


The article outlines ten essential conferences on medical devices for 2025, emphasizing their significance in networking, innovation, and regulatory insights within the Medtech sector. Each conference represents a vital opportunity for industry professionals to connect, learn about emerging trends, and navigate the complexities of medical device development and market entry. As the landscape of Medtech evolves, these conferences will serve as critical platforms for collaboration and knowledge exchange, addressing key challenges faced by the industry.
As the medical device industry evolves at an unprecedented pace, the significance of remaining informed and connected has reached new heights. Conferences emerge as crucial platforms for professionals to delve into the latest innovations, regulatory updates, and networking opportunities that are essential for success in this competitive arena.
With a multitude of events slated for 2025, the question arises: which conferences truly stand out as must-attend for those seeking to advance their careers and propel innovation?
This article reveals ten pivotal conferences that are poised to shape the future of medical technology, offering insights and connections that have the potential to redefine industry standards.
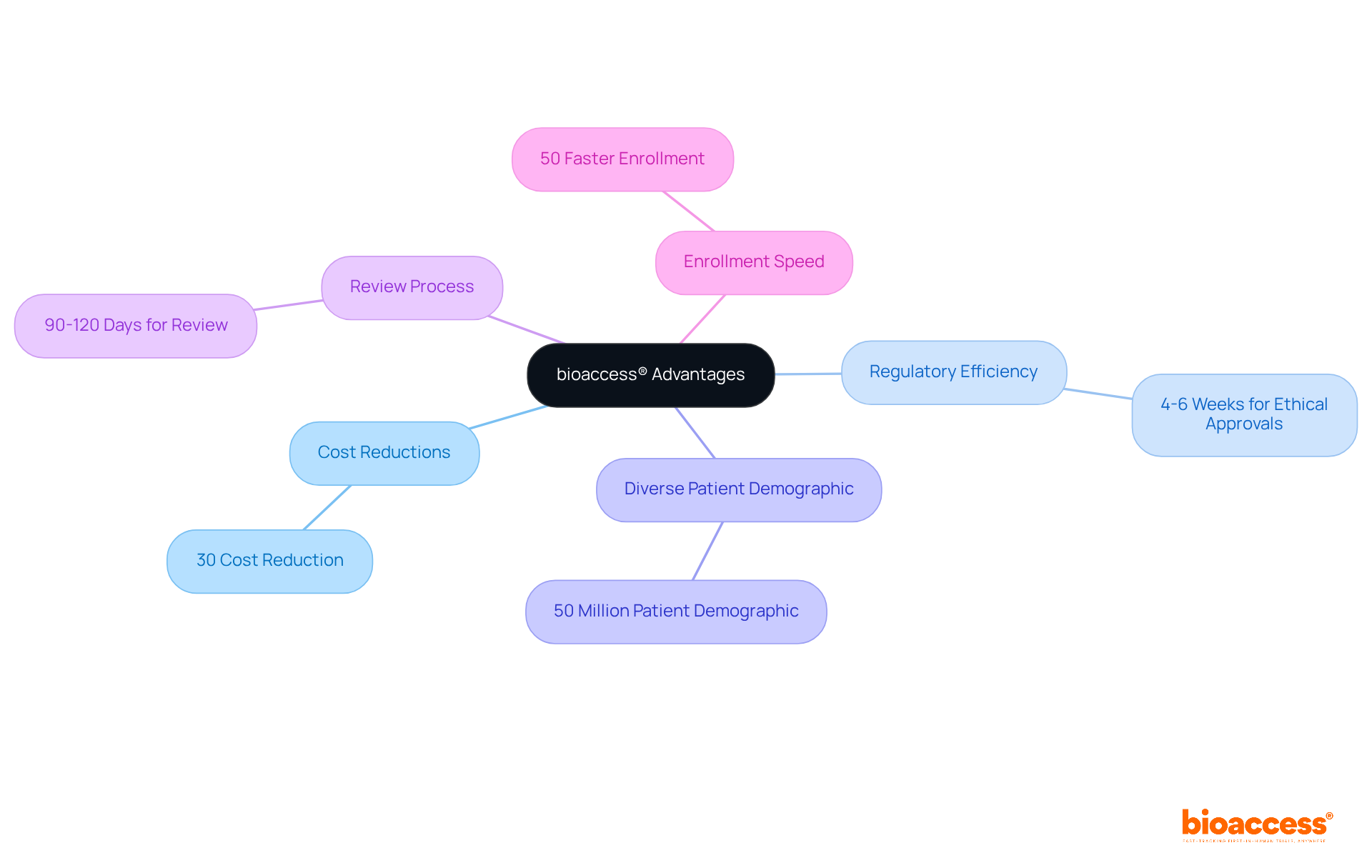
bioaccess® stands out as a leader in facilitating early-phase clinical research for healthcare devices throughout Latin America. By harnessing Colombia's competitive advantages—such as cost reductions exceeding 30% compared to North America and Western Europe, regulatory efficiency with ethical approvals secured in just 4-6 weeks, and a diverse patient demographic of over 50 million—bioaccess® empowers medical technology firms to execute studies effectively.
The comprehensive IRB/EC and MoH (INVIMA) review process in Colombia is completed within a mere 90-120 days, and the country is home to a healthcare system ranked among the top five globally. This swift turnaround is further enhanced by a 50% faster enrollment rate compared to traditional markets, positioning bioaccess® as an indispensable partner for innovators eager to expedite their products' market entry.
With over 15 years of experience, bioaccess® guarantees that each study is conducted to the highest quality standards, ultimately advancing medical technology and improving patient outcomes.
The J.P. Morgan Healthcare Conference is recognized as a premier gathering among the conferences on medical devices, drawing industry leaders, investors, and stakeholders from around the world. This event is celebrated for its outstanding networking opportunities, allowing participants to establish connections with potential investors and strategic partners.
Attendees can expect to gain invaluable insights into the latest trends in healthcare and medical technology at conferences on medical devices, which are essential for those aiming to advance their innovations and secure critical funding for their projects. The impact of networking at this conference is profound, often resulting in increased funding opportunities for medical technology initiatives, as industry experts emphasize the importance of these connections in driving growth and innovation.
Notably, bioaccess® enables the enrollment of treatment-naive cardiology or neurology cohorts at a rate 50% faster than Western sites, translating to substantial savings of $25K per patient with FDA-ready data—no rework, no delays. This efficiency not only accelerates clinical trials but also fosters economic growth in local communities by creating jobs and enhancing healthcare outcomes.
Justin Klein, Managing Partner at Vensana Capital, characterizes the MedTech Conference as one of the key conferences on medical devices, serving as an annual assembly for leaders within the medtech ecosystem, including CEOs and investors, and underscoring its role in promoting collaboration for innovation in patient care.
Furthermore, the conference will include comprehensive educational sessions and discussions on legal and compliance issues, enriching the experience for all attendees. With participation from over 1,600 companies, the J.P. Morgan Healthcare Conference, scheduled for October 5-8, 2025, is an indispensable event for those in the Medtech sector, particularly for those interested in conferences on medical devices and the broader implications of clinical studies on local economies and global health improvement.

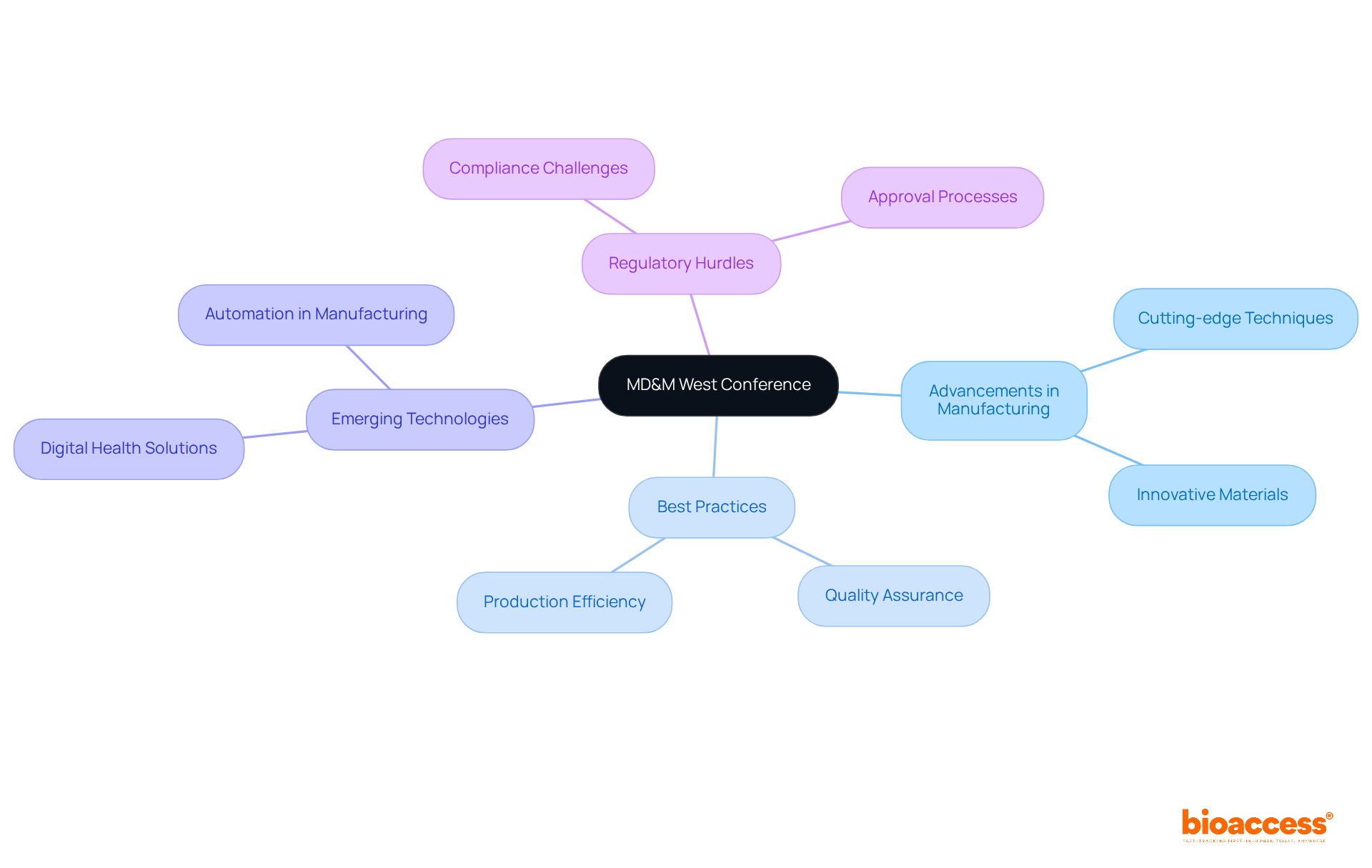
MD&M West is one of the key conferences on medical devices, highlighting the latest advancements in healthcare equipment manufacturing and innovation. This event is one of the key conferences on medical devices where industry leaders convene to deliberate on best practices, emerging technologies, and regulatory hurdles.
Attendees have the opportunity to delve into cutting-edge manufacturing techniques and materials, which are vital for the creation of high-quality healthcare devices. For Medtech professionals, conferences on medical devices offer invaluable insights aimed at refining production processes and ensuring adherence to industry standards.
The MedTech Forum serves as a premier platform for engaging discussions at conferences on medical devices regarding the future of medical technology. This conference on medical devices convenes thought leaders, innovators, and policymakers to delve into emerging trends and technologies poised to shape the industry. Participants are invited to engage in workshops and panel discussions focusing on crucial topics such as:
Notably, with bioaccess®'s expertise in accelerating clinical trials, attendees can uncover strategies to secure official approval in just 6-8 weeks and enroll treatment-naive cardiology or neurology groups 50% faster than traditional Western locations. This forum is essential for Medtech professionals seeking to stay informed and actively contribute to the industry's advancement while tackling patient recruitment challenges and navigating compliance hurdles.
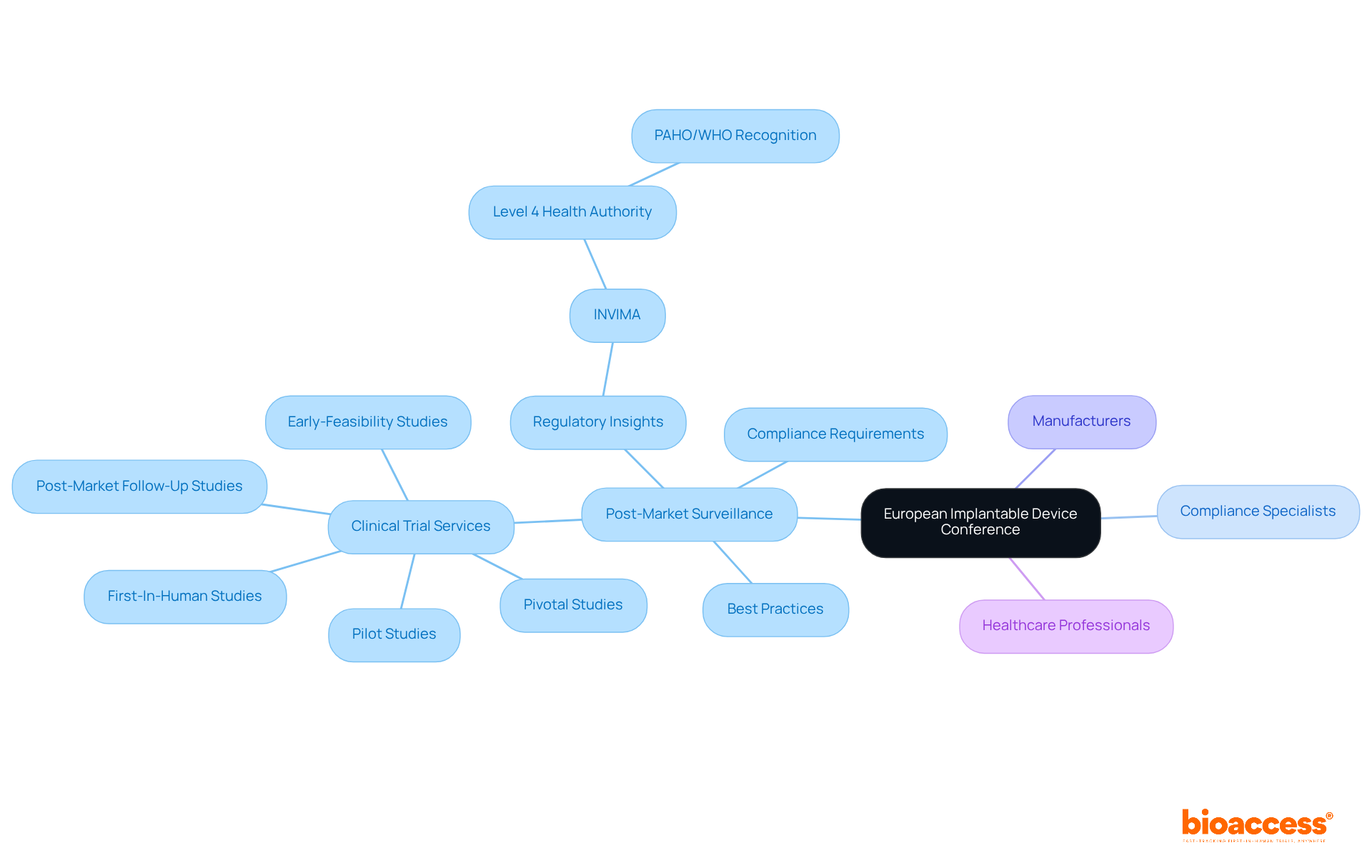
The European Implantable Product Conference underscores the essential components of post-market monitoring and adherence for medical instruments. This pivotal event convenes compliance specialists, manufacturers, and healthcare professionals to exchange best practices and strategies aimed at ensuring product safety following market entry. Participants will gain insights into the latest compliance requirements and learn how to effectively oversee the performance of implantable technologies, making this conference indispensable for Medtech firms committed to maintaining high safety standards.
With the expertise of bioaccess®, a leader in comprehensive clinical trial management services—including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—attendees will understand how these services bolster effective post-market surveillance.
Moreover, insights from regulatory bodies like INVIMA, recognized as a Level 4 health authority by PAHO/WHO, will be crucial for navigating the complexities of healthcare equipment oversight in Latin America.
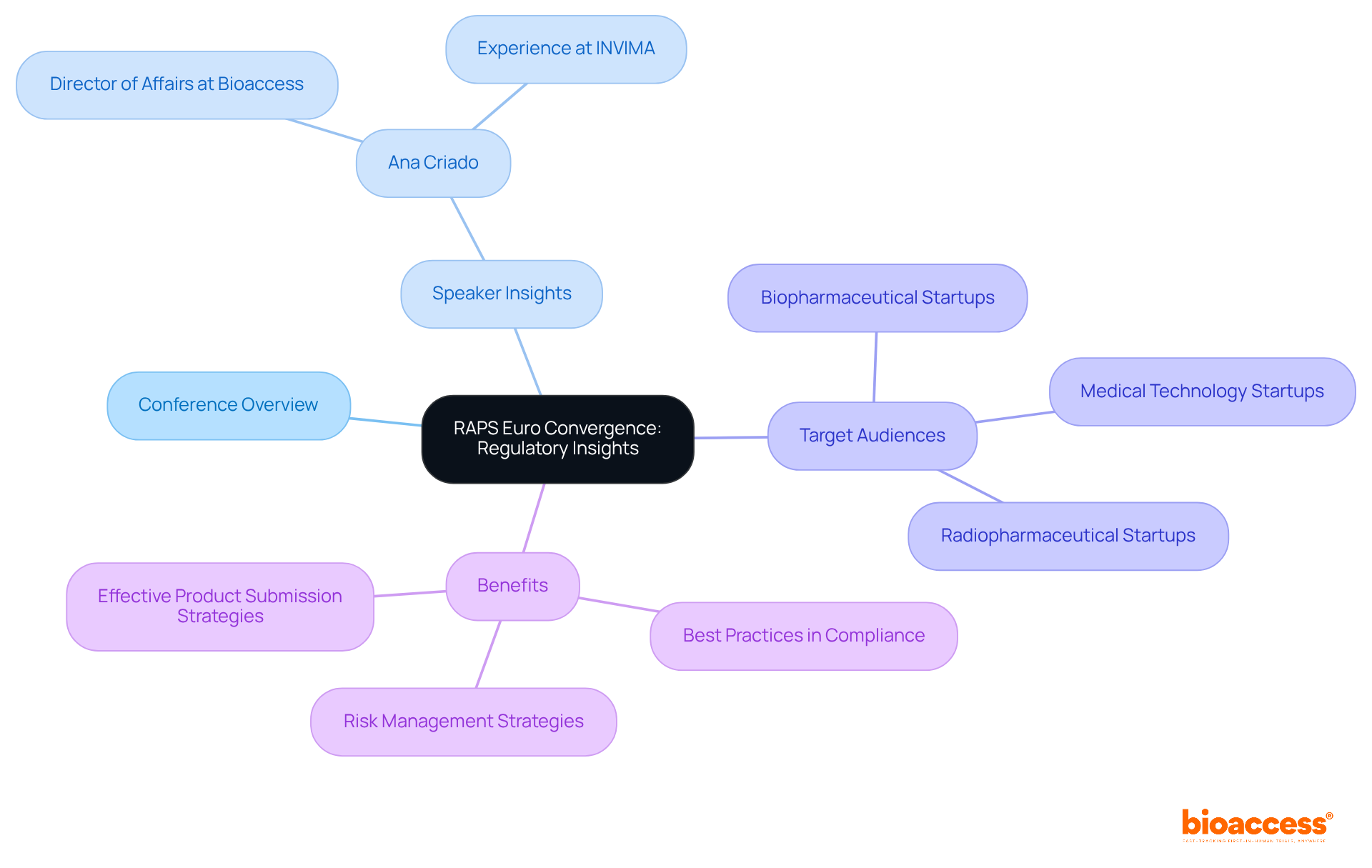
RAPS Euro Convergence is one of the key conferences on medical devices for healthcare equipment experts dedicated to deepening their understanding of the compliance landscape in Europe. This event features sessions led by compliance specialists, including insights from Ana Criado, Director of Affairs at bioaccess, who brings a wealth of experience from her leadership roles at Colombia’s oversight agency, INVIMA. Attendees will gain valuable knowledge about best practices in compliance, risk management, and effective strategies for product submissions. This conference on medical devices is essential for anyone engaged in medical device development. As regulatory complexities continue to grow, acquiring these insights is critical for:
Eager to accelerate their clinical trials and navigate the regulatory landscape with agility.
The Generis European Medical Device Summit serves as a pivotal platform for exploring strategies that drive innovation and market access, particularly in relation to conferences on medical devices within the Medtech sector. This summit convenes industry leaders for conferences on medical devices to engage in discussions surrounding the challenges and opportunities inherent in the introduction of new medical devices to the market.
Attendees will acquire invaluable insights into:
Furthermore, with the expertise of companies such as bioaccess®, which specializes in comprehensive clinical trial management services—including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—participants will gain knowledge of accelerated regulatory approval processes, significantly reducing timelines.
This summit serves as an advantageous platform for medical technology firms looking to enhance their market presence and foster innovation, particularly in the context of conferences on medical devices, while adeptly navigating the complexities of clinical trials.
The MedTech Innovation Expo stands as a pivotal event in the healthcare technology landscape, featuring conferences on medical devices that showcase groundbreaking advancements shaping the future of healthcare. This expo attracts a diverse array of exhibitors from across the Medtech sector, unveiling cutting-edge products and innovative solutions. Participants will have the opportunity to explore the latest healthcare tools and digital health innovations, including AI-driven instruments that enhance diagnostic precision and patient support.
With the global medical device market projected to exceed $600 billion by 2025, attending conferences on medical devices is essential for professionals striving to remain at the forefront of industry trends. The expo not only highlights successful innovations but also provides expert insights into emerging digital health technologies, establishing itself as an invaluable resource for advancing research and development efforts within the medical technology sector.

The American Medical Device Summit serves as a pivotal event, featuring conferences on medical devices that delve into the intricate design and product development challenges within the medical technology sector. This summit includes conferences on medical devices that feature discussions on best practices, compliance considerations, and innovative design strategies. Attendees will gain valuable insights into effectively navigating the design process to ensure compliance while optimizing product performance.
Importantly, the summit will address the unique challenges and opportunities present in the Latin American medical technology landscape, with a focus on strategies for market entry discussed at conferences on medical devices and the expedited approval processes facilitated by organizations like bioaccess®. With bioaccess®'s 6-8 week sprint method for regulatory approval, participants will learn how to enroll treatment-naive cardiology or neurology groups 50% faster than in Western locations. This makes the summit essential for professionals involved in conferences on medical devices and the development of new healthcare devices.
Medica stands as the global hub for medical technology networking and innovation. This premier trade fair attracts thousands of exhibitors and attendees from around the world, providing a unique opportunity for medical technology professionals to connect and collaborate.
Attendees can delve into groundbreaking innovations, such as bioaccess®'s revolutionary 6-8 week sprint approach for regulatory approval, which facilitates accelerated patient enrollment in cardiology and neurology cohorts—achieving results 50% faster than traditional methods in Western sites. This innovative solution not only tackles patient recruitment challenges but also significantly enhances the potential for economic growth and healthcare advancement within local communities.
By participating in Medica, attendees can engage in meaningful discussions with industry leaders about advancing global health improvement through international collaboration and innovation in Medtech, making this event essential for anyone seeking to expand their network and gain valuable insights from conferences on medical devices regarding the future of medical technology.
Attending the right conferences on medical devices in 2025 is crucial for professionals aiming to stay ahead in this rapidly evolving industry. Networking opportunities at the J.P. Morgan Healthcare Conference and insights on manufacturing at MD&M West illustrate the unique advantages each event offers, significantly impacting the future of medical technology. Additionally, the focus on regulatory compliance and post-market surveillance at events like the European Implantable Device Conference and RAPS Euro Convergence underscores the importance of maintaining high safety standards in the medical device sector.
Key discussions at these conferences will delve into:
These topics equip attendees with valuable knowledge to propel their projects forward. Events such as the MedTech Forum and Generis European Medical Device Summit will center on the future of healthcare technology, while the MedTech Innovation Expo will showcase the latest advancements shaping the industry.
As the global medical device market continues to expand, engaging with these conferences is not merely beneficial—it's essential. Professionals in the field are urged to participate actively, leveraging these platforms to foster collaboration, share knowledge, and drive innovation. By doing so, they contribute to the advancement of medical technology and ultimately enhance patient outcomes worldwide.
What is bioaccess® and what role does it play in clinical research for medical devices in Latin America?
bioaccess® is a leader in facilitating early-phase clinical research for healthcare devices in Latin America, leveraging Colombia's advantages such as cost reductions, regulatory efficiency, and a diverse patient demographic.
What are the cost advantages of conducting clinical research with bioaccess® in Colombia?
Conducting clinical research with bioaccess® in Colombia can result in cost reductions exceeding 30% compared to North America and Western Europe.
How quickly can ethical approvals be secured for clinical studies in Colombia?
Ethical approvals in Colombia can be secured in just 4-6 weeks.
What is the typical duration for the IRB/EC and MoH (INVIMA) review process in Colombia?
The IRB/EC and MoH (INVIMA) review process in Colombia is completed within 90-120 days.
How does bioaccess® improve patient enrollment rates for clinical studies?
bioaccess® achieves a 50% faster enrollment rate compared to traditional markets, allowing for quicker study execution.
How does the J.P. Morgan Healthcare Conference benefit medtech innovators?
The J.P. Morgan Healthcare Conference provides premier networking opportunities, allowing participants to connect with potential investors and strategic partners while gaining insights into the latest trends in healthcare and medical technology.
What impact does networking at the J.P. Morgan Healthcare Conference have on funding opportunities?
Networking at the conference often leads to increased funding opportunities for medical technology initiatives, as connections made are crucial for driving growth and innovation.
What are the expected savings per patient when enrolling cohorts through bioaccess®?
Enrolling treatment-naive cardiology or neurology cohorts through bioaccess® can result in substantial savings of $25K per patient with FDA-ready data.
What is MD&M West and what does it focus on?
MD&M West is a key conference on medical devices that highlights advancements in healthcare equipment manufacturing and innovation, where industry leaders discuss best practices, emerging technologies, and regulatory challenges.
What can attendees expect to learn at MD&M West?
Attendees can gain insights into cutting-edge manufacturing techniques and materials vital for creating high-quality healthcare devices, as well as refining production processes and ensuring adherence to industry standards.