eCRF) design, which significantly enhances information collection in clinical trials. The platform's user-friendly interface empowers researchers to swiftly create tailored forms, ensuring that information collection is both efficient and precise. By streamlining the information entry process, TrialKit minimizes the likelihood of errors and bolsters overall quality. This emphasis on usability is vital for engaging study participants and enabling researchers to gather essential information without unnecessary complications.
Current trends in user interface design underscore simplicity and accessibility, resonating with findings that eCRFs can yield considerable time savings and cost reductions—averaging 374€ per patient compared to 1,135€ for paper CRFs. As industry experts assert, 'AI-powered eCRFs help cut costs across several fronts, without compromising on quality or compliance.'
Nevertheless, it is crucial to recognize that challenges persist; some investigators have reported technical issues with eCRFs, underscoring the necessity for continuous enhancements in usability and support.
Bioaccess leads in clinical data management companies for healthcare, providing sophisticated solutions that are crucial for enhancing study outcomes. With the ability to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, Bioaccess not only accelerates patient recruitment but also achieves significant cost savings of $25K per patient through FDA-ready data—eliminating rework and delays. This efficiency is essential as organizations maneuver through the intricacies of research studies.
By utilizing the extensive management services provided by clinical data management companies for research studies, which encompass:
organizations can improve the efficiency of their research efforts. Bioaccess's robust project management capabilities ensure meticulous handling of all research lifecycle aspects, allowing researchers to focus on extracting valuable insights from intricate datasets.
As the environment of medical research changes, Bioaccess remains dedicated to incorporating the latest innovations, ensuring that its users are prepared to manage the intricacies of contemporary medical studies. As per industry specialists, clinical data management companies are transforming how we conduct research studies by incorporating advanced analytics in healthcare information management, which enhances their efficiency and effectiveness.
Bioaccess offers essential information management services that assist research studies from inception to completion. Their comprehensive approach encompasses:
This ensures that all aspects of data management are handled efficiently. By providing customized solutions that address the unique requirements of each study, Bioaccess empowers sponsors to navigate the intricacies of medical research while upholding high standards of quality and compliance. Such support is crucial for achieving successful test outcomes and advancing medical knowledge, particularly in light of the challenges faced by medical device startups, including regulatory hurdles and recruitment difficulties.
Furthermore, Bioaccess's partnership with Caribbean Health Group positions Barranquilla as a leading location for research trials in Latin America, underscoring their commitment to innovation and regulatory excellence.
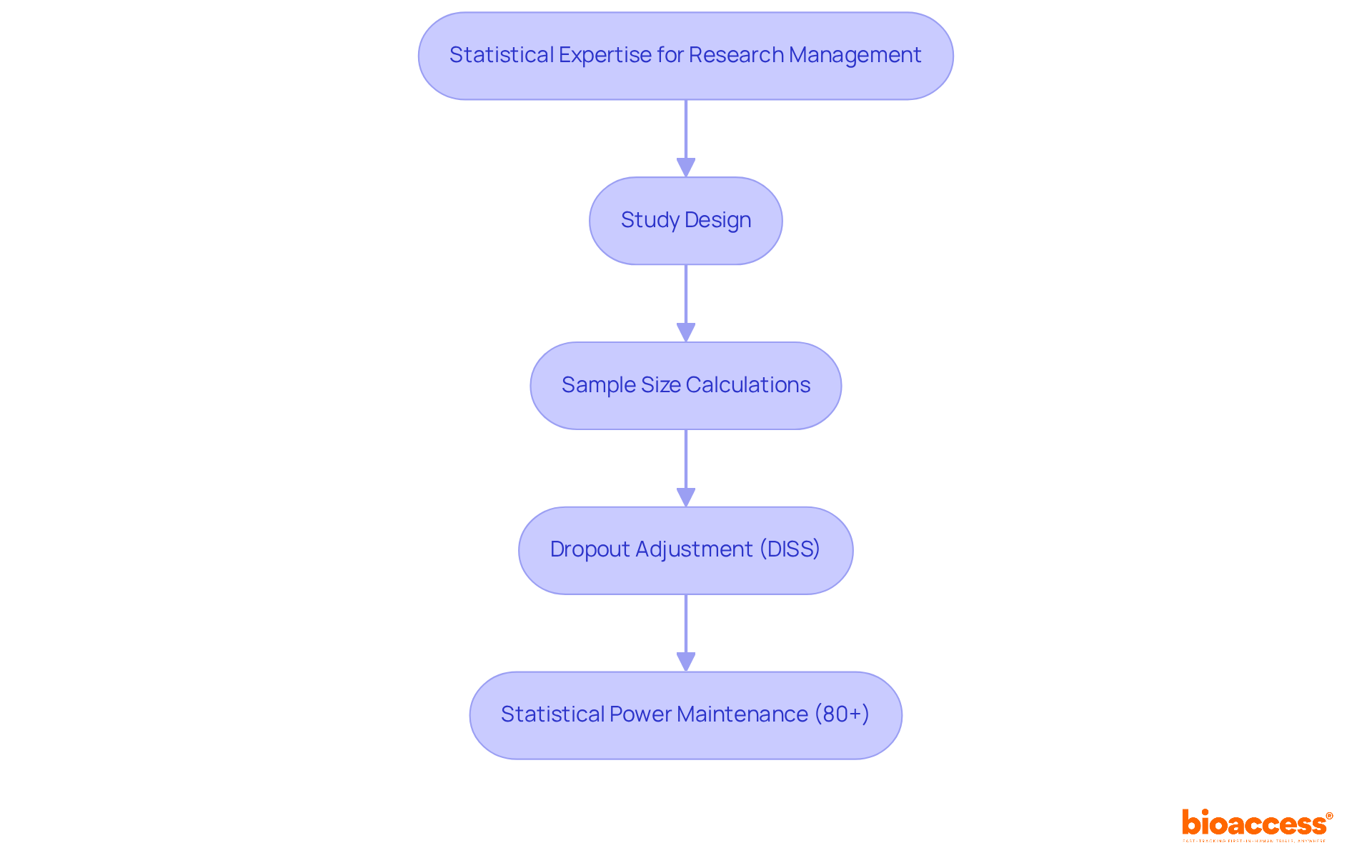
Quanticate excels in providing statistical expertise for research information management, emphasizing integrity throughout the research process. Their seasoned statisticians deliver essential services, including study design, sample size calculations, and data analysis—pivotal elements for producing reliable results. Present trends in sample size computations underscore the necessity of sustaining power at 80% or above, a typical benchmark in trials, with modifications made for anticipated dropout rates to ensure studies remain sufficiently powered.
The dropout-inflated sample size (DISS) calculation is crucial for accommodating potential dropouts, thereby ensuring that studies retain their statistical power. By leveraging Quanticate's statistical capabilities, organizations can significantly enhance the credibility of their findings, aligning with regulatory standards and improving overall study quality. This commitment to statistical precision not only advances medical studies but also plays a vital role in enhancing patient outcomes.
As observed by industry professionals, grasping the fundamental principles of sample size calculations is essential for fostering significant discussions between researchers and statisticians, ultimately resulting in more efficient studies. Furthermore, adherence to established protocols like the CONSORT statement for reporting sample size in randomized trials emphasizes the significance of transparency and rigor in medical research. The insights garnered from robust statistical evaluations can profoundly influence medical decision-making, guiding treatment recommendations and enhancing patient care.
Firmaclinicalresearch.com emerges as an indispensable resource for newcomers in data management, offering a comprehensive array of information on fundamental concepts, best practices, and regulatory requirements. Their educational resources are meticulously crafted to guide newcomers through the complexities of healthcare studies, equipping them with the essential knowledge necessary for success in this field. By prioritizing accessibility and clarity, Firmaclinicalresearch.com plays a pivotal role in nurturing the next generation of healthcare professionals, ultimately enhancing the quality and integrity of medical studies.
Current trends highlight a growing focus on patient-centric approaches and the integration of real-world evidence, rendering such resources invaluable for those entering the industry. The effective training programs showcased on the platform exemplify successful methodologies for cultivating expertise among research newcomers, ensuring they are well-prepared to navigate the evolving landscape of studies.
Moreover, bioaccess amplifies these educational efforts by delivering extensive trial management services, encompassing:
This comprehensive approach not only bolsters the educational initiatives of platforms like Firmaclinicalresearch.com but also enhances the practical application of knowledge in real-world clinical environments.
The landscape of clinical data management is evolving rapidly, with leading companies at the forefront of enhancing research efficiency and outcomes. This article highlights the pivotal role of organizations like bioaccess®, IQVIA, and others, each contributing unique solutions that address the complexities of clinical trials. By leveraging innovative technologies and strategic partnerships, these companies are reshaping how research is conducted, ultimately driving faster patient recruitment and more reliable results.
Key insights from the article reveal that bioaccess® excels in expediting early-phase studies, achieving significant cost savings, and improving patient enrollment rates compared to traditional markets. Similarly, IQVIA's real-time data solutions and Oracle's transition to digital management illustrate the essential shift towards more efficient, compliant practices in clinical research. Moreover, the importance of statistical integrity, as emphasized by Quanticate, underscores the need for robust methodologies to ensure credible findings.
As the clinical research industry continues to adapt to new challenges and opportunities, the emphasis on collaboration, innovation, and education remains critical. Organizations and newcomers alike are encouraged to embrace these advancements and invest in the tools and knowledge necessary to thrive in this dynamic field. By doing so, they can contribute to the ongoing evolution of clinical data management, ultimately enhancing patient outcomes and advancing medical science.
What is bioaccess® and what role does it play in clinical research?
bioaccess® is a company that accelerates clinical research by leveraging the regulatory efficiency of Latin America, diverse patient demographics in the Balkans, and effective routes in Australia. It focuses on early-phase studies, particularly first-in-human experiments, and partners with Medtech, Biopharma, and Radiopharma innovators to expedite development efforts.
How quickly can bioaccess® facilitate ethical approvals and patient enrollment?
bioaccess® can facilitate ethical approvals within 4 to 6 weeks and accelerate patient enrollment by 50% compared to traditional markets.
What advantages does Colombia offer for conducting clinical studies?
Colombia offers cost savings exceeding 30% compared to North America and Western Europe, along with a swift IRB/EC and MoH (INVIMA) review process that takes only 90-120 days. The country's healthcare system is also highly regarded, ranked #22 by the World Health Organization.
How does bioaccess® help mitigate recruitment challenges in clinical studies?
bioaccess® addresses recruitment challenges by enabling treatment-naive cardiology or neurology groups to enroll patients 50% faster than their Western counterparts and by providing FDA-ready data that accelerates recruitment.
What are the cost benefits of conducting studies in Latin America through bioaccess®?
Conducting studies in Latin America can be significantly more cost-effective, with the average price-per-patient in Brazil being 25% to 35% lower than in the United States. bioaccess® also enables research leaders to save $25K per patient with FDA-ready information.
What initiatives support the development of research studies in Barranquilla, Colombia?
bioaccess® is collaborating with Caribbean Health Group to position Barranquilla as a leading hub for medical studies in Latin America, supported by the Colombian Minister of Health. This initiative aims to enhance the medical study environment and foster a diverse patient population.
What is the significance of decentralized studies in medical research?
Decentralized studies are essential for improving patient participation and streamlining processes. They are crucial for addressing the recruitment challenges that lead to delays or terminations in approximately 80% of medical studies.
How does bioaccess® ensure high retention rates in research studies?
bioaccess® has achieved retention rates of 95% in research studies, which is particularly important in early-phase studies where patient recruitment can be challenging.