


The primary focus of the article titled "10 Strategies for Clinical Research Directors to Enhance rdc" is to delineate effective strategies that Clinical Research Directors can implement to bolster research and development capabilities. It underscores the significance of:
These strategies aim to enhance the efficiency and outcomes of clinical research. This approach not only supports the overarching goal of improving patient care but also accelerates market access for innovations.
The landscape of clinical research is evolving rapidly, driven by the imperative for efficiency and effectiveness in bringing innovative therapies to market. Clinical Research Directors stand at the forefront of this transformation, confronting the dual challenge of navigating complex regulatory environments while ensuring diverse patient participation and robust data management. This article delves into ten strategic approaches that empower these leaders to enhance their research capabilities, streamline processes, and ultimately improve patient outcomes.
What innovative practices can be adopted to overcome existing barriers and leverage the full potential of clinical research?
bioaccess® effectively leverages the regulatory speed of Latin America, the diverse patient populations of the Balkans, and the streamlined pathways of Australia to secure ethical approvals within a remarkable timeframe of just 4-6 weeks. This innovative strategy empowers research directors to significantly shorten timelines and enhance efficiency, establishing bioaccess® and rdc as leading models in the industry.
With a proven track record of over 20 years in Medtech, bioaccess® specializes in managing a variety of research projects, including:
Notably, the median time for regulatory authority approval in Latin America averages a mere 51 days, enabling organizations to capitalize on this speed to accelerate their clinical trials. Furthermore, studies conducted in Latin America can yield savings exceeding 30% in operational expenses compared to North America or Western Europe, rendering it a cost-effective choice for Medtech innovators.
The incorporation of diverse patient groups not only enhances information gathering but also fosters comprehensive insights into drug effectiveness across various demographics, as highlighted by industry leaders. As noted by the Bioaccess Content Team, 'The advantages include cost-effectiveness, diverse patient populations for comprehensive data collection, and streamlined approval processes.'
This capability to navigate expedited processes effectively translates into significant advantages for Medtech innovators, ultimately driving faster access to market and improved patient outcomes with the help of rdc.

Navigating the regulatory landscape in Latin America requires a comprehensive understanding of local laws and guidelines. Clinical Research Directors must prioritize early engagement with local regulatory bodies, utilizing their insights to streamline processes. Staying informed about recent legislative changes, particularly the updates anticipated in 2025, is essential for ensuring compliance and expediting approvals.
Effective strategies include:
As Julio G. Martinez-Clark, CEO of bioaccess®, states, "With their profound grasp of the local environment, they connect the divide between your creative concepts and the practical aspects of research studies."
By implementing these strategies, organizations can enhance their compliance initiatives and simplify the clinical research process in this dynamic region.

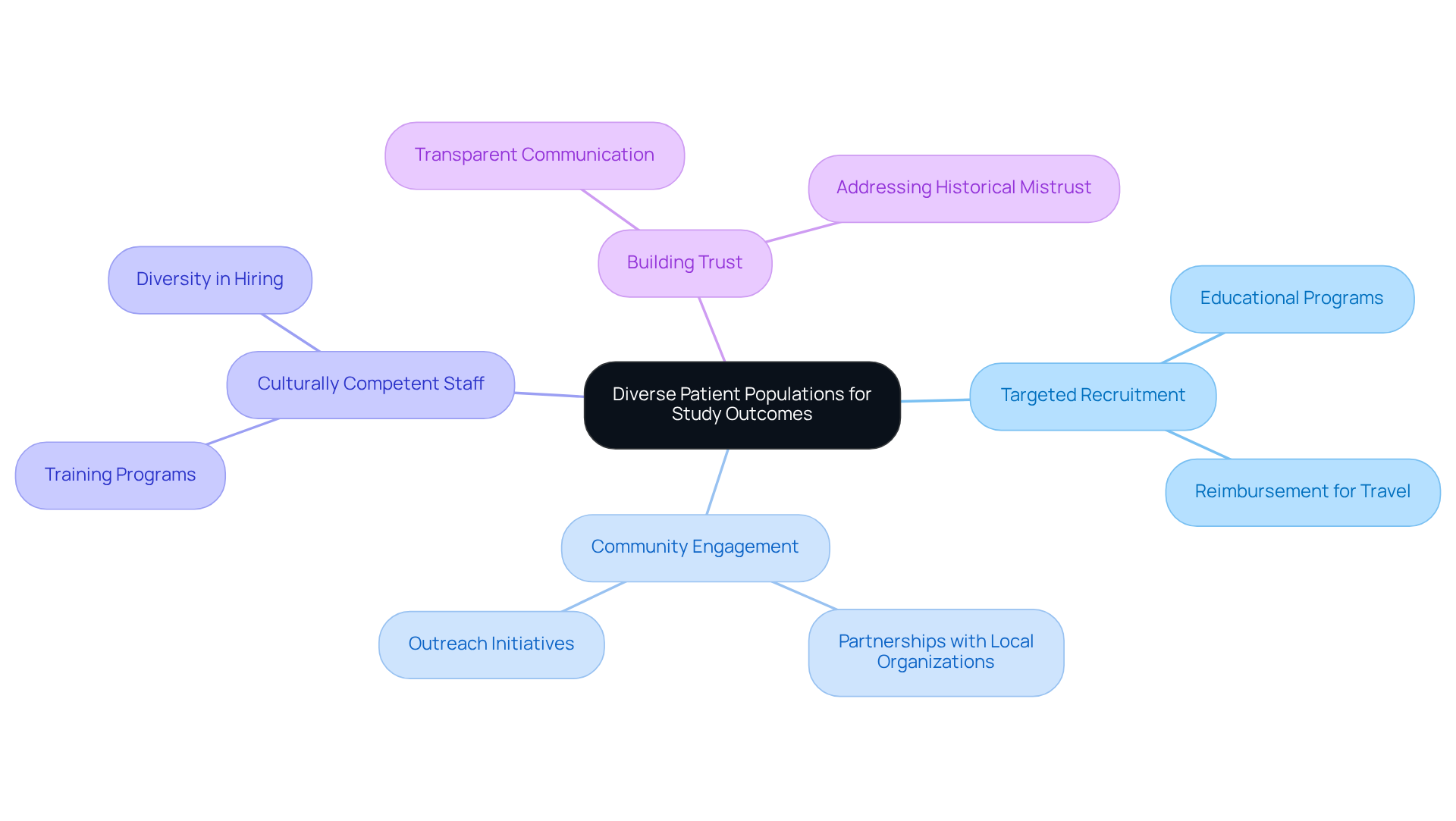
Leveraging diverse patient populations significantly enhances study outcomes, ensuring that findings are relevant across a broader demographic spectrum. Clinical Research Directors must adopt targeted recruitment strategies, which include engaging with community organizations and utilizing culturally competent staff. For instance, educational programs and reimbursement for travel expenses have proven successful in boosting participation rates among underrepresented populations. Notably, in Alzheimer's studies, the involvement of Black patients rose by more than 100% through these strategies.
Furthermore, fostering trust through transparent communication and addressing historical mistrust—such as the legacy of the Tuskegee Syphilis Study—can further encourage diverse participation. As emphasized by specialists, including Stephanie Monroe, ensuring that medical studies reflect the demographics of the population is not merely a question of fairness; it is crucial for the safety and effectiveness of treatments.
Additionally, it is essential to acknowledge that Black Americans constituted only 7% of the Moderna Covid-19 vaccine study, compared to 13% of the US population, highlighting the persistent issue of underrepresentation in research. By prioritizing diversity in recruitment initiatives and addressing obstacles such as mistrust, discomfort with the research process, and insufficient information, Clinical Research Directors can enhance the relevance and applicability of their results. Ultimately, this approach leads to more effective healthcare solutions for all demographic groups.
Initial clinical research is paramount for assessing the safety and effectiveness of new therapies, laying the groundwork for subsequent trials. These studies yield vital information that informs the design of later experiments and significantly impacts the overall success of drug development initiatives. For instance, the probability of a drug advancing from Phase I to later stages is approximately 66.4%, underscoring the importance of robust early-phase data in mitigating risks associated with later evaluations.
Clinical Research Directors must prioritize these early-phase investigations, as they offer invaluable insights that can boost the likelihood of success in subsequent phases. Successful early-stage studies have shown an increase in the probability of medications progressing to later phases, with success rates for drugs that have undergone thorough early-stage assessments being markedly higher. Research indicates that academic projects collaborating with industry partners achieve greater success rates, particularly in later phases, highlighting the critical role of early-stage research in shaping the trajectory of drug development.
In addition to Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), bioaccess provides comprehensive management services for studies, including:
Insights from research directors emphasize the significance of these studies: "Early-phase investigations are crucial for understanding the pharmacokinetics and biodistribution of new compounds, which can profoundly influence later-stage study designs." By focusing on early-phase trials, Clinical Research Directors can gather essential data and enhance the overall success rates of their drug development programs, ultimately leading to more effective therapies reaching the market.

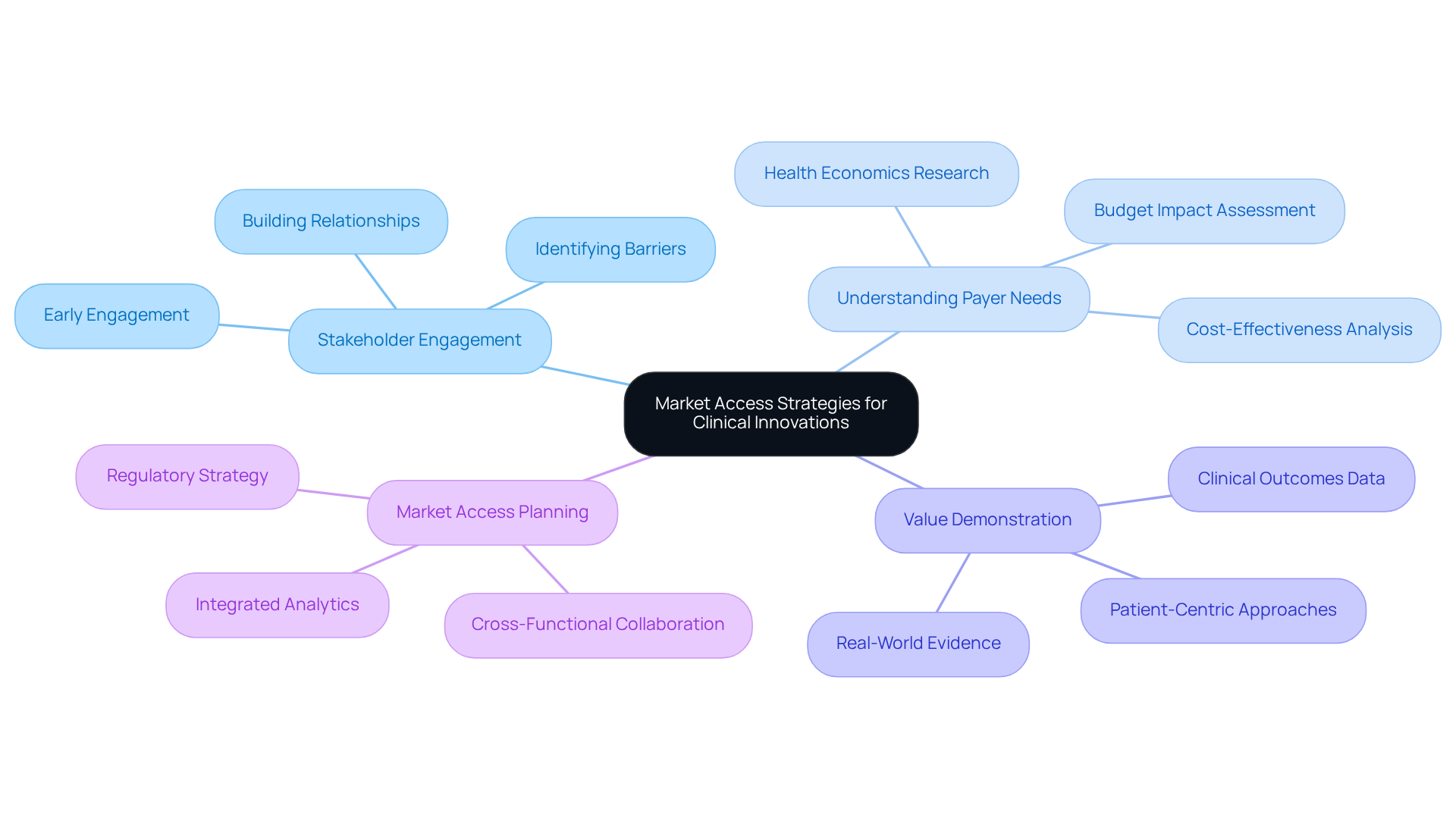
Establishing successful market access strategies requires proactive engagement with stakeholders, a deep understanding of payer needs, and a clear demonstration of the value of healthcare innovations. Research Directors must prioritize the development of comprehensive market access plans that are intricately aligned with their overall rdc development strategies. This alignment not only facilitates successful product launches but also enhances the overall efficiency of the research process.
For instance, early stakeholder engagement can significantly influence product launch success, as it enables the identification of potential barriers and fosters supportive relationships with payers and healthcare providers. Companies that incorporate market access considerations early in the development process frequently experience improved outcomes, including expedited approvals and better alignment with market demands.
By leveraging insights from successful product launches and examples of stakeholder engagement, Research Directors can devise robust rdc strategies that enhance their innovations' market readiness and acceptance. Furthermore, bioaccess® specializes in overseeing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies, ensuring that healthcare innovations are developed efficiently and positioned effectively for market access.
With over 20 years of experience in Medtech, bioaccess® achieves 50% faster enrollment compared to traditional markets, underscoring the advantages of early engagement.
Establishing strategic alliances with research entities, educational institutions, and industry participants is essential for enhancing the success of medical research. Clinical Research Directors must actively pursue collaborations with rdc that align with their objectives, facilitating the sharing of resources and expertise.
For instance, bioaccess™ has partnered with Caribbean Health Group to position Barranquilla as a leading hub for medical studies in Latin America, a collaboration supported by Colombia's Minister of Health. Such partnerships not only foster innovation through rdc joint initiatives but also enhance the overall effectiveness of medical studies.
Evidence from successful collaborations shows that aligning goals and pooling resources can lead to accelerated study timelines and improved outcomes. As Helen Keller aptly stated, 'Alone we can do so little; together we can do so much.'
Furthermore, statistics reveal that 82% of B2B business leaders planned to expand their network of partners in 2022, highlighting the increasing acknowledgment of the significance of partnerships. By prioritizing these strategic alliances, organizations can navigate the complexities of medical trials more effectively and achieve greater success in their rdc-related research endeavors.

Ensuring ethical compliance in medical research is paramount, involving strict adherence to established guidelines, obtaining informed consent, and maintaining transparency throughout the research process.
Clinical Research Directors play a crucial role in this endeavor by implementing comprehensive training programs that cultivate a culture of ethical awareness and accountability among their teams. Research indicates that effective ethics training can significantly enhance participants' comprehension of ethical standards, leading to improved decision-making and compliance with regulatory requirements.
Furthermore, the importance of informed consent cannot be overstated; it is a fundamental ethical principle that protects participants' rights and fosters trust in the research process. Openness in research is also crucial, as it guarantees that all parties involved, including ethics committees, are kept updated about the project's advancement and any possible concerns that emerge.
By prioritizing these elements, Clinical Research Directors can significantly enhance the ethical landscape of their research practices, ultimately leading to more reliable and credible outcomes.

The incorporation of technology—especially electronic information capture (EDC) systems, telemedicine, and AI-driven analytics—is transforming clinical research processes. Effective applications of EDC systems have shown considerable enhancements in information-gathering efficiency and precision, with some research indicating up to an 80% speedup in study timelines. Furthermore, AI technologies are increasingly utilized to enhance patient recruitment and engagement, with tools like Amgen's ATOMIC reducing patient enrollment time by as much as 50%. Telemedicine also plays a crucial role, enabling remote patient monitoring and consultations, which can lead to higher retention rates and improved participant experiences.
Industry leaders emphasize the necessity of adopting these technologies to remain competitive. For example, a recent survey revealed that 79% of global leaders in healthcare have interacted with generative AI, underscoring its increasing significance in study design and implementation. The effect of AI and telemedicine on research efficiency cannot be overstated; studies using integrated data management solutions have demonstrated the ability to finish patient enrollment 30% quicker, while AI-driven analytics can forecast potential adverse events, ensuring timely interventions and improving participant safety.
As medical research progresses, investing in innovative tools that simplify study management and enhance patient involvement will be crucial for RDCs seeking to improve their operations and results.

Addressing recruitment obstacles in research studies necessitates a comprehensive approach that integrates focused outreach, community involvement, and online recruitment platforms. Research Directors must develop robust recruitment strategies that not only identify and tackle barriers to participation but also elevate awareness about the significance of rdc in relation to clinical trials.
Effective community engagement plays a pivotal role in enhancing participation rates, fostering trust, and encouraging meaningful dialogue between researchers and potential participants. By leveraging local networks and resources, directors can create tailored outreach initiatives that resonate with diverse populations.
Furthermore, utilizing digital platforms for recruitment can expand reach and streamline the process, facilitating easier access to information and expression of interest for potential participants.
In summary, a holistic approach prioritizing community involvement and innovative outreach methods is essential for improving recruitment outcomes in research.

Ensuring information integrity in clinical research is paramount for achieving reliable outcomes. It begins with implementing robust information management practices, which necessitate establishing clear protocols for collection, validation, and storage. Clinical Research Directors must prioritize information integrity by leveraging advanced management systems that support rdc for real-time monitoring and compliance with regulatory standards. Regular audits are essential to identify discrepancies and ensure adherence to established protocols.
The impact of information reliability on clinical study results is significant. Research indicates that trials with unified information management workflows finalize patient enrollment 30% faster and achieve database lock in 45% less time compared to those using disjointed systems. This efficiency not only accelerates timelines but also enhances the overall quality of information collected.
Experts in information management emphasize the importance of a strategic approach to integrity. For instance, Sarah Lee notes that pharmaceutical companies implementing robust research management systems can reduce study costs by an average of 25%. Additionally, the adoption of end-to-end information management solutions has been linked to a 23% increase in the likelihood of regulatory approval, underscoring the financial and operational benefits of prioritizing rdc for information integrity.
In the realm of comprehensive trial management services, bioaccess offers capabilities such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. These services not only bolster information integrity but also contribute to economic growth and healthcare improvements in local communities through job creation and international collaboration.
Effective examples of information integrity protocols in medical research highlight the efficacy of thorough monitoring strategies. In a study evaluating the effectiveness and safety of oral salmon calcitonin, a structured monitoring plan was crucial for maintaining information integrity, resulting in an overall error rate of just 0.45% across 2566 subjects and over 3 million information fields. This case illustrates how rigorous validation procedures can significantly enhance the reliability of clinical study results. Moreover, comprehensive oversight led to a notably reduced error rate of 0.27% compared to partial monitoring at 0.53%, reinforcing the argument for extensive information management practices.
In conclusion, cultivating a culture of data integrity through advanced management practices is vital for Clinical Research Directors seeking to enhance trial outcomes and ensure compliance with regulatory standards.

The strategies outlined for Clinical Research Directors highlight the critical importance of enhancing research efficiency and outcomes through innovative practices. By leveraging the unique advantages of regions like Latin America and implementing robust data management techniques, directors can streamline processes and foster a more effective clinical research environment. The emphasis on diversity in patient populations, early-phase studies, and strategic partnerships further illustrates how comprehensive approaches can lead to more meaningful and impactful research results.
Key insights from the article underscore the necessity of early engagement with regulatory bodies, the value of diverse patient recruitment, and the integration of technology to transform clinical research processes. Each strategy contributes to a holistic framework that not only improves compliance and operational efficiency but also enhances the overall quality of clinical trials. The focus on ethical practices and effective market access strategies is equally vital, ensuring that innovations are not only developed but also successfully introduced to the market.
Ultimately, the call to action for Clinical Research Directors is clear: embracing these strategies is essential for achieving success in clinical trials. By prioritizing agility, diversity, and ethical compliance, research leaders can significantly improve patient outcomes and drive advancements in healthcare. The future of clinical research hinges on the ability to adapt and innovate, making these strategies not just beneficial but necessary for the evolving landscape of medical research.
What is bioaccess® and what role does it play in clinical research?
bioaccess® is an organization that leverages the regulatory speed of Latin America, diverse patient populations of the Balkans, and streamlined pathways of Australia to secure ethical approvals for clinical research within 4-6 weeks. It specializes in managing various research projects, enhancing efficiency and shortening timelines in clinical trials.
What types of research projects does bioaccess® manage?
bioaccess® manages a variety of research projects, including Early-Feasibility Assessments (EFS), First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and Post-Market Clinical Follow-Up Assessments (PMCF).
How does the regulatory approval timeline in Latin America compare to other regions?
The median time for regulatory authority approval in Latin America averages 51 days, which is significantly faster than many other regions, allowing organizations to accelerate their clinical trials effectively.
What are the financial benefits of conducting studies in Latin America?
Studies conducted in Latin America can yield savings exceeding 30% in operational expenses compared to conducting similar studies in North America or Western Europe.
Why is leveraging diverse patient populations important in clinical research?
Leveraging diverse patient populations enhances study outcomes and ensures findings are relevant across a broader demographic spectrum. This approach allows for comprehensive data collection and improves the safety and effectiveness of treatments.
What strategies can Clinical Research Directors use to recruit diverse patient populations?
Effective strategies include engaging with community organizations, employing culturally competent staff, providing educational programs, and reimbursing travel expenses to increase participation among underrepresented populations.
How can historical mistrust affect participation in clinical studies?
Historical mistrust, such as the legacy of the Tuskegee Syphilis Study, can discourage diverse participation in clinical research. Addressing this mistrust through transparent communication is essential for improving recruitment from diverse demographics.
What are some challenges in achieving diversity in clinical research?
Challenges include underrepresentation of certain demographic groups, discomfort with the research process, and insufficient information about the studies. Overcoming these obstacles is crucial for enhancing the relevance and applicability of research results.
What is the significance of ensuring that medical studies reflect the demographics of the population?
Ensuring that medical studies reflect the demographics of the population is important not only for fairness but also for the safety and effectiveness of healthcare solutions across all demographic groups.