


The article presents ten strategies for effective minority recruitment in clinical trials, underscoring the critical importance of:
to engage underrepresented populations. Evidence supports these strategies, indicating that tailored approaches and community partnerships can significantly enhance participation rates and ensure diverse representation in medical research. By implementing these strategies, researchers can address key challenges and foster a more inclusive environment in clinical trials.
The landscape of clinical trials is evolving, yet the underrepresentation of minority populations remains a significant challenge that impacts the validity and applicability of research outcomes. By implementing effective recruitment strategies, organizations can not only enhance diversity but also ensure that clinical studies reflect the realities of the populations they aim to serve.
However, what are the most effective methods to engage these communities and build trust? This article delves into ten innovative strategies for minority recruitment in clinical trials, offering insights into how to foster inclusivity and improve participation rates among underrepresented groups.
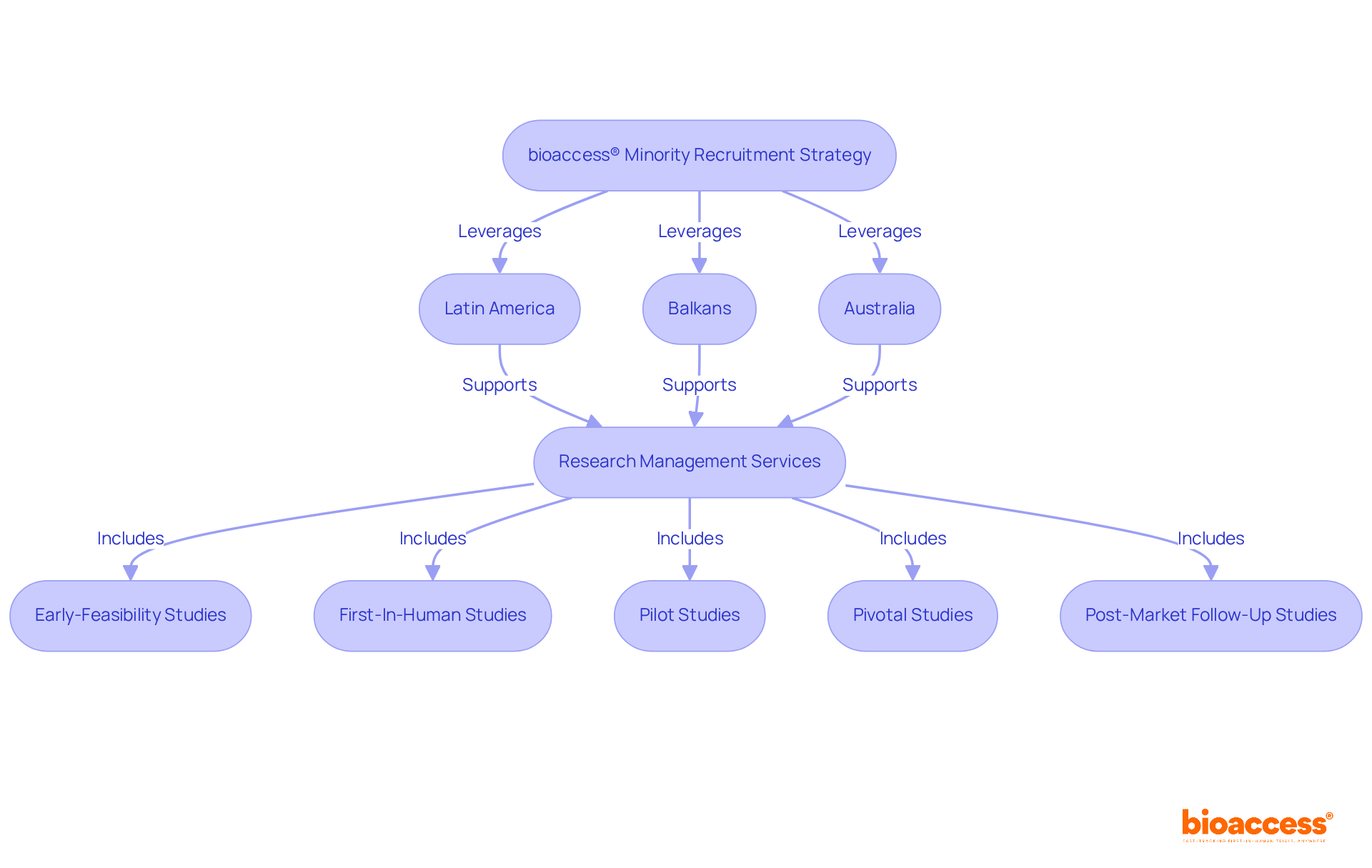
bioaccess® strategically leverages the regulatory pace of Latin America, the diverse patient demographics of the Balkans, and Australia's efficient ethical approval processes to significantly enhance minority recruitment clinical trials. With ethical approvals secured in merely 4-6 weeks and enrollment rates that are 50% faster than traditional markets, bioaccess® empowers Medtech, Biopharma, and Radiopharma innovators to effectively engage underrepresented populations.
Our comprehensive research management services include:
This ensures a tailored approach for each project. This agility not only accelerates research timelines but also fosters inclusivity in studies, especially through minority recruitment clinical trials, addressing the pressing need for diverse participant representation in medical research.
Recent advancements in hiring strategies underscore the importance of understanding demographic nuances and employing tailored outreach techniques, guaranteeing that studies reflect the diversity of the populations they aim to serve. By establishing strong partnerships with local healthcare providers and utilizing digital tools for engagement, bioaccess® is at the forefront of enhancing diverse participant enrollment, ultimately contributing to more equitable healthcare outcomes while achieving substantial cost savings of $25K per patient with FDA-ready data.
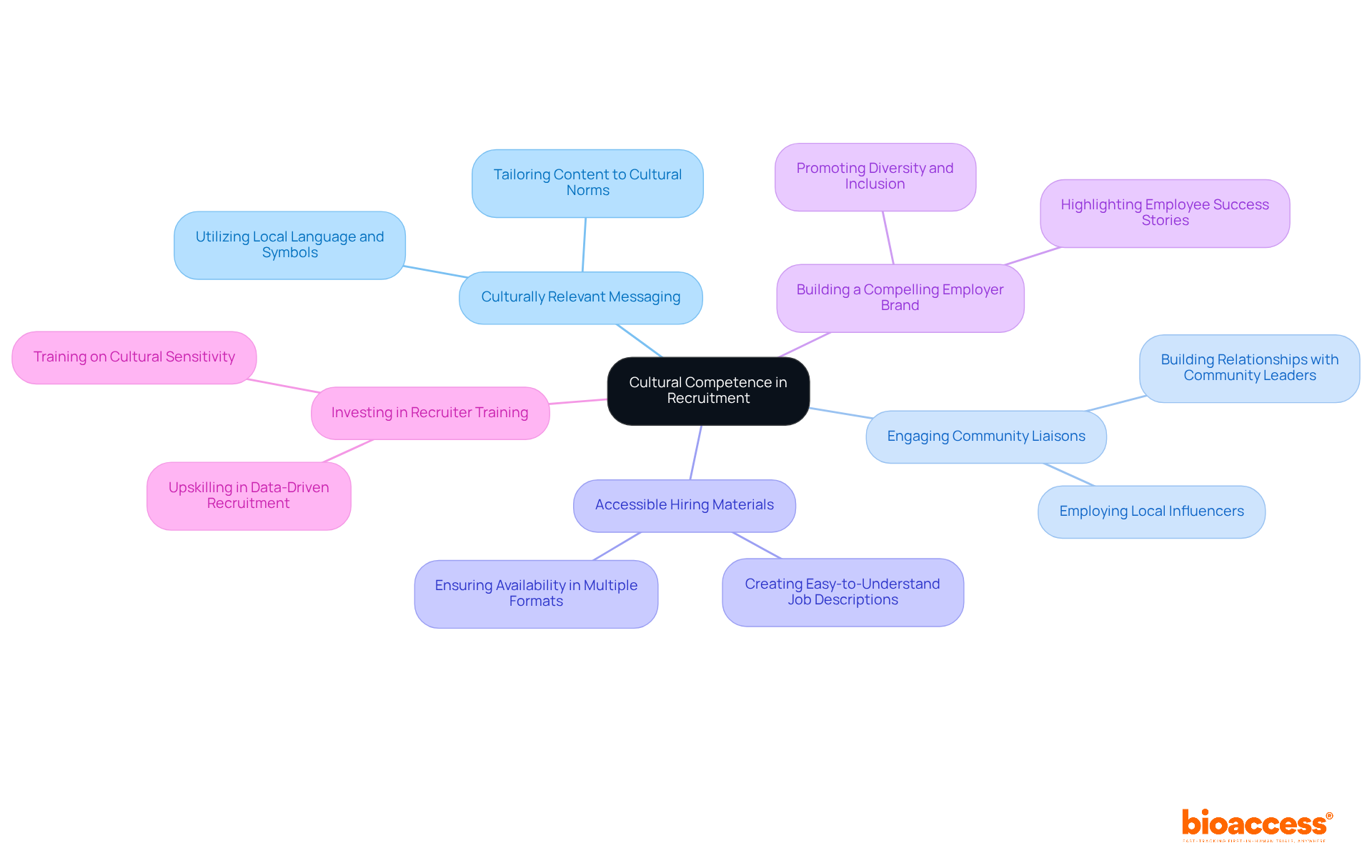
Cultural competence is paramount in understanding and respecting the diverse backgrounds of potential participants in clinical trials. By customizing hiring strategies to align with the cultural values, beliefs, and practices of minority populations, organizations significantly enhance engagement. This can involve:
Demonstrating cultural sensitivity fosters trust and rapport, which is crucial for increasing participation rates among underrepresented groups. For instance, employing local influencers or community leaders in outreach efforts creates a sense of familiarity and comfort, encouraging more individuals to participate. Furthermore, adjusting communication styles and formats to match the preferences of various groups can further improve hiring effectiveness.
A compelling employer brand is vital; organizations with strong reputations as 'employers of choice' experience higher application rates. Investing in recruiter training and development equips recruiters with the skills necessary to engage effectively with diverse populations. Overall, a culturally tailored approach not only improves engagement but also contributes to the overall success of minority recruitment clinical trials by ensuring a more representative participant pool. Research indicates that possessing a more varied workforce can result in a 35% boost in productivity, underscoring the significance of diversity in hiring strategies.
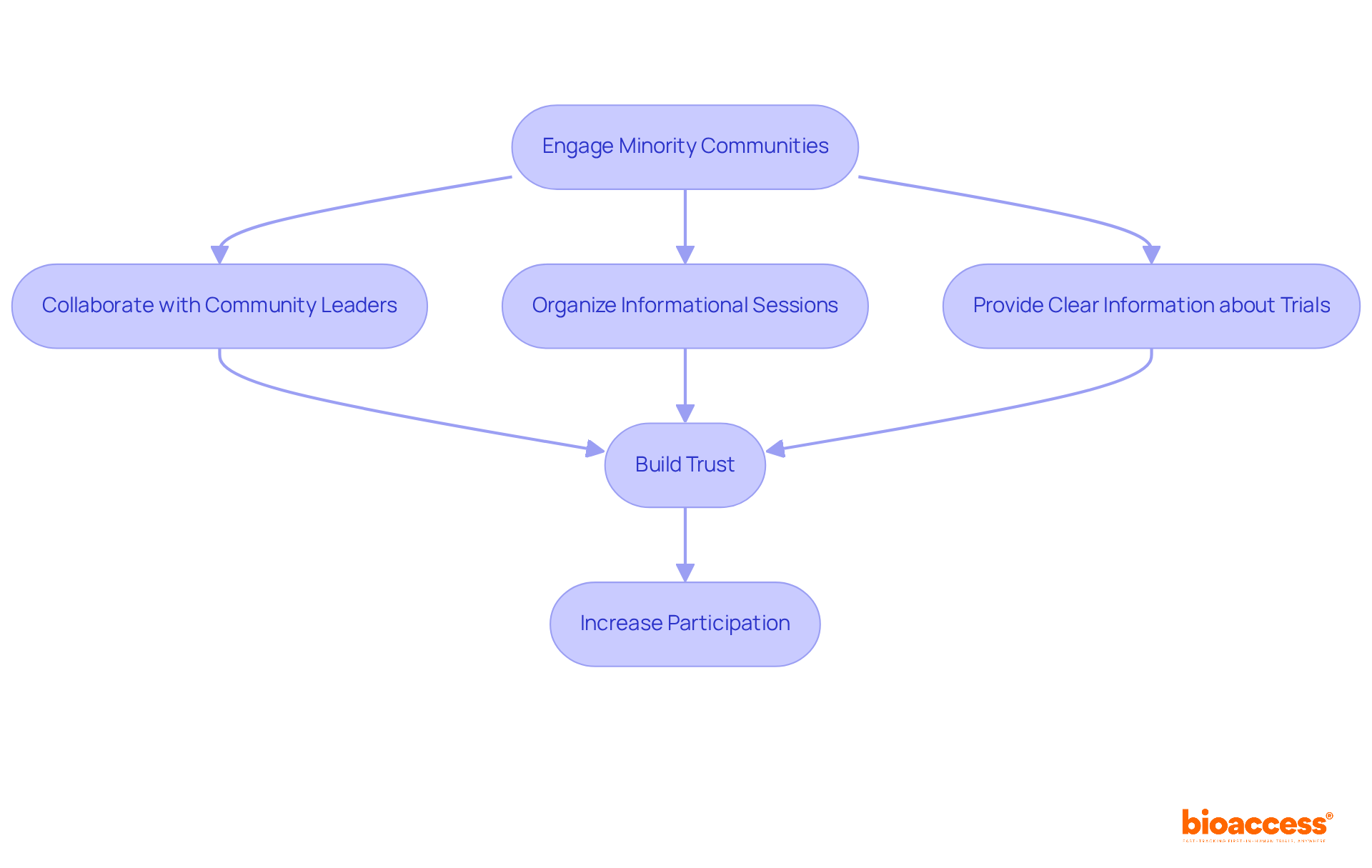
Engaging minority communities for minority recruitment clinical trials necessitates transparent communication and sustained outreach efforts to cultivate trust. It is essential for researchers to acknowledge the effects of historical injustices and actively address concerns regarding medical mistrust, particularly in the context of minority recruitment clinical trials. This can be achieved through:
Notably, media coverage, such as that from Clinical Leader, underscores the significance of research in Latin America and Colombia, which can further inform and engage these communities. By fostering a climate of trust, researchers can significantly enhance participation rates in minority recruitment clinical trials and ensure that underrepresented viewpoints are adequately represented in health studies.
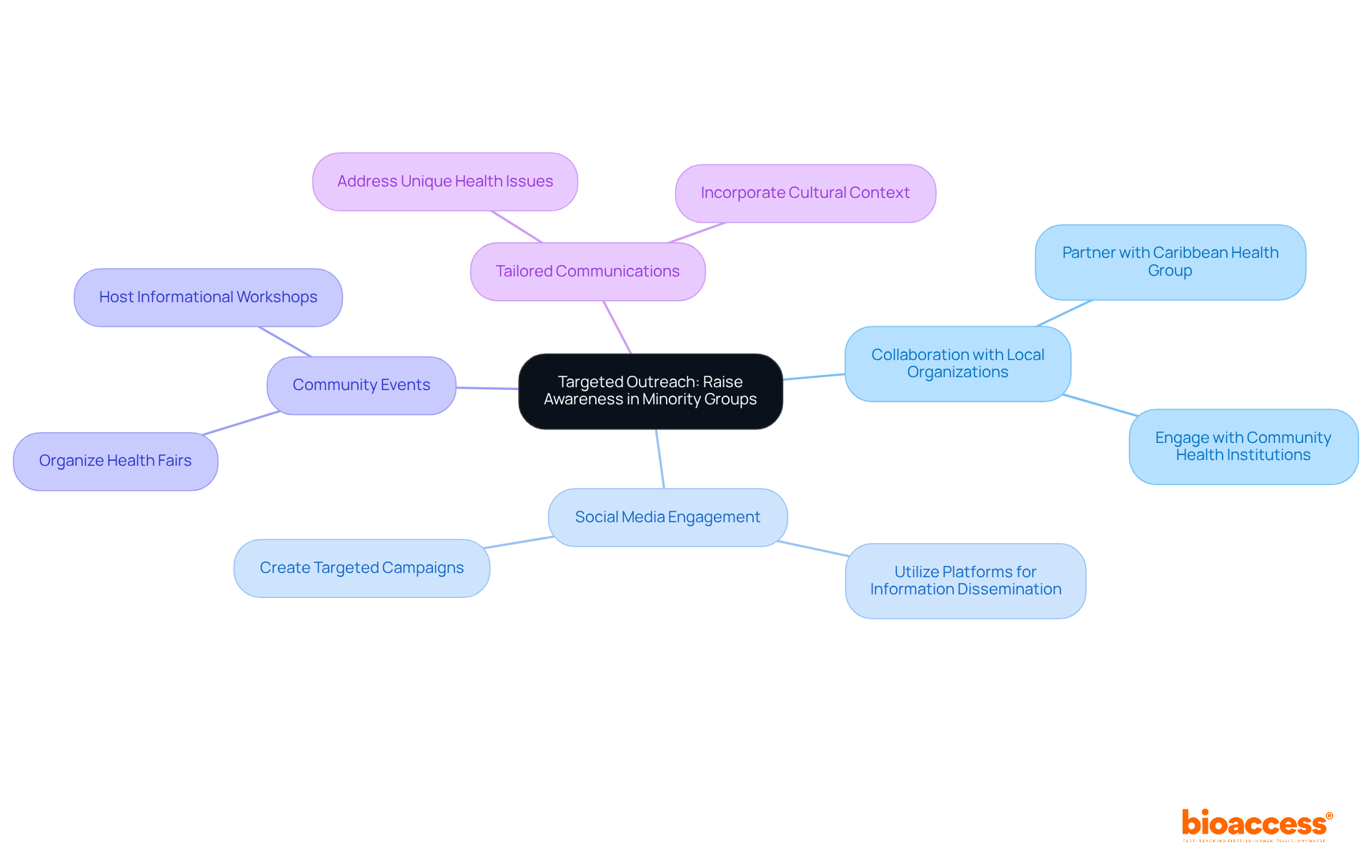
Executing targeted outreach initiatives that focus on minority populations significantly enhances awareness and engagement in minority recruitment clinical trials. This approach necessitates collaboration with local organizations, such as the Caribbean Health Group, which consists of leading healthcare institutions in Barranquilla. Together, these efforts aim to position the city as a premier destination for medical research in Latin America.
Leveraging social media platforms and organizing community events to disseminate information can further bolster engagement. By tailoring communications to address the unique health issues and cultural backgrounds of these groups, researchers can effectively involve potential participants in minority recruitment clinical trials and inspire them to consider joining research studies.
This initiative is endorsed by Colombia's Minister of Health, underscoring the critical importance of inclusivity in medical research. Moreover, the economic impact of medical studies can facilitate job creation and enhance healthcare within the region.
Forming alliances with nearby entities, such as community health facilities, faith-based organizations, and advocacy groups, significantly enhances efforts to attract participants. These entities often hold established trust within their communities, rendering them invaluable allies in disseminating information about clinical trials.
Collaborating with these groups allows researchers to leverage existing networks, gain insights into community needs, and develop culturally tailored engagement strategies that resonate with potential participants. For example, community health centers have demonstrated an ability to improve minority recruitment by nurturing relationships that encourage participation.
By prioritizing these collaborations, researchers can foster a more inclusive environment that not only boosts enrollment in minority recruitment clinical trials but also ensures that studies accurately reflect the diverse communities they aim to serve. This approach aligns with the growing recognition that equitable representation in research studies is essential for generating valid and relevant findings.
Utilizing digital resources and platforms significantly enhances hiring initiatives for minority recruitment clinical trials in medical studies, especially when faced with challenges such as disengaged research facilities and participant eligibility concerns. By leveraging social media, mobile applications, and online forums, researchers can reach a broader audience and engage potential participants in real-time.
These digital engagement strategies not only facilitate the dissemination of information about clinical studies but also address the concerns of prospective participants, fostering a sense of involvement in the process. Embracing technology allows researchers to create more inclusive hiring processes that resonate with diverse populations, ultimately facilitating minority recruitment clinical trials and overcoming the hurdles that Medtech and Biopharma startups often face in securing site involvement and managing studies efficiently.
Implementing adaptable study designs that cater to the needs of minority participants is crucial for enhancing recruitment and retention rates in clinical research. This approach may involve:
By prioritizing participant comfort and accessibility, researchers can create a more inclusive atmosphere that encourages diverse populations to engage in clinical studies. Such strategies not only improve participation rates but also contribute to the overall integrity and relevance of clinical research.
Providing language support resources, including translation services and bilingual staff, is essential for facilitating communication with minority participants in minority recruitment clinical trials who may have limited English proficiency. Industry leaders emphasize that making study materials accessible in various languages and ensuring culturally aware communications can significantly help overcome language barriers. This strategy not only cultivates a more inclusive environment but also recognizes that local knowledge and cultural awareness are critical for successful market access in Latin America. By prioritizing language accessibility and engaging local communities, researchers can enhance participant comprehension and involvement in minority recruitment clinical trials, ultimately contributing to the advancement of health equity.
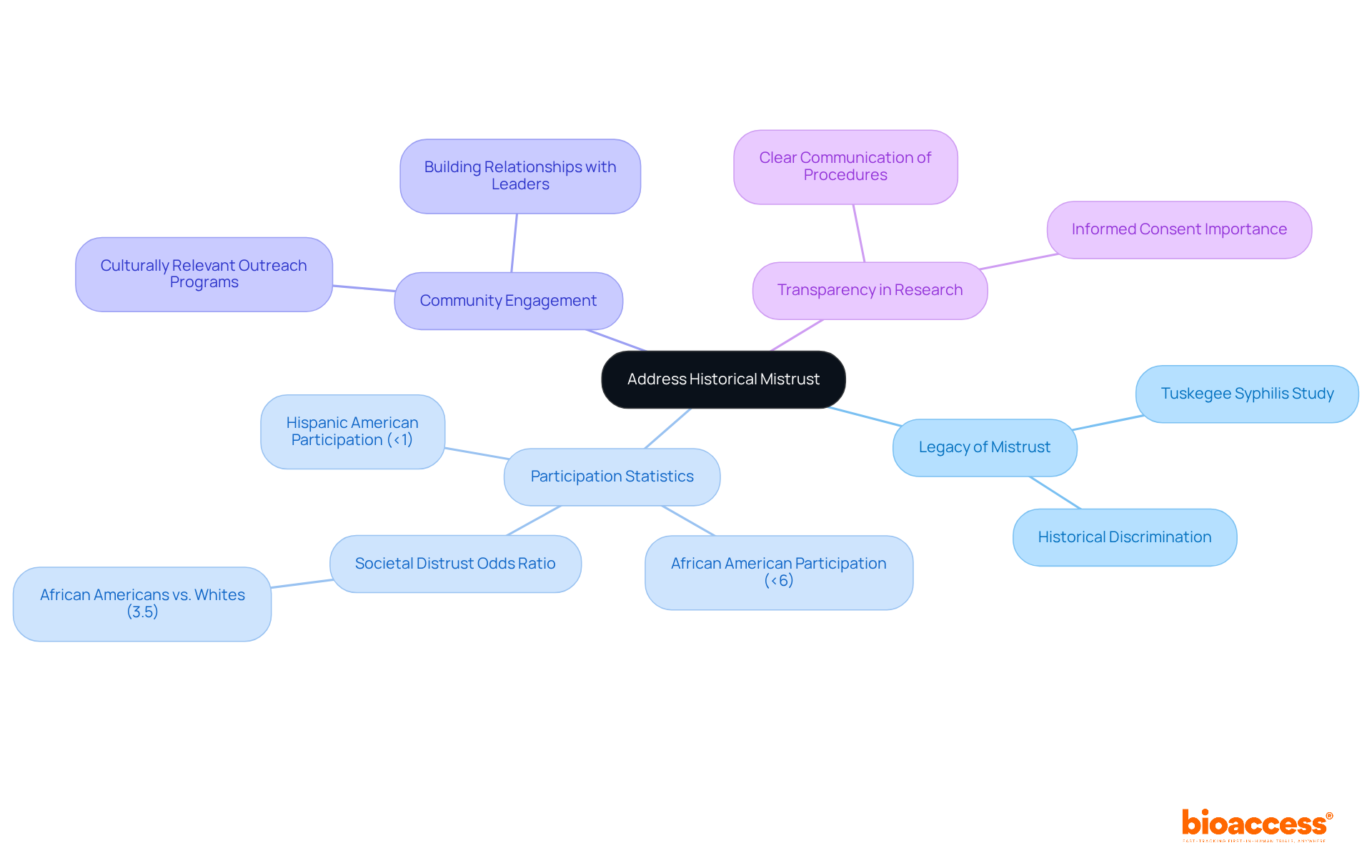
To effectively support minority recruitment clinical trials, it is essential to acknowledge and address the historical mistrust that exists within these communities. Researchers must engage in open discussions regarding historical wrongs, such as the legacy of the Tuskegee Syphilis Study, which has fostered profound skepticism towards medical research.
Statistics indicate that under 6% of African Americans and under 1% of Hispanic Americans participate in cancer clinical studies, which underscores the urgent need for minority recruitment clinical trials to address this mistrust. Building relationships with community leaders can facilitate trust-building efforts, as these leaders often serve as credible intermediaries.
Furthermore, providing clear and transparent information regarding testing procedures is crucial; research shows that over 80% of individuals from underrepresented backgrounds would consider joining studies if they were adequately informed about them. Raegan W Durant, MD, MPH, emphasizes that societal distrust is a significant barrier to research participation among underrepresented groups.
By prioritizing openness and ethical standards, researchers can significantly enhance participation rates in minority recruitment clinical trials, ensuring that underrepresented voices are both acknowledged and valued in research studies. This commitment to inclusivity enriches the research landscape and contributes to more equitable healthcare outcomes.
Implementing fair compensation and incentive programs is crucial for enhancing participation rates in minority recruitment clinical trials among minority populations. This approach may encompass:
By ensuring that incentives are both equitable and transparent, researchers can effectively address financial barriers and foster greater engagement in minority recruitment clinical trials from diverse populations. This, in turn, leads to more inclusive clinical research, reinforcing the importance of collaboration in advancing medical knowledge.

Implementing effective strategies for minority recruitment in clinical trials is essential for achieving equitable healthcare outcomes. By prioritizing inclusivity and understanding the unique needs of diverse populations, researchers can foster a more representative participant pool. This approach not only enhances the integrity of clinical research but also ensures that the benefits of medical advancements are accessible to all communities.
Key insights from the article highlight the importance of:
Leveraging digital engagement and flexible trial designs further empowers researchers to connect with minority populations. Additionally, addressing historical mistrust and providing fair compensation can significantly boost participation rates, leading to more comprehensive and relevant study results.
The significance of these strategies extends beyond mere recruitment; they contribute to a broader movement towards health equity. By actively working to involve underrepresented groups in clinical trials, the medical research community can help dismantle barriers and create a healthcare landscape that reflects the diversity of the populations it serves. Embracing these practices is not just a responsibility but a vital step towards ensuring that all voices are heard and valued in the pursuit of medical knowledge.
What is bioaccess® and how does it enhance minority recruitment in clinical trials?
bioaccess® leverages the regulatory pace of Latin America, diverse patient demographics of the Balkans, and Australia's efficient ethical approval processes to enhance minority recruitment in clinical trials. It secures ethical approvals in 4-6 weeks and achieves enrollment rates that are 50% faster than traditional markets, helping Medtech, Biopharma, and Radiopharma innovators engage underrepresented populations effectively.
What services does bioaccess® provide for clinical trials?
bioaccess® offers comprehensive research management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies, ensuring a tailored approach for each project.
Why is cultural competence important in clinical trial recruitment?
Cultural competence is crucial for understanding and respecting the diverse backgrounds of potential participants. By customizing hiring strategies to align with cultural values, organizations can significantly enhance engagement and increase participation rates among underrepresented groups.
What strategies can organizations use to improve engagement with minority populations?
Organizations can improve engagement by utilizing culturally relevant messaging, engaging community liaisons, ensuring accessibility of hiring materials, employing local influencers, and adjusting communication styles to match the preferences of various groups.
How does trust building impact minority recruitment in clinical trials?
Trust building is essential for engaging minority communities. Researchers can enhance participation rates by acknowledging historical injustices, collaborating with community leaders, organizing informational sessions, and providing clear, honest information about trial objectives and benefits.
What are the potential cost savings associated with bioaccess® services?
bioaccess® can achieve substantial cost savings of $25K per patient while providing FDA-ready data, making it a cost-effective solution for clinical trials focused on minority recruitment.
How does diversity in hiring strategies affect productivity?
Research indicates that having a more varied workforce can lead to a 35% boost in productivity, highlighting the significance of diversity in hiring strategies for successful minority recruitment in clinical trials.