


The primary focus of the article titled "10 Strategies for Enhancing Clinical Trials Diversity" is to delineate effective methods for augmenting diversity in clinical trial participation. It emphasizes that the implementation of strategies such as:
can significantly enhance the representation of underrepresented populations. This, in turn, leads to more reliable and applicable research outcomes, underscoring the critical importance of diversity in clinical research.
Enhancing diversity in clinical trials transcends mere ethical obligation; it stands as a pivotal factor in advancing medical research and improving health outcomes across all populations. Historical inequities have frequently resulted in the underrepresentation of marginalized groups in clinical studies, rendering the need for inclusive practices more urgent than ever. This article explores ten effective strategies that organizations can implement to promote diversity in clinical trials, tackling participation barriers and underscoring the substantial benefits of a more representative research landscape.
How can stakeholders collaborate to ensure that clinical trials mirror the rich tapestry of the global population, ultimately leading to safer and more effective medical treatments?
bioaccess® harnesses the regulatory efficiency of Latin America, the diverse patient demographics of the Balkans, and streamlined processes in Australia, significantly enhancing clinical trials diversity in research studies. With ethical approvals achieved in just 4-6 weeks and enrollment rates 50% faster than conventional markets, bioaccess® empowers innovators in Medtech, Biopharma, and Radiopharma to conduct studies that promote clinical trials diversity and accurately reflect the global population. This agility accelerates the advancement of medical technologies while ensuring clinical trials diversity, which results in more comprehensive and relevant findings.
Enhancing clinical trials diversity in research studies is essential for addressing historical inequities and fostering trust within marginalized communities through targeted outreach and engagement strategies. Such representation is vital, as it ultimately enhances the efficacy and safety of new treatments by ensuring clinical trials diversity across diverse demographic groups.

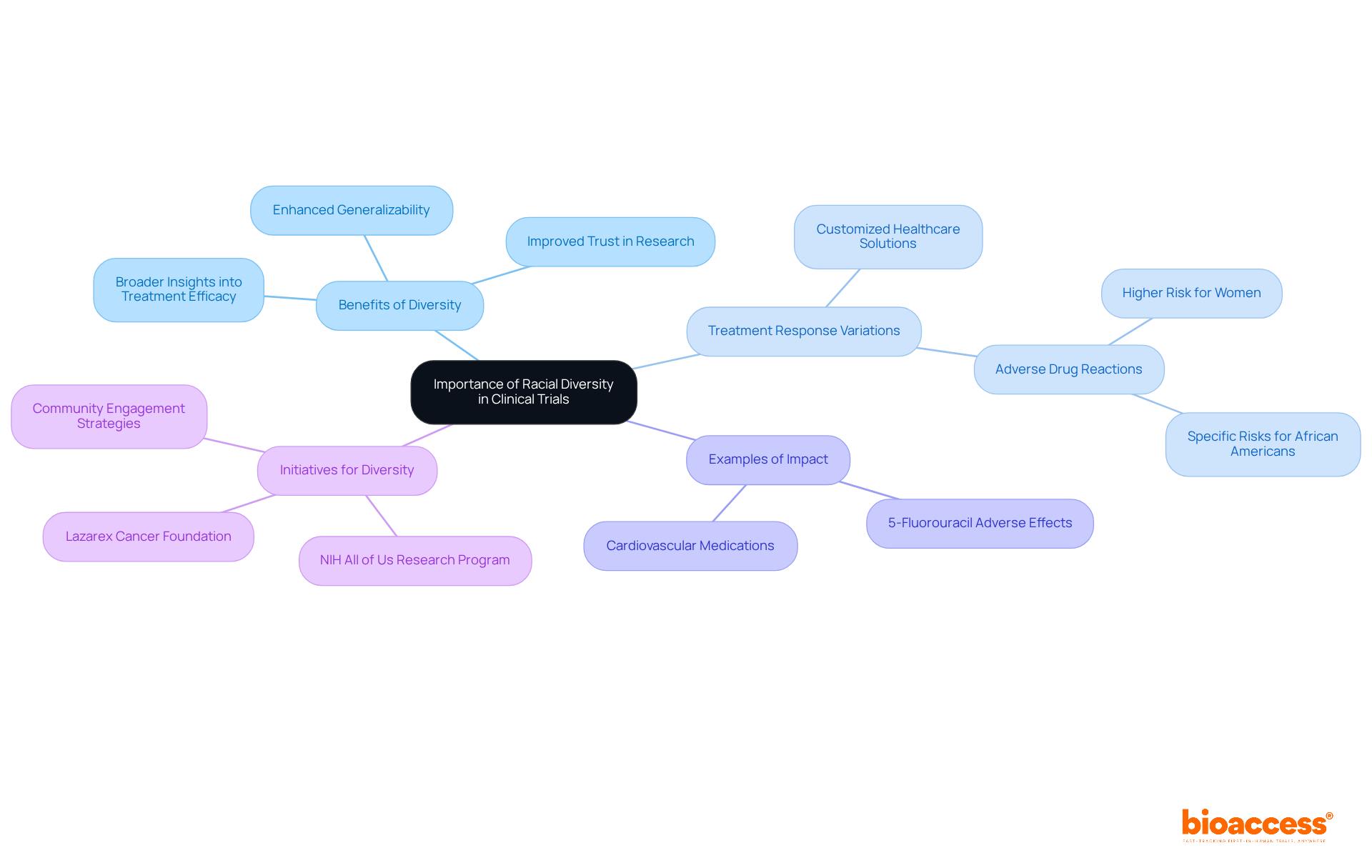
Enhancing study results requires clinical trials diversity, which ensures racial variety in trials. By incorporating clinical trials diversity through diverse participant groups, researchers can identify variations in treatment responses influenced by genetic, environmental, and lifestyle factors associated with various demographics.
For instance, certain cardiovascular medications exhibit varying medical outcomes between individuals of European descent and African Americans, underscoring the necessity for customized healthcare solutions. Additionally, studies indicate that women are more likely to experience adverse drug reactions compared to men, emphasizing the importance of representative participation.
The clinical trials diversity not only enhances the generalizability of findings but also fosters trust within the medical research community. Initiatives like the NIH All of Us Research Program and the Lazarex Cancer Foundation illustrate effective strategies for increasing diversity by addressing barriers to participation and ensuring that historically underrepresented groups benefit from advancements in personalized medicine.
Ultimately, clinical trials diversity ensures a broader range of racial and ethnic representation in studies, leading to more comprehensive insights into treatment efficacy and safety, and paving the way for improved healthcare outcomes for all populations.
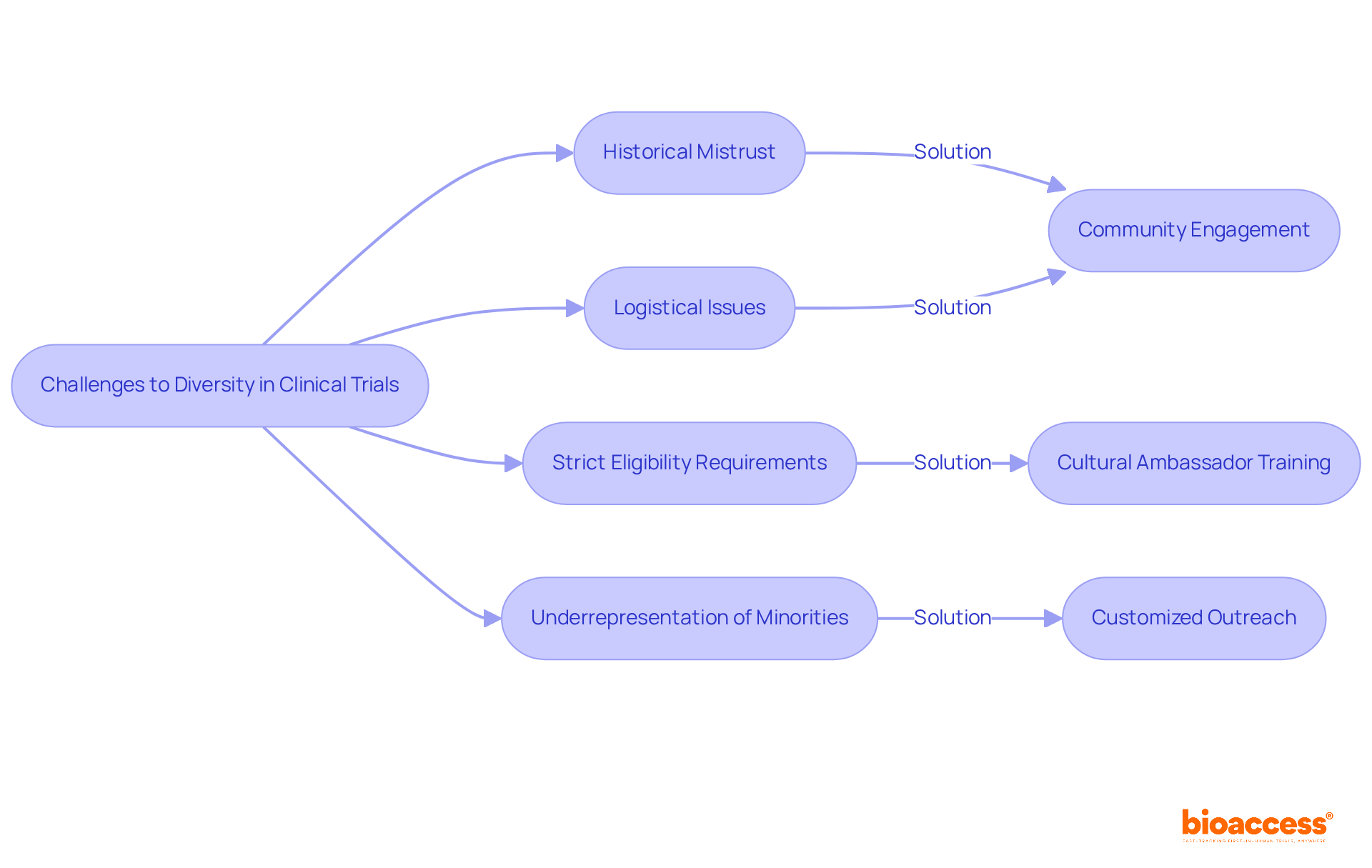
Variety in medical study participation encounters multiple considerable obstacles. Historical mistrust of medical studies, particularly among minority communities, stems from past injustices and ethical breaches, deterring individuals from participating. For instance, African Americans are disproportionately impacted by heart disease, with 48% of African American women and 44% of African American men experiencing some form of the condition. Despite this, they continue to be underrepresented in clinical trials diversity, as participation rates frequently fall short of goals. Logistical challenges, including transportation issues and financial constraints, further complicate participation. Additionally, strict eligibility requirements can unintentionally exclude potential participants from various backgrounds, which restricts the clinical trials diversity necessary for thorough study results.
To effectively address these barriers, a multifaceted approach is essential. Community engagement initiatives, such as informational sessions and health fairs, can raise awareness about available trials and the importance of participation. Training cultural ambassadors to communicate the benefits of clinical research can help build trust and encourage involvement. Effective approaches, exemplified by COUCH Health's initiative, demonstrate that customized outreach and collaborations with local organizations can significantly improve recruitment rates. In one notable instance, the enlistment of African American participants in a heart disease study surged from 15% to 900 within a 9-month period, exceeding initial goals and enhancing overall diversity in the research. By promoting an inclusive atmosphere and creating more accessible study designs, the research community can ensure that clinical trials diversity is achieved, ultimately resulting in improved health outcomes for all patient groups.
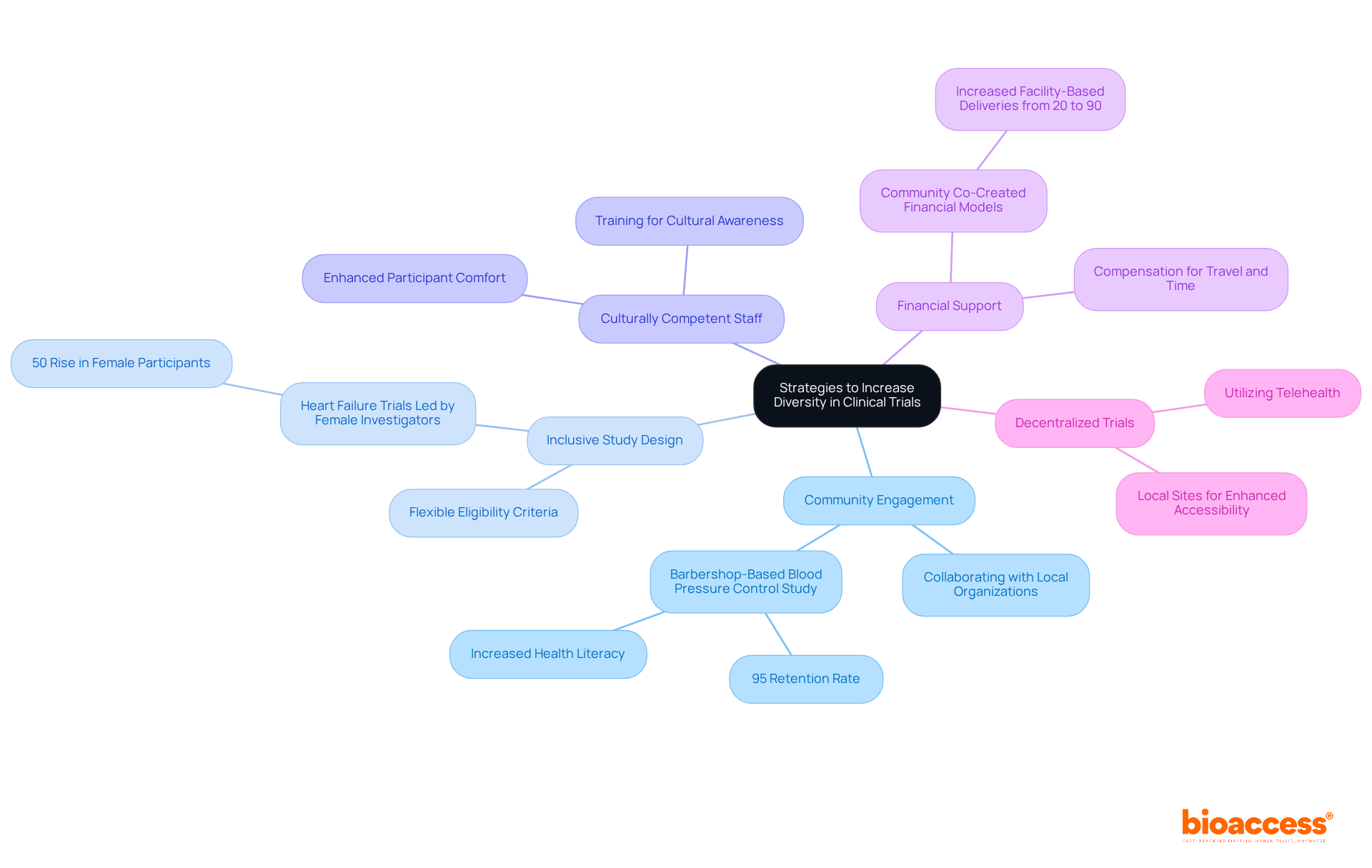
Organizations can adopt several effective strategies to enhance clinical trials diversity.
Community Engagement: Collaborating with local organizations is essential for increasing awareness about clinical studies and their advantages. Successful initiatives, like the barbershop-based blood pressure control study, demonstrated that engaging reliable local partners can significantly enhance recruitment and retention rates, achieving a remarkable retention rate of 95% among participants. Enhanced adherence to treatment plans and improved health literacy have also been observed as measurable outcomes of public involvement.
Inclusive Study Design: Creating research protocols that accommodate diverse populations is crucial. This includes implementing flexible eligibility criteria that consider the unique needs of various communities, thereby increasing participation rates among underrepresented groups. Notably, heart failure research trials led by female investigators experienced a 50% rise in female participants, underscoring the importance of varied researchers in improving trial diversity.
Culturally Competent Staff: Training teams to understand and respect cultural differences fosters participant comfort and trust. This approach has been shown to enhance engagement, as culturally aware staff can better address the concerns of diverse populations. The NIH emphasizes that enhancing diversity in medical research is both an ethical and scientific necessity, highlighting the critical role of such training.
Financial Support: Providing compensation for travel and time can alleviate financial barriers that often deter participation. For instance, jointly developed financial models have successfully increased facility-based deliveries from 20% to 90% in various health initiatives, demonstrating the impact of reducing economic barriers.
Decentralized Trials: Utilizing telehealth and local sites enhances accessibility for participants. Decentralized studies have successfully engaged with communities through reliable local collaborators, thus improving recruitment results and ensuring that studies are more inclusive.
By implementing these strategies, organizations can significantly enhance clinical trials diversity in medical studies, ultimately leading to more equitable health outcomes and improved representation in healthcare research.
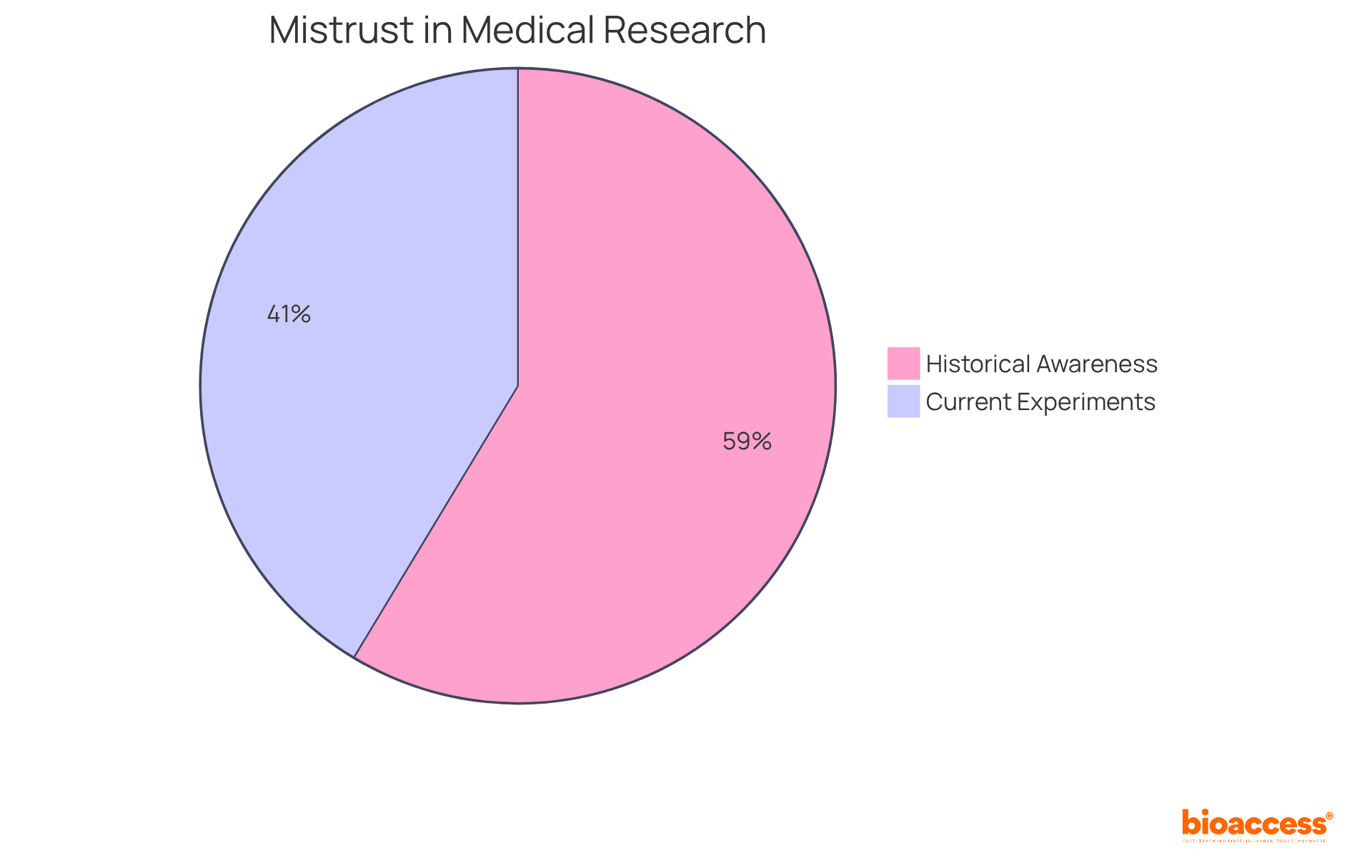
Historical mistrust in medical research, particularly among minority populations, originates from unethical practices such as the Tuskegee Syphilis Study and other instances of exploitation. This legacy has cultivated widespread skepticism regarding medical studies, evidenced by the fact that 78% of Black adults have heard the notion that medical researchers experiment on Black individuals without their knowledge or consent. Furthermore, 55% of Black Americans believe that nonconsensual experiments are currently being conducted on Black people, highlighting a broader mistrust in the healthcare system.
To effectively build trust, researchers must prioritize transparency and ethical practices. Involving local leaders and advocates is essential in bridging the gap between researchers and minority groups. Successful instances, such as the case study on distrust in the U.S. healthcare system, demonstrate that when researchers actively collaborate with local organizations, they can surmount historical skepticism and foster participation in research studies.
By promoting open discussions and engaging communities in the research process, we can enhance clinical trials diversity, making medical studies more inclusive and representative. Researchers should consider implementing community engagement strategies, including:
to enhance trust, participation, and ultimately improve clinical trials diversity.
Integrating genetic ancestry into research designs is essential for comprehending how genetic variations influence drug metabolism and effectiveness. Research indicates that distinct populations demonstrate varied responses to medications due to their unique genetic backgrounds. For instance, studies reveal that individuals of Sub-Saharan African ancestry face a higher risk of adverse drug reactions (ADRs), whereas those of East Asian descent frequently exhibit a protective genetic profile. As Patrinos, G.P. asserts, "population pharmacogenomics has a significant impact on public health and drug development."
By analyzing ancestry data, researchers can identify variations in drug response, paving the way for more personalized and effective treatments. This approach not only enhances the safety and effectiveness of new therapies but also ensures that clinical trials diversity is prioritized, making medical studies relevant and beneficial to diverse demographic groups.
The integration of pharmacogenomic knowledge into research can significantly improve therapeutic outcomes, as evidenced by the development of population-specific pharmacogenomic guidelines that account for the unique genetic profiles of different groups. Ultimately, embracing clinical trials diversity in medical studies is a crucial step toward optimizing drug therapies and mitigating risks associated with adverse reactions.
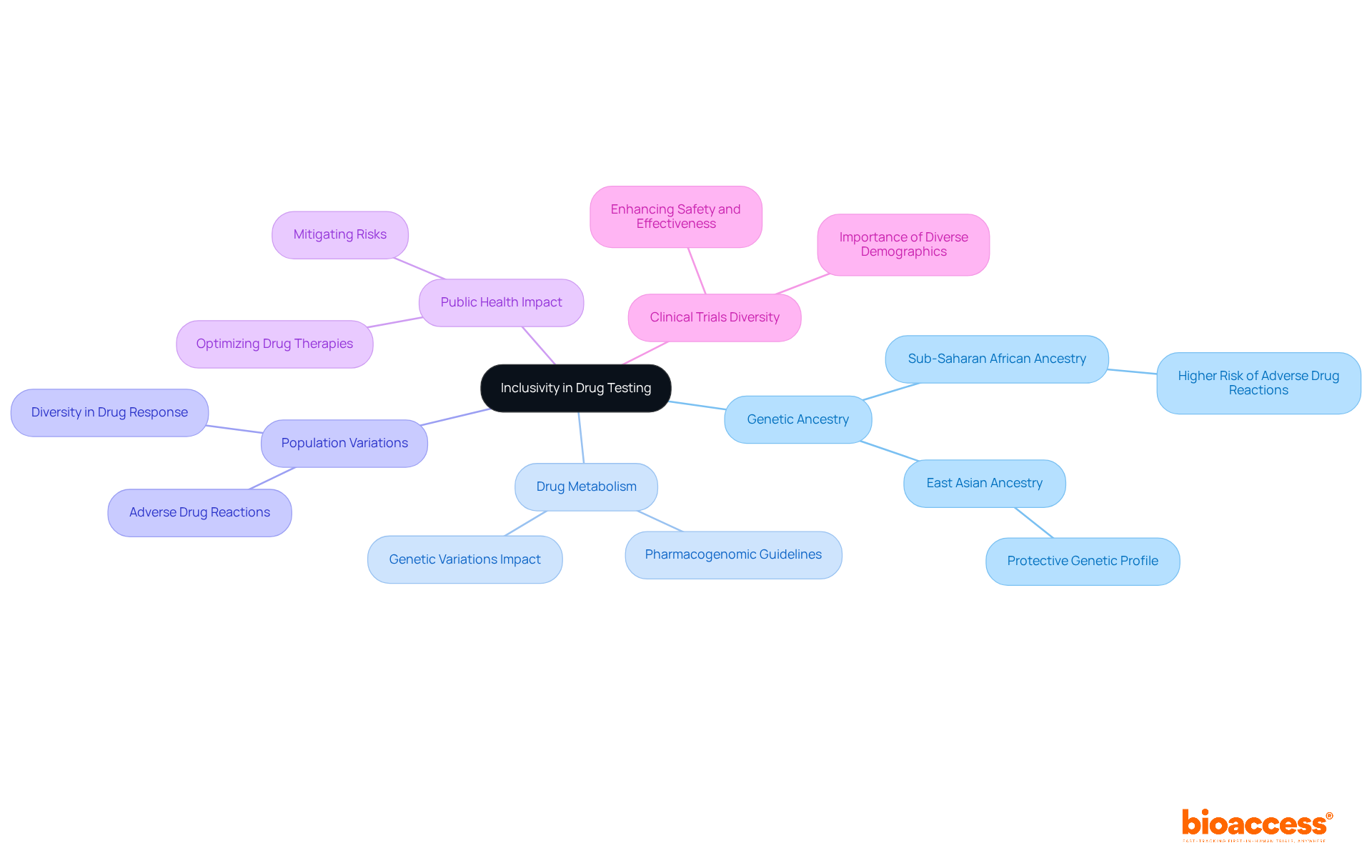
Promoting legislative actions that enhance diversity in research studies is crucial for fostering systemic change. Policies mandating research sponsors to submit diversity action plans are essential in ensuring the inclusion of varied populations in studies. For instance, the FDA's recent guidelines require sponsors to develop these plans, demonstrating a commitment to addressing health disparities. Notably, the revised recommendations issued in January 2024 underscore the importance of precise demographic information in research studies.
Furthermore, funding initiatives aimed at outreach and education within underrepresented communities can significantly elevate participation rates. Statistics reveal that racial and ethnic minorities constitute 39% of the U.S. population yet represent only 2% to 16% of research participants, highlighting the urgent need for targeted strategies.
A compelling case study exemplifying successful strategies is Walgreens' commitment to involving women in health studies, showcasing effective implementation of diversity initiatives. By endorsing such policies, stakeholders can contribute to more equitable healthcare outcomes and enhance the overall quality of medical research, ultimately leading to treatments that are effective across diverse demographics.
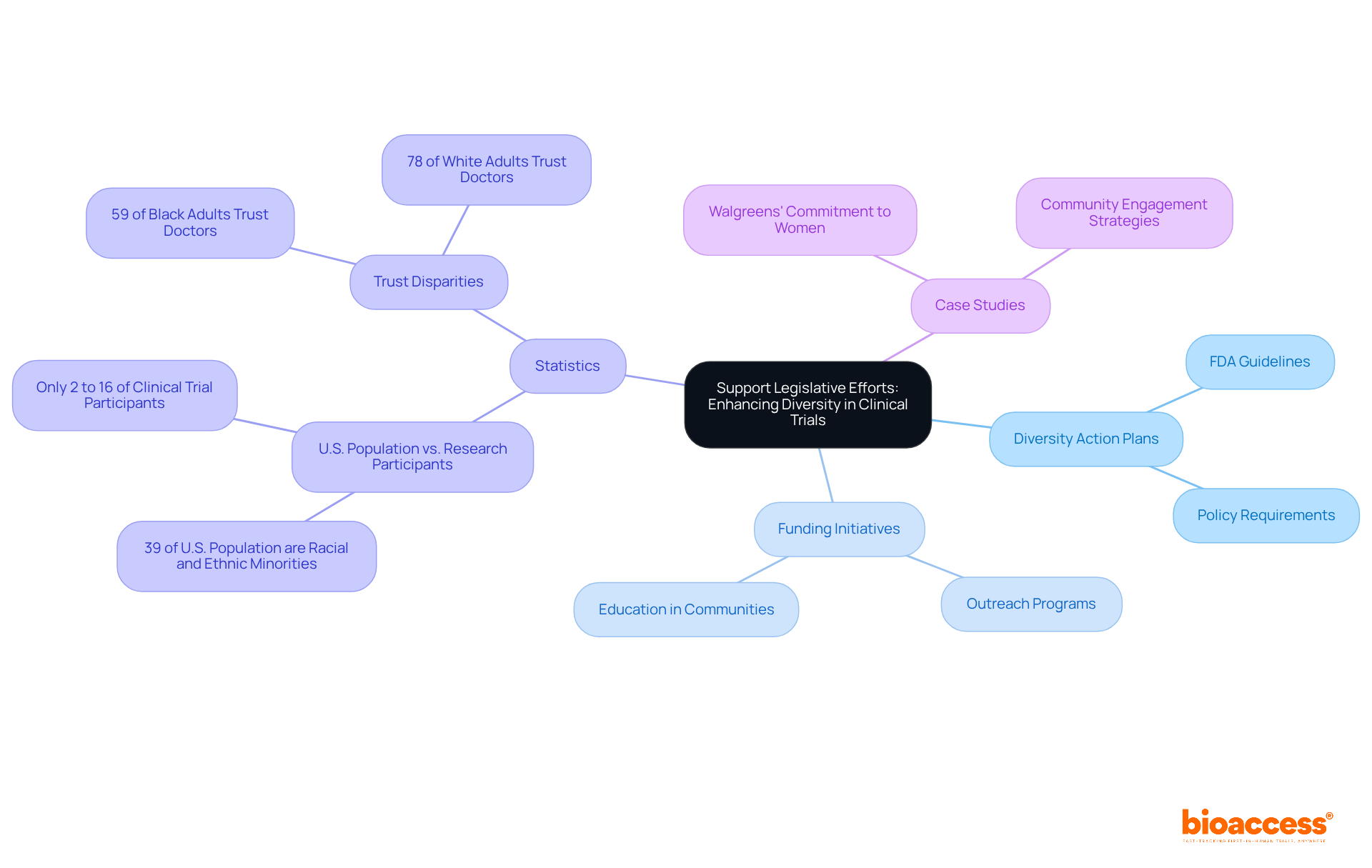
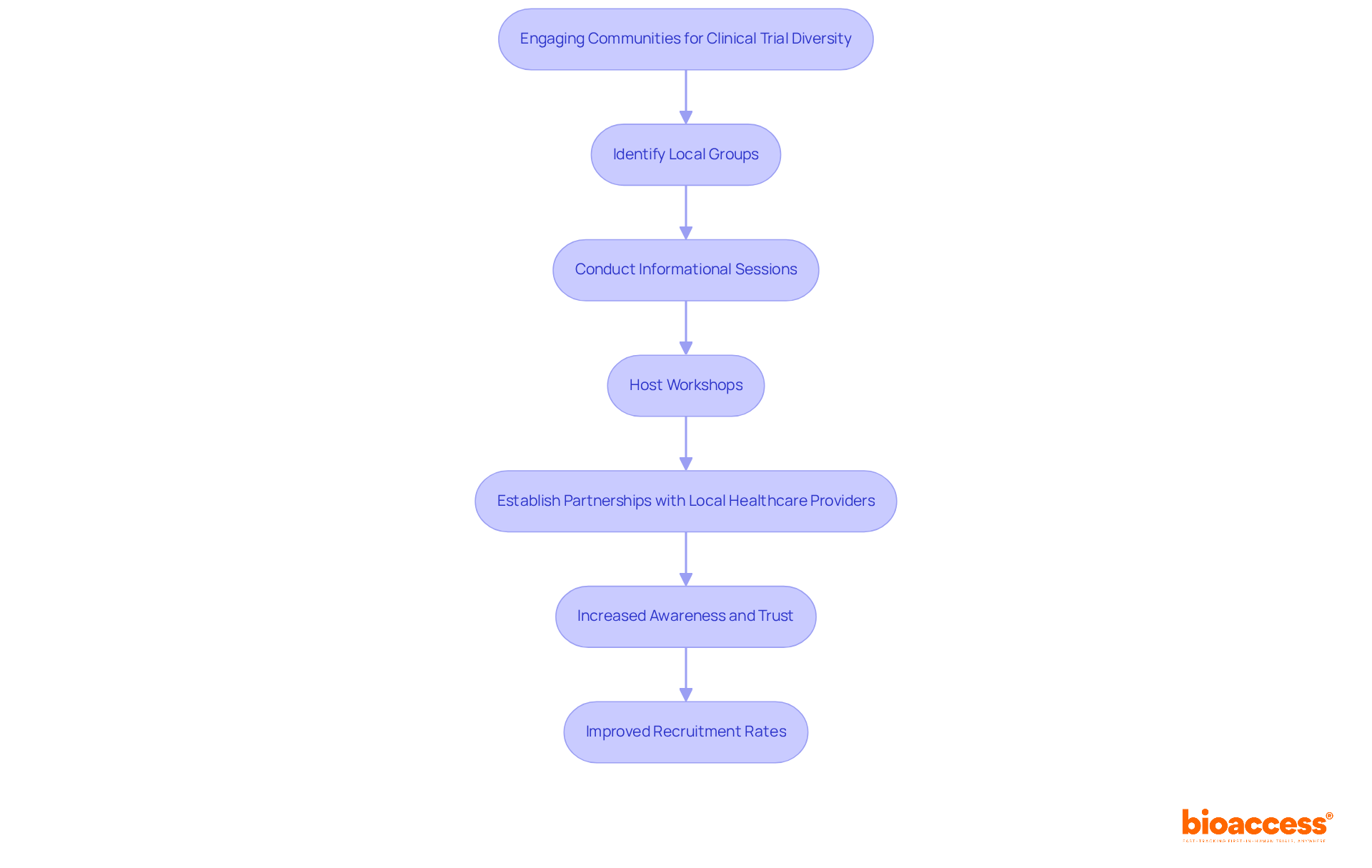
Involving local groups is crucial for enhancing clinical trials diversity in medical studies. By collaborating with community leaders and organizations, researchers gain valuable insights into the specific needs and concerns of potential participants. This involvement manifests through:
Such initiatives not only increase awareness about research studies but also build trust and relationships with historically underrepresented groups. Furthermore, the extensive research management services offered by bioaccess—including feasibility studies, site selection, study setup, and project oversight—are essential in structuring studies to address the needs of diverse populations.
Data indicates that the recruitment rate of African American participants decreased from 15% to 10% over a 9-month period, underscoring the urgent need to enhance clinical trials diversity in research studies. The integration of digital health tools can improve outreach and simplify participation, making studies more accessible.
As Michelle D. Kelsey from Duke University emphasizes, enhancing clinical trials diversity is both an ethical and scientific imperative. Ultimately, local involvement, supported by robust project oversight and compliance assessments, not only improves the recruitment process but also ensures that clinical trials diversity accurately reflects the varied populations they aim to assist. This, in turn, enhances the validity and relevance of study results.
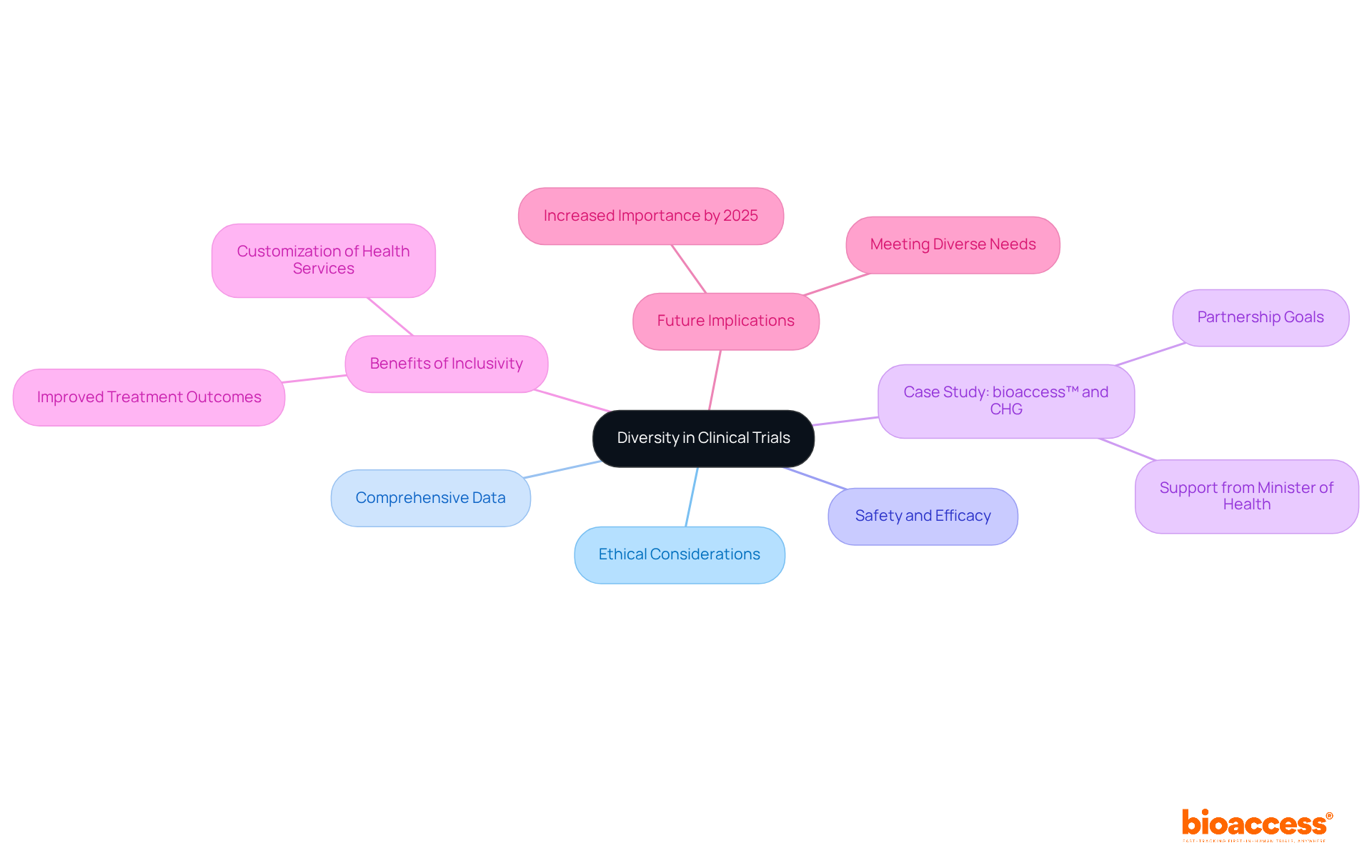
Variety in medical studies extends beyond ethical considerations; it is a crucial component for advancing medical knowledge. Engaging diverse participant groups is essential for clinical trials diversity, as it yields more comprehensive data and enhances the safety and efficacy of treatments across various populations. A prime example is the partnership between bioaccess™ and Caribbean Health Group (CHG), which aims to establish Barranquilla as a leading hub for medical studies in Latin America, supported by Colombia's Minister of Health. This initiative exemplifies how strategic collaborations can bolster the inclusivity of medical research.
Furthermore, studies reveal that clinical trials diversity allows researchers to gain invaluable insights into how different groups respond to therapies when they reflect the demographics of the general population. Such inclusivity elevates the quality of healthcare and fosters the development of more effective medical interventions. Additionally, local engagement initiatives have demonstrated tangible benefits, such as increasing facility-based deliveries from 20% to 90%.
As we approach 2025, the importance of clinical trials diversity in research will only amplify, shaping the future of medical advancements and ensuring that innovations meet the needs of all groups. As noted, 'a significant benefit lies in the capacity to customize health services to the requirements of local populations,' underscoring the essential role of diversity in enhancing healthcare outcomes.
Encouraging cooperation among various participants—including researchers, healthcare providers, community organizations, and regulatory bodies—is vital for improving diversity in research studies. The collaboration between bioaccess™ and Caribbean Health Group demonstrates how joint efforts can establish Barranquilla as a prominent location for medical trials in Latin America, with robust backing from Colombia's Minister of Health. By collaborating, these groups can share resources, knowledge, and best practices to create more inclusive academic environments.
For instance, GlobalCare Clinical Trials' collaboration with bioaccess™ has led to an impressive over 50% reduction in recruitment time and a remarkable 95% retention rate, showcasing the effectiveness of strategic partnerships. Moreover, endorsements from industry figures such as Dr. John B. Simpson, CEO of Avinger, emphasize the positive experiences in carrying out studies in Colombia, further strengthening the significance of cooperation.
These efforts not only address common barriers to participation but also ensure that diverse populations are represented in clinical research. As Stuart Milk stated, 'We are less when we don't include everyone,' reinforcing the message of inclusivity and collaboration. This synergy not only improves trial outcomes but also strengthens the overall healthcare system, promoting equity and access for all.
Enhancing diversity in clinical trials transcends ethical responsibility; it is a vital element for advancing medical research and developing effective treatments for all populations. By prioritizing diverse participant groups, researchers can ensure that clinical findings remain relevant and applicable across various demographics, ultimately leading to improved healthcare outcomes.
This article delineates several key strategies for achieving this objective, including:
It is imperative to address historical mistrust and logistical barriers to encourage participation from underrepresented populations. Furthermore, integrating genetic ancestry into research can significantly enhance our understanding of drug responses, making treatments both safer and more effective.
As the importance of diversity in clinical trials continues to escalate, it is essential for stakeholders—researchers, healthcare providers, and policymakers—to collaborate and implement these strategies with efficacy. By fostering an inclusive research environment, the medical community can not only enhance the efficacy of treatments but also build trust within marginalized communities, paving the way for a healthier future for all.
What is bioaccess® and how does it enhance clinical trials diversity?
bioaccess® leverages the regulatory efficiency of Latin America, diverse patient demographics in the Balkans, and streamlined processes in Australia to significantly enhance clinical trials diversity. It achieves ethical approvals in 4-6 weeks and enrollment rates that are 50% faster than conventional markets, enabling Medtech, Biopharma, and Radiopharma innovators to conduct studies that reflect the global population.
Why is clinical trials diversity important?
Clinical trials diversity is essential for addressing historical inequities and fostering trust within marginalized communities. It enhances the efficacy and safety of new treatments by ensuring representation across diverse demographic groups, leading to more comprehensive and relevant findings.
How does racial diversity impact research outcomes in clinical trials?
Racial diversity in clinical trials allows researchers to identify variations in treatment responses influenced by genetic, environmental, and lifestyle factors. This is crucial for developing customized healthcare solutions, as evidenced by differing medical outcomes for certain medications among various racial groups.
What challenges exist in achieving diversity in clinical trial participation?
Key challenges include historical mistrust of medical studies among minority communities, logistical issues like transportation and financial constraints, and strict eligibility requirements that can exclude potential participants from diverse backgrounds.
What strategies can be employed to overcome barriers to diversity in clinical trial participation?
A multifaceted approach is needed, including community engagement initiatives, training cultural ambassadors, and customized outreach efforts. Successful examples include COUCH Health's initiative, which significantly improved recruitment rates for African American participants in a heart disease study.
How can enhancing clinical trials diversity benefit healthcare outcomes?
By ensuring a broader range of racial and ethnic representation in studies, clinical trials diversity leads to more comprehensive insights into treatment efficacy and safety, ultimately paving the way for improved healthcare outcomes for all populations.