


The article titled "10 Strategies for Effective CRF Clinical Trial Design" focuses on outlining key strategies that enhance the design and implementation of Case Report Forms (CRFs) in clinical trials. It emphasizes the critical importance of:
These essential strategies significantly improve data quality, streamline processes, and ensure adherence to regulatory standards. Ultimately, this leads to more efficient and reliable clinical trials, underscoring the necessity for these approaches in the evolving landscape of clinical research.
The landscape of clinical trials is rapidly evolving, driven by the need for efficiency and accuracy in data collection. As researchers seek to navigate the complexities of Case Report Form (CRF) design, understanding and implementing effective strategies becomes paramount. This article delves into ten key approaches that not only streamline the design process but also enhance data integrity and regulatory compliance. However, with so many evolving standards and technologies, what are the most critical elements that can make or break a clinical trial's success?
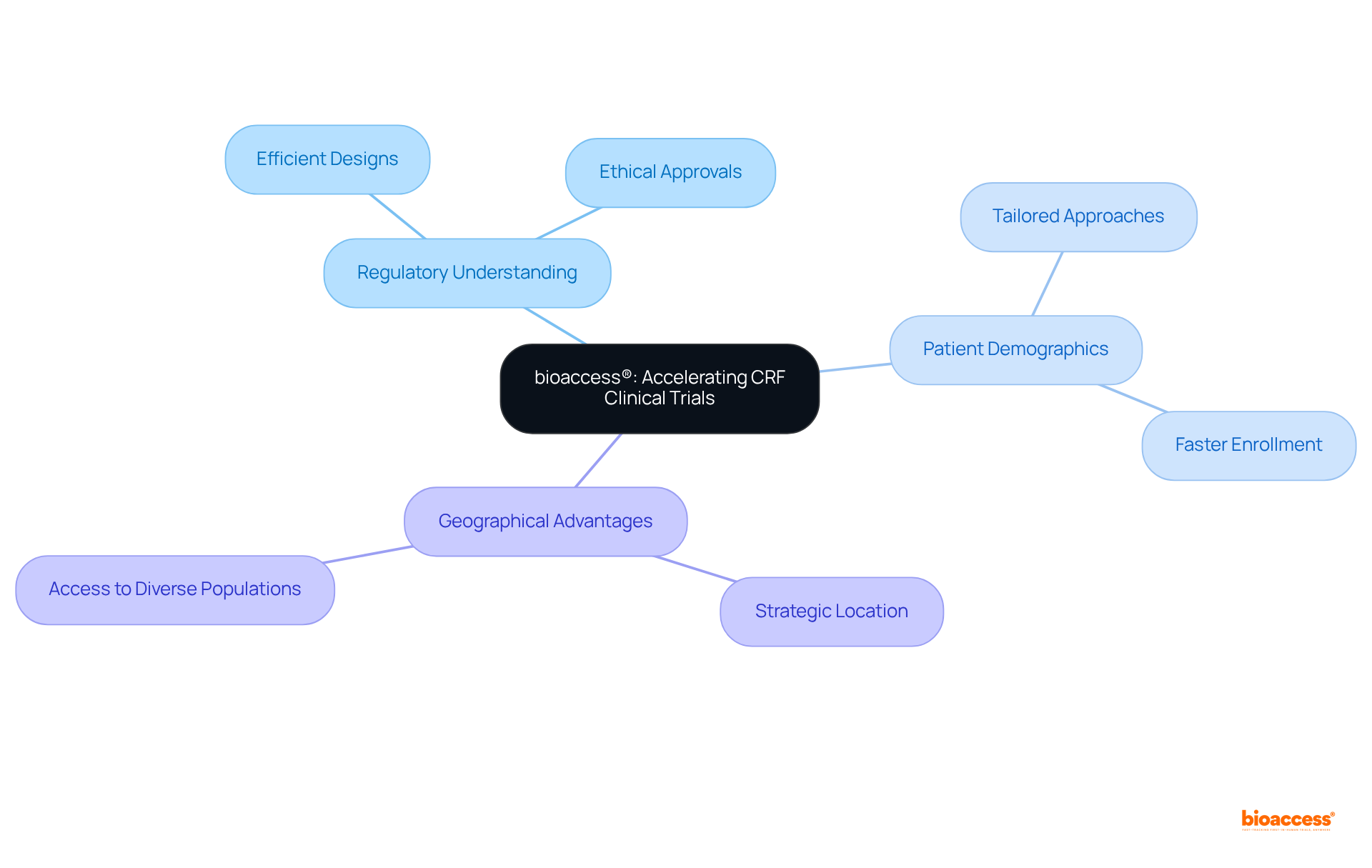
bioaccess® accelerates research processes by leveraging its deep understanding of regulatory environments and patient demographics. By capitalizing on its unique geographical advantages, bioaccess® ensures that its CRF clinical trial research designs are not only efficient but also tailored to meet the specific needs of Medtech, Biopharma, and Radiopharma innovators. This strategic framework facilitates expedited ethical approvals and patient enrollment, ultimately resulting in faster study outcomes and significant advancements in medical technology.
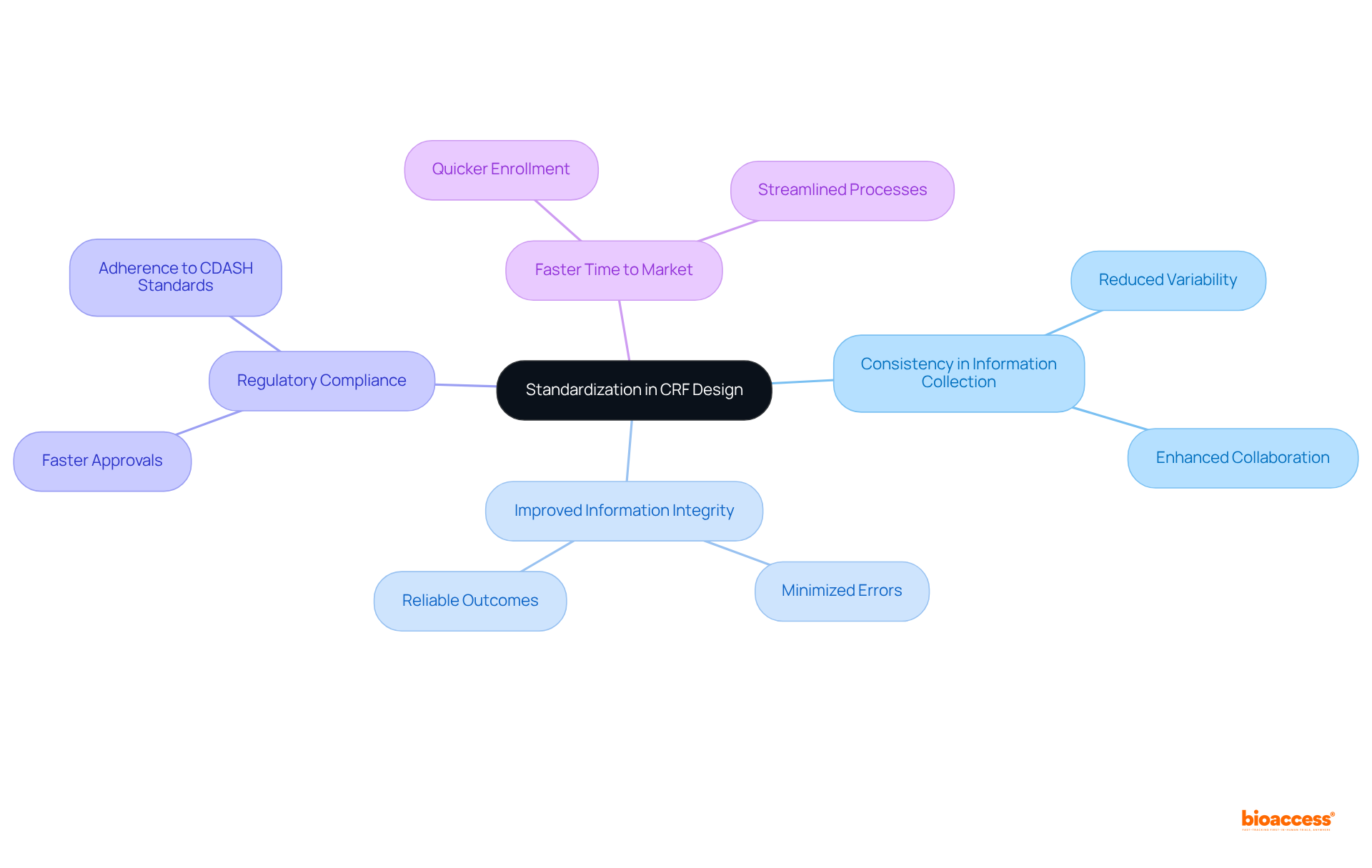
Implementing standardized Case Report Forms (CRFs) across CRF clinical trials is essential for ensuring consistent information collection, which is vital for preserving the integrity of the study. This standardization significantly reduces variability, facilitating easier comparisons of results across diverse sites and populations. By adhering to established guidelines and templates, researchers can streamline the design process, minimize errors, and enhance the overall quality of the information gathered. Recent trends indicate a growing acknowledgment of the significance of information standards in clinical research, with over 500 organizations currently following CDISC standards. Such extensive adoption not only improves information integrity but also aids in regulatory compliance, ultimately resulting in more effective analysis and faster approvals.
Clinical research experts emphasize that reducing variability in information collection is crucial for attaining dependable results. Standardized CRFs used in a CRF clinical trial provide a structure that enhances collaboration and interoperability among research teams. Furthermore, standardization allows for flexibility in research studies, ensuring robustness while preserving reproducibility. The advantages of standardization extend beyond information quality; they also facilitate quicker enrollment and shorter time to market for new treatments, making it a strategic necessity in the evolving landscape of CRF clinical trials. As Khone Saysana notes, in an era of increasing regulations and study complexity, standardization is critical for managing diverse and complex studies.
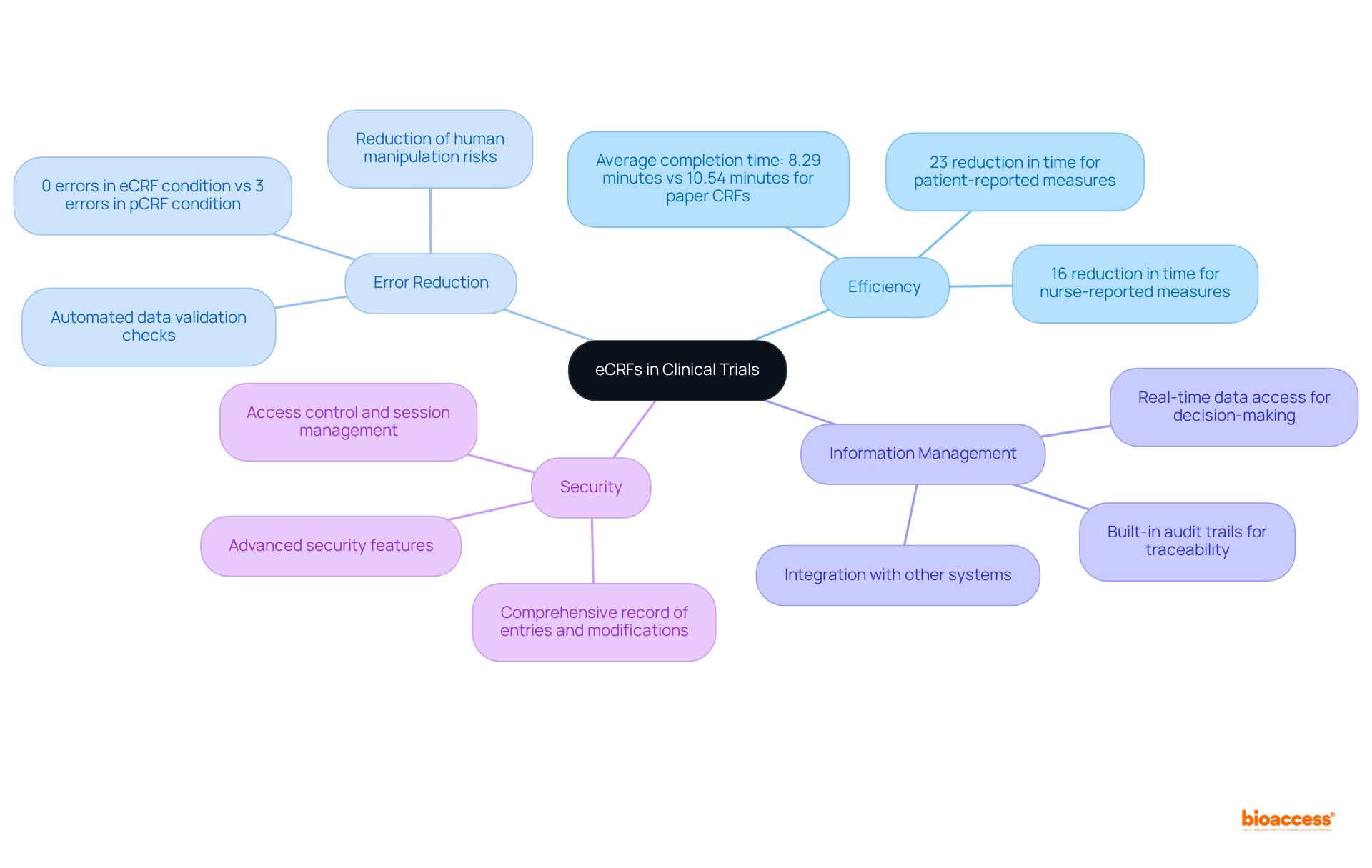
eCRFs have fundamentally transformed information collection in clinical studies by facilitating real-time data entry and monitoring. This digital approach significantly minimizes transcription errors, with studies indicating that eCRFs can virtually eliminate mistakes, in stark contrast to traditional paper forms that are prone to multiple errors. For example, a comparative study revealed that eCRFs resulted in a 23% reduction in time for patient-reported measures and a 16% reduction for nurse-reported measures, underscoring their efficiency. Moreover, information gathering via eCRFs required an average of 8.29 minutes, compared to 10.54 minutes for paper CRFs, further highlighting the time-saving benefits of eCRFs.
Additionally, eCRFs enhance information management and analysis, allowing researchers to swiftly access and interpret data, which is crucial for timely decision-making during trials. The integration capabilities of eCRFs with other systems improve information accessibility, thereby boosting operational efficiency. As industry experts have noted, the ability to input data directly into electronic forms not only accelerates the collection process but also ensures that the information is organized and standardized, facilitating higher quality submissions to regulatory agencies.
The advantages of eCRFs extend beyond mere efficiency; they also bolster information security and traceability. With built-in audit trails, eCRFs provide a comprehensive record of entries and modifications, reducing the risks associated with manual handling and potential data loss. This level of oversight is vital for maintaining the integrity of crf clinical trial information, ultimately leading to more reliable outcomes and expedited regulatory approvals.
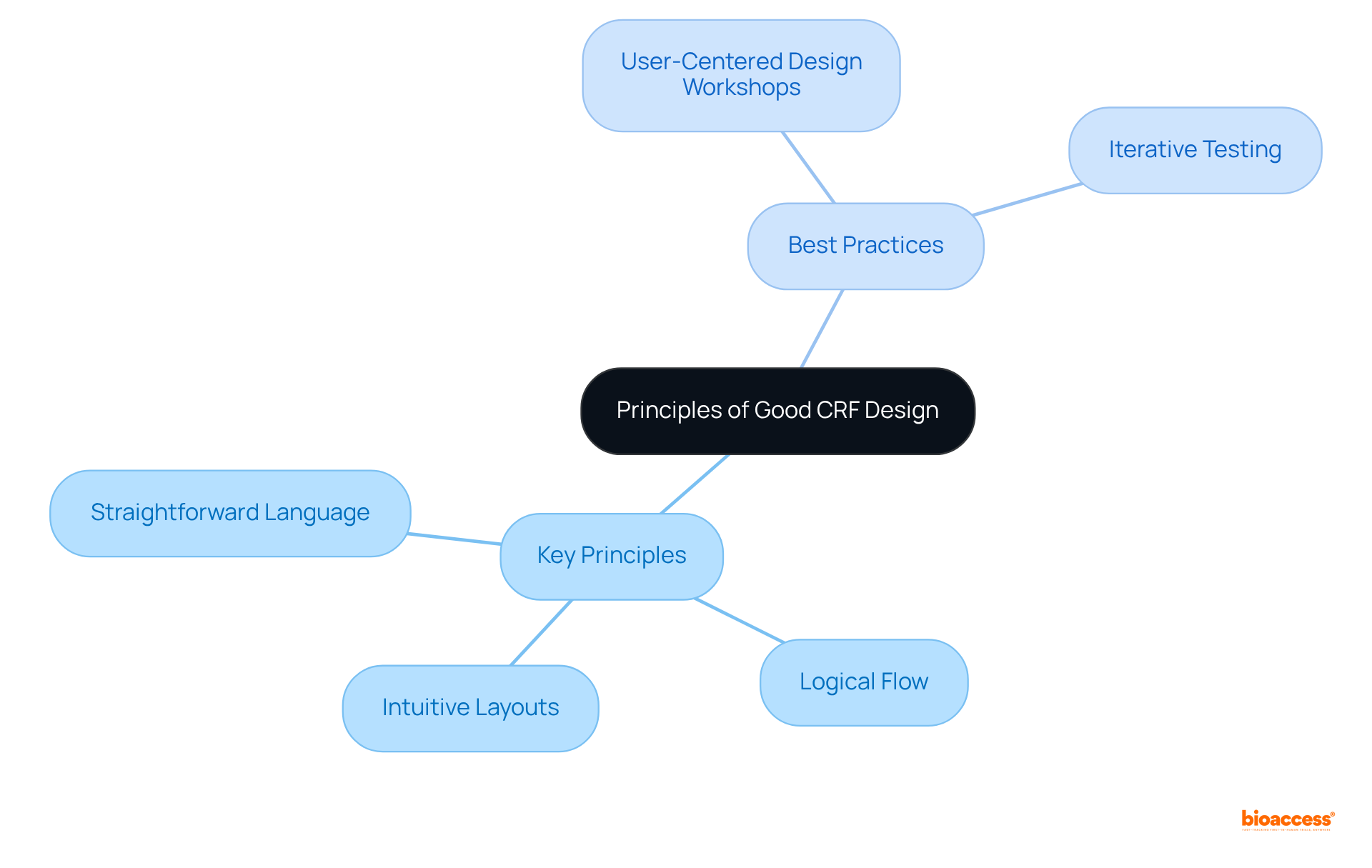
Effective CRF design must prioritize clarity, simplicity, and usability to enhance information quality and participant compliance. Key principles include:
Forms ought to be customized for the end-user, ensuring that entry is smooth and reduces the chance of mistakes. Integrating user input during the design stage is essential; research shows that usability problems in CRF design can result in considerable inaccuracies, with error rates for Medical Record Abstraction (MRA) reaching a combined rate of 6.57%.
Best practices for ensuring usability include:
These practices can significantly improve the overall effectiveness of CRFs. For example, organizations that have adopted user-centered design in their CRF clinical trial processes have reported improved information integrity and decreased error rates, showcasing the concrete advantages of emphasizing usability in clinical research.
As Maryam Y. Garza observes, "High quality information provides the foundation from which study conclusions may be drawn, and, in contrast, poor quality threatens the validity and generalizability of study findings." This underscores the essential requirement for effective CRF design in preserving information quality and adherence to industry standards.
Creating Case Report Forms with regulatory compliance as a priority is essential for the success of the CRF clinical trial. Understanding the specific requirements established by regulatory bodies such as the FDA and EMA is crucial. Case report forms must effectively gather all essential information required for regulatory submissions, ensuring that studies can withstand thorough audits and evaluations. Compliance safeguards the integrity of the research and significantly enhances the credibility of the findings.
The FDA highlights that case report forms should contain sections for essential information such as:
These elements aid in precise information management and traceability. Likewise, the EMA mandates that case report forms comply with stringent guidelines to guarantee quality and consistency across studies. Adopting CDASH standards can streamline this process, as they provide a framework for creating case report forms that meet these regulatory expectations. The latest version of CDASH, v1.3, released in September 2023, further refines these standards to enhance compliance.
Experts in the field emphasize that the implementation of standardized CRFs not only reduces the time spent on development but also minimizes data queries and enhances the overall quality of the data collected. As noted by Jagadeeswara Rao Gaddale, "By implementing the CDASH standards, the number of hours spent for CRF development, edit check specifications, programming screens, edits, and SDTM mapping will be reduced." By aligning the design of a CRF clinical trial with regulatory requirements, researchers can enhance the effectiveness of the study process and accelerate the path to market for innovative therapies. Moreover, it is vital to acknowledge that projections of early discontinuation of research studies vary from 3% to 46%, highlighting the significance of appropriate CRF design in averting study failures.
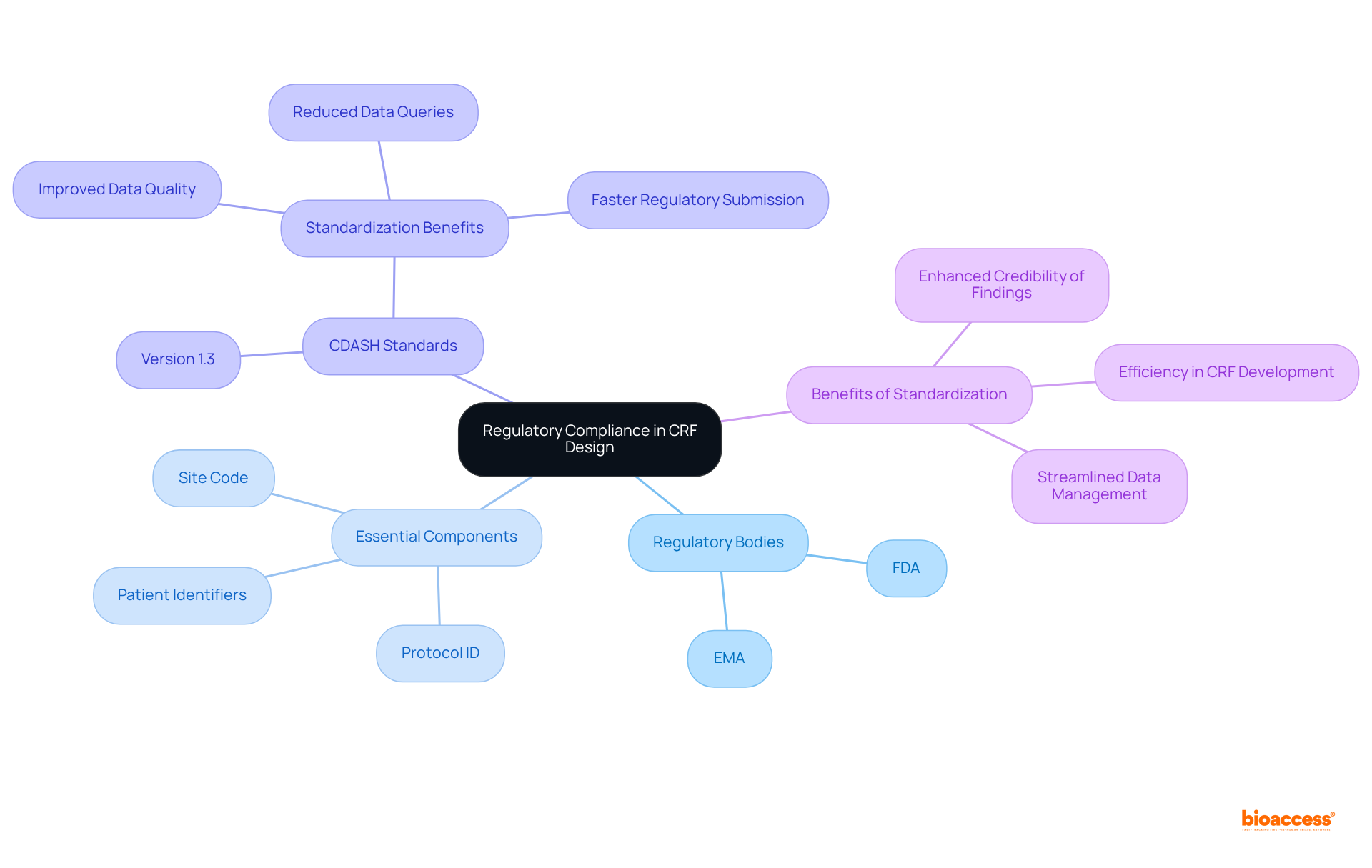
Cloud-based storage for Case Report Forms (CRFs) significantly enhances both accessibility and security in CRF clinical trials. Researchers can access information from any location, fostering collaboration among teams spread across various regions. This adaptability is crucial in today's global research landscape, where timely information sharing can accelerate testing procedures.
Moreover, cloud solutions come equipped with advanced security features, including encryption and regular backups, which protect sensitive information from potential breaches. The average cost of a cloud information breach is approximately $4.88 million, highlighting the urgent need for robust security measures. As Richard Clarke aptly states, 'If you spend more time on coffee than on IT security, you will be hacked.'
By adopting cloud storage, organizations not only optimize their information management processes but also ensure compliance with strict protection regulations, thereby fostering trust and transparency in CRF clinical trials. With 94% of businesses projected to utilize cloud solutions by 2025 and the cloud computing market anticipated to reach $947.3 billion by 2026, the integration of these technologies in CRF clinical trials is increasingly vital for maintaining information integrity and participant safety.
As William Malik emphasizes, a business will have strong security if its corporate culture is appropriate, underscoring the importance of leadership in cultivating a secure environment for information management.
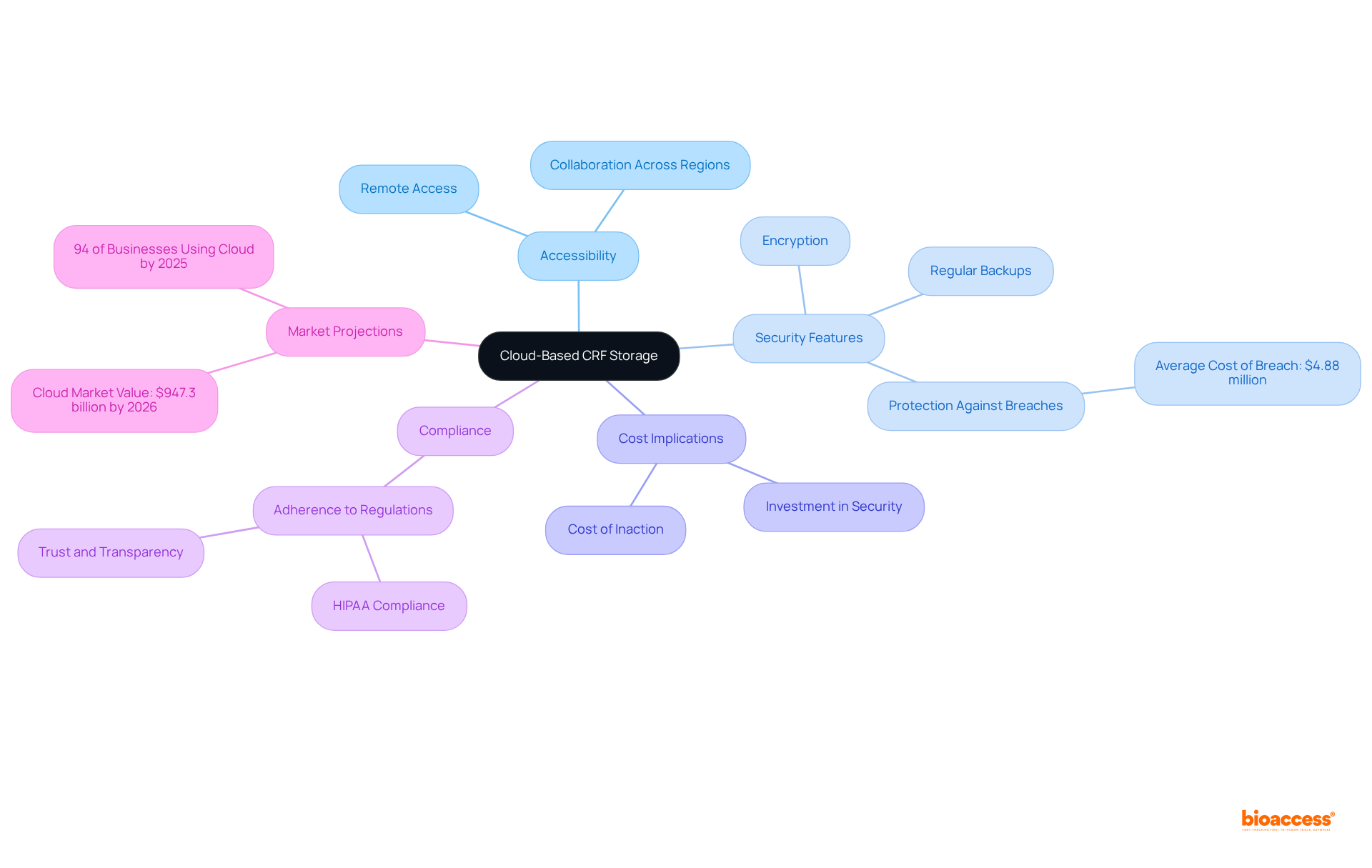
Common mistakes in CRF design often stem from:
These complicated structures can perplex users, resulting in entry mistakes that jeopardize study integrity. Additionally, neglecting to carry out pilot tests can expose unexpected problems that may arise during the actual experiment, potentially hindering the study's advancement. Furthermore, disregarding user input obstructs the recognition of usability issues that could impede efficient information gathering. By proactively addressing these challenges, researchers can create more efficient and user-friendly CRFs, which will ultimately enhance the quality and dependability of results in a CRF clinical trial.
Pilot testing is essential; it enables the evaluation of usability and information integrity of the CRF clinical trial prior to full-scale implementation. This iterative process not only improves the CRF design but also ensures that it aligns with the practical requirements of clinical workflows. Consequently, this alignment facilitates smoother information collection and analysis, reinforcing the importance of collaboration in overcoming key challenges in clinical research.
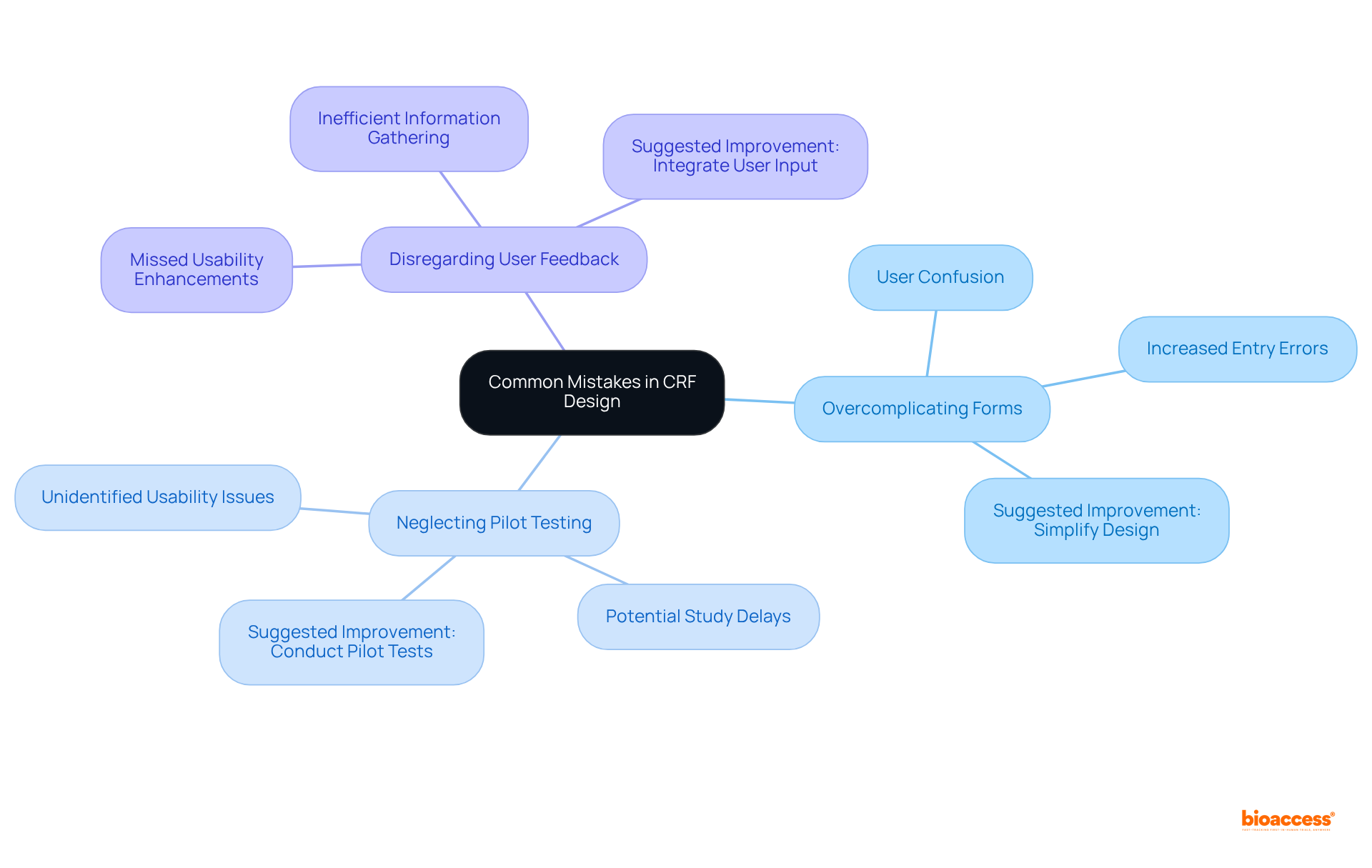
Annotated case report forms are pivotal in enhancing clarity and the interpretation of information, providing detailed explanations and definitions for each data field. This contextual information guarantees that researchers collect data consistently and accurately, which is essential for upholding high-quality standards.
Studies reveal that clear guidance and structured annotations can drastically lower error rates, with pooled error rates for single-data entry methods reaching as low as 0.29%. Furthermore, annotated conditional random fields serve as vital resources for reviewers, fostering a deeper understanding of the collected data and yielding more reliable study outcomes.
A meta-analysis demonstrated that well-crafted forms, inclusive of annotations, can improve user comprehension and streamline the information-gathering process, ultimately elevating the overall quality of medical research. Additionally, annotated case report forms are crucial for FDA submissions, underscoring their importance in medical studies.
By diminishing uncertainty and providing explicit guidelines, these forms not only facilitate precise data entry but also enhance the effective interpretation of CRF clinical trial results. Moreover, automated CRF annotations can conserve time and lessen manual effort, while minimizing free-text responses aids in maintaining data consistency.
Investing in training and resources for researchers is crucial for successful CRF design. Workshops, online courses, and access to design templates empower researchers with the knowledge and skills necessary to create effective CRFs. Training programs that include hands-on learning and real-world simulations, backed by over 30 years of experience in trials, significantly enhance the practical application of theoretical concepts, leading to improved CRF quality.
Furthermore, workshops and role-playing exercises simulate real-world challenges, providing practical tools that aid in CRF design. Continuous education guarantees that teams remain informed about best practices and regulatory changes. Research professionals must complete continuing education courses to maintain certification, promoting a culture of ongoing enhancement in medical research.
As industry leaders highlight, providing researchers with the appropriate tools and training not only improves their abilities but also aids in the overall success of medical studies.
As technology advances, the design of Case Report Forms (CRFs) must evolve to leverage innovative tools and methodologies. The combination of artificial intelligence (AI) and machine learning (ML) is transforming information gathering and examination in medical studies. For instance, AI-driven site selection has been shown to enhance the identification of top-enrolling sites by 30 to 50 percent, significantly accelerating enrollment by 10 to 15 percent. Notably, bioaccess® achieves enrollment 50% faster than traditional markets, underscoring the efficiency of these advancements. Furthermore, generative AI has enabled a reduction in document generation time, cutting the process costs by up to 50 percent through automation. The CSR drafting process has been notably reduced from 8-14 weeks to just 5-8 weeks, illustrating significant efficiency gains.
Mobile information gathering is another essential trend that improves research efficiency. By enabling real-time information entry and observation, mobile solutions enhance accuracy and participant involvement, ultimately resulting in improved study outcomes. Studies indicate that the application of AI/ML techniques can compress development timelines by an average of six months per asset, allowing innovative therapies to reach patients faster.
In this rapidly changing landscape, researchers must remain agile and open to integrating these advancements into their CRF clinical trial design processes. Embracing these innovations not only enhances efficiency and data quality but also contributes to the overall success of CRF clinical trials, ensuring that they meet the evolving needs of the healthcare sector.
The effectiveness of clinical trial designs is fundamentally rooted in the strategic implementation of various methodologies, particularly concerning Case Report Forms (CRFs). By emphasizing standardization, leveraging electronic solutions, and adhering to regulatory compliance, researchers can significantly enhance the quality and efficiency of clinical trials. The insights presented throughout this article underscore the critical importance of these strategies in not only improving data integrity but also expediting the overall research process.
Key arguments highlighted include:
Furthermore, the significance of user-centered design and comprehensive training for researchers has been emphasized, illustrating how these elements contribute to the development of effective CRFs that meet the evolving needs of the clinical research landscape.
As the field of clinical trials continues to evolve with technological advancements, it is imperative for researchers and organizations to adopt these best practices. Embracing innovations such as cloud-based storage and AI-driven tools will not only enhance data management and security but also foster a culture of continuous improvement in clinical research. By prioritizing these strategies, stakeholders can ensure that clinical trials are conducted efficiently, ultimately leading to faster access to life-saving therapies and improved patient outcomes.
What is bioaccess and how does it enhance CRF clinical trial design?
bioaccess accelerates CRF clinical trial design by leveraging its understanding of regulatory environments and patient demographics, ensuring research designs are efficient and tailored to the needs of Medtech, Biopharma, and Radiopharma innovators. This approach facilitates expedited ethical approvals and patient enrollment, leading to faster study outcomes.
Why is standardization important in CRF design for clinical trials?
Standardization of Case Report Forms (CRFs) is essential for consistent information collection, preserving study integrity and reducing variability. It enhances collaboration among research teams, facilitates easier comparison of results, and improves regulatory compliance, resulting in more effective analysis and faster approvals.
How has the adoption of CDISC standards impacted clinical research?
Over 500 organizations currently follow CDISC standards, which improves information integrity and aids in regulatory compliance. This extensive adoption enhances the quality of information gathered, making it easier to analyze results and expedite approvals.
What are the benefits of using standardized CRFs in clinical trials?
Standardized CRFs provide a structured approach that reduces variability, enhances information quality, and allows for flexibility in research studies. They facilitate quicker patient enrollment and shorter time to market for new treatments, making them strategically necessary in clinical trials.
How do eCRFs transform data collection in clinical trials?
eCRFs facilitate real-time data entry and monitoring, significantly minimizing transcription errors compared to traditional paper forms. They improve efficiency, reduce the time needed for patient-reported and nurse-reported measures, and enhance information management and analysis.
What advantages do eCRFs offer over traditional paper CRFs?
eCRFs offer time-saving benefits, improved information management, enhanced security and traceability, and built-in audit trails. They ensure organized and standardized data collection, leading to higher quality submissions to regulatory agencies.
How do eCRFs contribute to regulatory approvals?
By providing a comprehensive record of data entries and modifications, eCRFs maintain the integrity of clinical trial information, leading to more reliable outcomes and expedited regulatory approvals.