


This article examines the strategies that Clinical Research Directors can employ to enhance human trials in clinical research. It underscores the significance of:
as pivotal areas that can substantially elevate the efficiency and success rates of clinical studies. Such improvements are essential for expediting market access for innovative therapies.
In the high-stakes realm of clinical research, the imperative to deliver innovative therapies swiftly and efficiently has reached unprecedented levels. Clinical Research Directors encounter a multitude of challenges, ranging from navigating intricate regulatory landscapes to ensuring diverse patient recruitment and upholding ethical integrity.
This article delves into ten strategic approaches that can significantly enhance human trials, empowering directors to optimize their processes and improve outcomes. As the landscape of clinical research evolves, it raises a crucial question: how can directors effectively adapt and implement these strategies not only to meet regulatory demands but also to ensure the success of their trials?
bioaccess® harnesses a unique blend of regulatory speed, diverse patient populations, and streamlined pathways to achieve ethical approvals in an impressive 4-6 weeks. This global-first agility empowers Clinical Research Directors to initiate studies more rapidly, significantly shortening the time-to-market for innovative medical solutions. By partnering with bioaccess®, organizations can enhance operational efficiency and seize emerging opportunities in the Medtech, Biopharma, and Radiopharma sectors.
The capability to navigate intricate regulatory landscapes efficiently is vital; for instance, nations such as Brazil, Mexico, and Argentina have emerged as frontrunners in outsourced medical studies due to their robust regulatory systems and larger patient groups. Moreover, the success rates of medical studies are closely linked to these factors, with quicker approvals associated with greater success rates in introducing innovative treatments to the market.
As the biopharma environment evolves, the significance of global-first agility in research cannot be overstated, as it enables organizations to adapt swiftly to shifting market needs and regulatory frameworks.
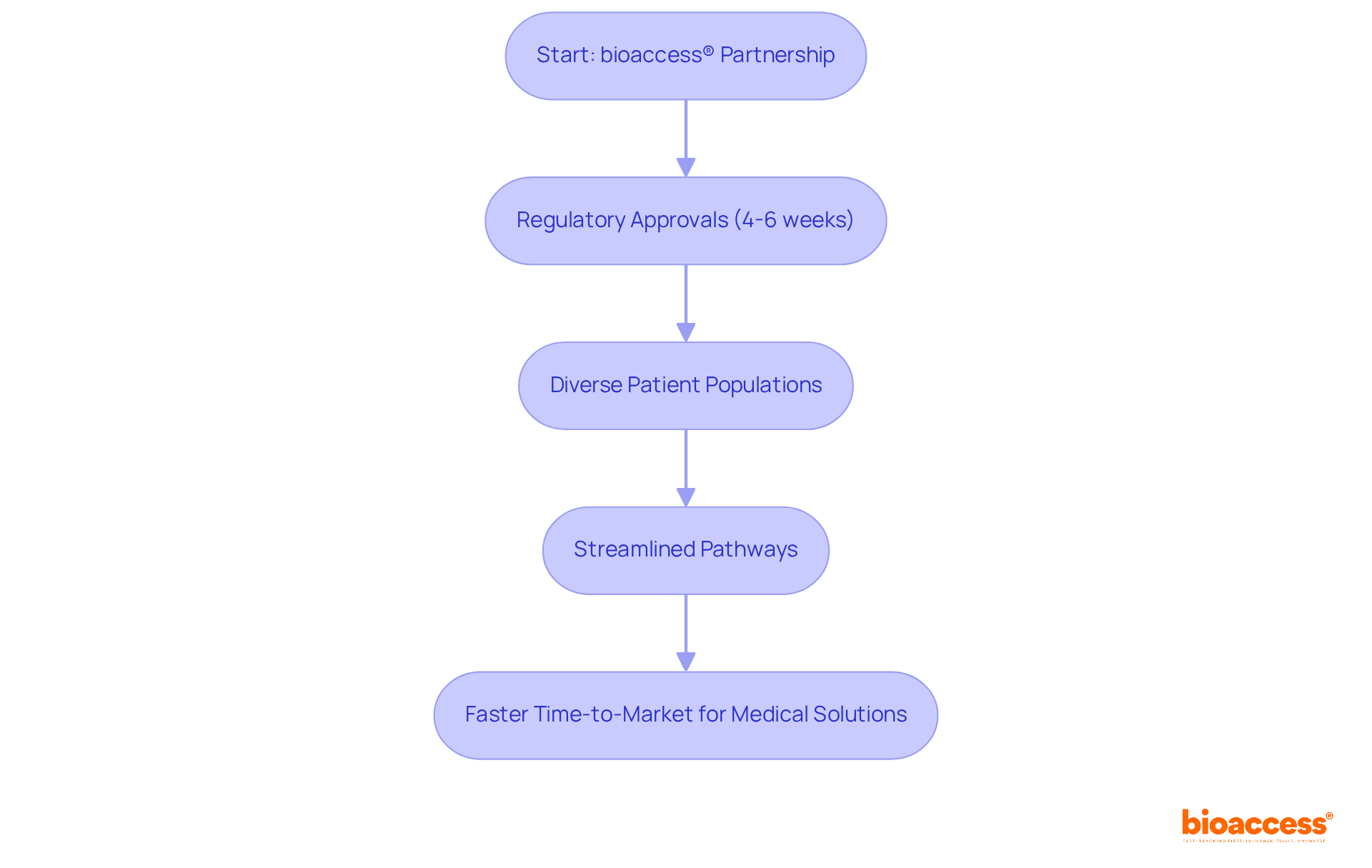
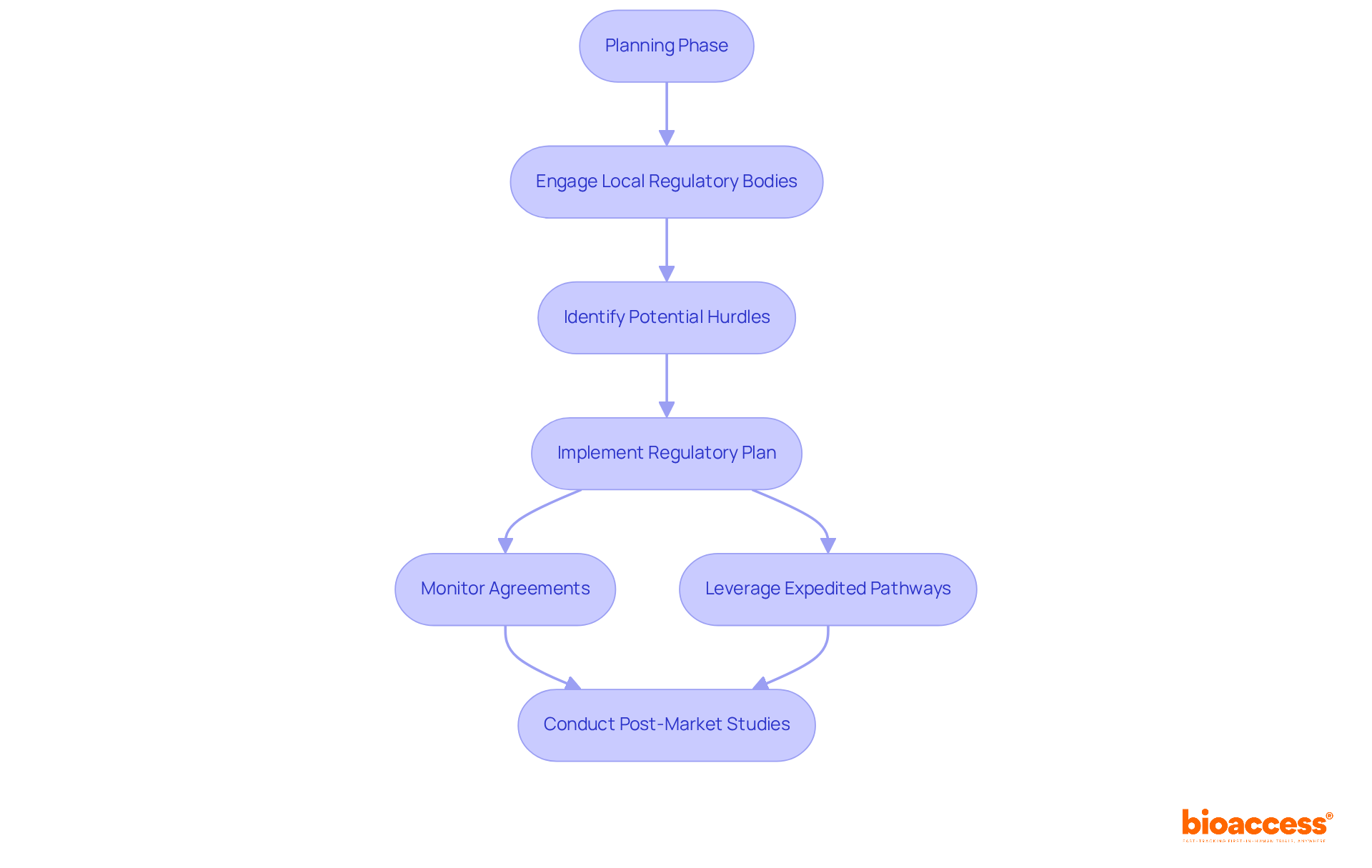
Navigating the regulatory environment effectively is essential for Clinical Research Directors aiming to accelerate research studies. Understanding the specific requirements of regions like Latin America, particularly Colombia, can significantly streamline the approval process. With regulatory approvals typically taking only 90-120 days, Colombia presents a compelling advantage, markedly faster than the average wait times in other regions. Engaging with local regulatory bodies early in the planning phase enables directors to identify potential hurdles and address them proactively. This approach not only accelerates approvals but also enhances patient access to innovative therapies.
For instance, Colombia boasts a population exceeding 50 million, with 95 percent covered by universal healthcare, providing a robust foundation for patient recruitment. This ensures prompt access to diverse patient groups for research studies. Moreover, Colombia's healthcare system is highly esteemed, ranked #22 by the World Health Organization, and its hospitals are recognized as some of the finest in Latin America, guaranteeing high-quality care during assessments. Additionally, the nation offers considerable R&D tax benefits, including a 100% tax deduction for investments in science and technology, which can alleviate the financial strain on research initiatives.
By creating a thorough regulatory plan that includes monitoring agreements with health agencies and addressing preclinical and research requirements, directors can facilitate a smoother start to their studies. Furthermore, leveraging expedited pathways established by the FDA can provide quicker access to new treatments. However, it is crucial to ensure that post-market studies are conducted to validate long-term safety and effectiveness. Engaging external regulatory strategists, who typically hold advanced science degrees and possess extensive experience in drug development disciplines, can bridge the gap between scientific and commercial needs, enhancing internal strategies and communication with health authorities.
This multifaceted approach is vital for managing the complexities of regulatory demands and achieving favorable outcomes.
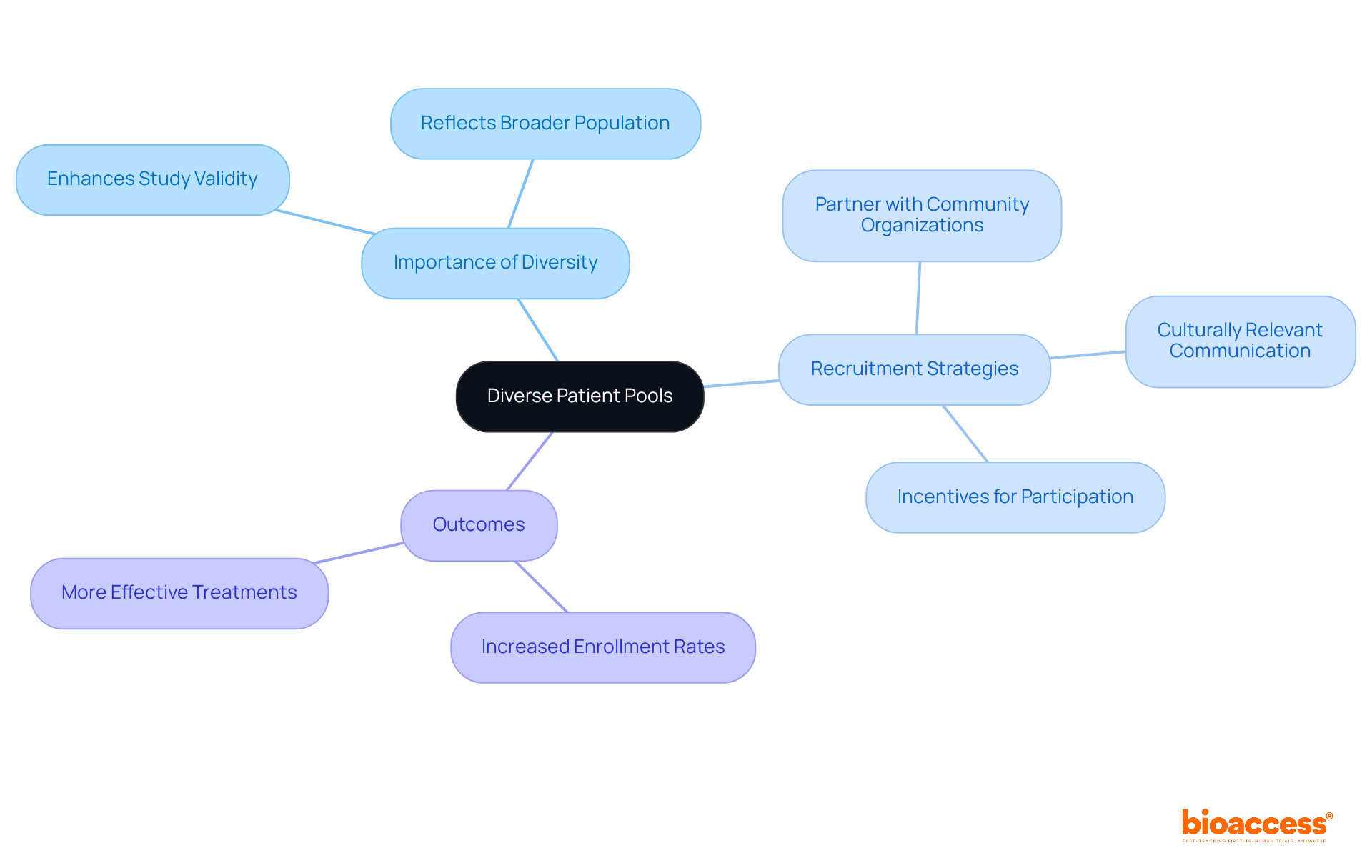
Utilizing varied patient groups significantly enhances the reliability of clinical studies, ensuring that results are applicable to a broader population. Clinical Study Directors must prioritize inclusive recruitment strategies that accurately reflect the demographics of the target market. Engaging with diverse communities and leveraging local networks can substantially boost enrollment rates.
For example, studies have demonstrated that targeted outreach efforts—such as covering transportation costs and providing educational resources—can increase participation among underrepresented groups. In 2023, initiatives led by organizations like Johnson & Johnson showcased that employing AI to identify diverse patient populations resulted in a remarkable increase in Black patient enrollment, more than doubling historical averages. Specifically, the average enrollment of Black patients in five Johnson & Johnson multiple myeloma studies exceeded twice the historical industry standard.
Furthermore, the FDA's 2020 guidance underscores the necessity of broadening eligibility criteria and enhancing recruitment strategies to mirror the population likely to utilize the drug. It is also critical to acknowledge that Hispanic and Black patients were 28% and 29% less likely to enroll than white patients, after adjusting for various factors. This highlights the disparities in enrollment and emphasizes the need for targeted outreach efforts.
By adopting these inclusive hiring strategies, Clinical Study Directors can ensure that study outcomes accurately reflect the intended patient demographic, ultimately leading to more effective and equitable healthcare solutions. As Bibbins-Domingo K. stated, 'A better understanding of study enrollment and results reported for specific demographic populations is likely to accelerate scientific advancement and ultimately lead to more effective treatment options for all patients.'
To further enhance recruitment efforts, Clinical Study Directors should consider actionable strategies such as:
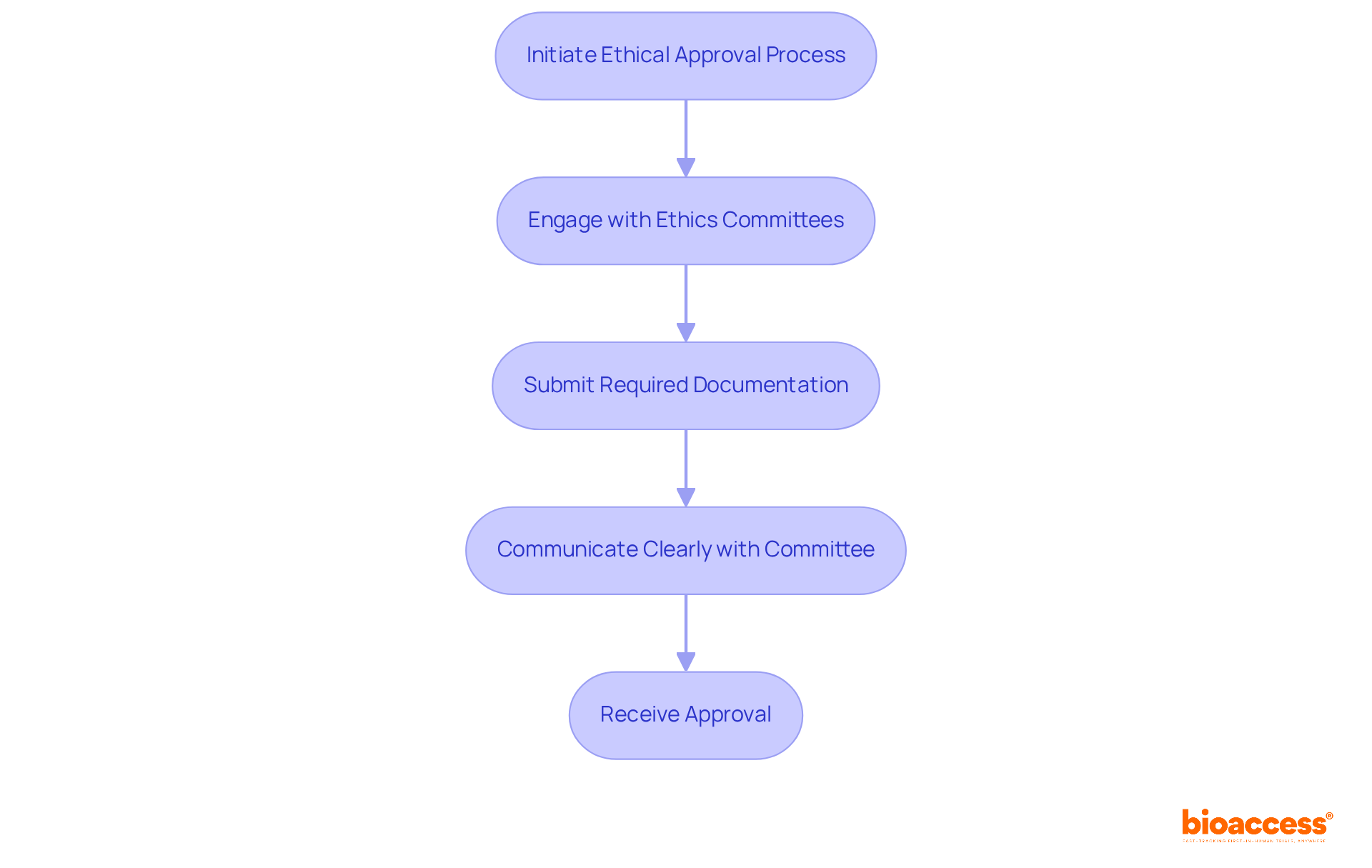
Securing ethical approvals is a pivotal aspect of the clinical trial process. Clinical Investigation Directors must ensure that all studies adhere to ethical guidelines to protect participant rights and maintain integrity in the field. Engaging with ethics committees early in the planning phase not only streamlines the approval process but also builds trust among stakeholders, significantly enhancing the credibility of the study.
Successful engagement can lead to reduced turnaround times for ethics reviews, with some institutions reporting median review times as low as 15 days. Furthermore, ethics committee members emphasize the necessity of clear communication and adherence to submission guidelines to avoid unnecessary delays.
As noted by experts, maintaining a focus on the ethical principles of autonomy, beneficence, and justice is essential in fostering a collaborative environment that prioritizes participant welfare. By actively engaging ethics committees, Clinical Research Directors can navigate the complexities of study approvals more effectively, ultimately contributing to the success of their research initiatives.
Early-phase studies, particularly first-in-human (FIH) and early-feasibility studies (EFS), are pivotal in assessing the safety and efficacy of new medical interventions prior to human trial. These studies serve as the foundation for subsequent phases, providing essential data that can significantly influence the trajectory of clinical development. For example, successful FIH studies not only validate the treatment's safety profile but also lay the groundwork for further exploration in human trials involving larger populations. Statistics indicate that approximately 75% of Phase I studies achieve success, underscoring the critical role these preliminary investigations play in the overall drug development process.
Moreover, the outcomes from early-phase studies can substantially enhance the likelihood of success in later-stage evaluations, particularly in the context of human trials. Research shows that studies with robust early-phase data are more inclined to meet their enrollment objectives and timelines. This is particularly crucial given that nearly 80% of clinical studies fail to achieve initial enrollment targets, resulting in considerable financial repercussions. By prioritizing early-stage investigations, organizations can mitigate risks and bolster their chances of favorable results in the human trial phases.
Industry leaders emphasize the transformative role of early-stage research: "Clinical studies are the best method to understand what is effective in addressing diseases such as cancer," highlighting the importance of these investigations in advancing medical knowledge and treatment options. As the landscape of clinical investigation evolves, the focus on early-phase studies remains vital for fostering innovation and ensuring the effective progression of new therapies. Companies like bioaccess™ are instrumental in navigating these challenges, optimizing the selection of principal investigators and the submission of regulatory dossiers, thereby expediting the approval process. By investing in robust early-phase studies and leveraging the expertise of partners like bioaccess™, organizations can significantly enhance the probability of successful outcomes in later-stage trials.
Recruiting qualified researchers presents a formidable challenge for Clinical Research Directors. To tackle this issue, forging robust partnerships with academic institutions and professional organizations is essential, as these collaborations provide access to a diverse pool of skilled researchers. Partnerships with universities not only enhance recruitment efforts but also foster knowledge exchange and innovation in research methodologies pertinent to healthcare. For instance, collaborations can lead to joint training initiatives that prepare researchers for the specific demands of trials, thereby improving the quality of study implementation.
Moreover, offering competitive compensation packages is vital for attracting top talent. Research indicates that financial incentives, combined with a supportive and collaborative work environment, significantly boost recruitment success. A recent study underscored this point, revealing that organizations with strong academic ties experienced a 30% increase in the recruitment of qualified researchers, highlighting the value of these partnerships.
Academic leaders emphasize the importance of these collaborations, noting that they facilitate the connection between theoretical knowledge and practical application in healthcare settings. By leveraging the expertise of academic institutions, research organizations can not only identify qualified researchers but also cultivate a culture of continuous learning and improvement within their teams. As the landscape of clinical studies evolves, establishing and maintaining these collaborations will be crucial for ensuring the success of human trials.

Clinical Research Directors frequently oversee multiple projects concurrently, rendering effective project management crucial for achieving success. The implementation of project management tools and methodologies can substantially streamline processes, enhance resource allocation, and facilitate progress tracking across diverse experiments.
For example, organizations that embrace structured project management practices attain an impressive 92% success rate in fulfilling their project objectives.
Regular team meetings and well-established communication channels are essential for maintaining alignment among team members, which is instrumental in keeping projects on course. Moreover, tools such as Gantt charts and project management applications aid in visualizing timelines and responsibilities, ultimately leading to improved outcomes in medical studies.
As the landscape of medical trials evolves, adopting these strategies becomes imperative for navigating the complexities of modern study initiatives.
Effective communication is paramount for the success of research teams in the medical field. Clinical Study Directors must cultivate an open communication environment, encouraging team members to share ideas and concerns freely. By implementing collaborative tools, such as project management software and communication platforms, team cohesion can be significantly enhanced, ensuring that all members remain informed about project developments. Regular check-ins and feedback sessions foster a sense of belonging and accountability—elements crucial for maintaining motivation and engagement.
Research indicates that efficient team communication correlates directly with improved research outcomes, reducing the likelihood of errors and enhancing patient safety. For instance, groups utilizing organized communication methods, such as daily meetings or online collaboration tools, report higher satisfaction levels among participants and encounter fewer issues during tests. In fact, patients receiving care in environments characterized by poor teamwork are nearly five times more likely to experience complications or death, underscoring the vital role of effective communication in healthcare settings.
As we look toward 2025, promoting a culture of open communication remains a priority in human trials, as it not only enhances team dynamics but also drives innovation and efficiency. As Andrew Carnegie aptly stated, "Teamwork is the ability to work together toward a common vision." Furthermore, Patrick Lencioni noted, "Teamwork requires determination from everyone involved, because when one person wavers, the whole team falters." By prioritizing communication and addressing obstacles such as inadequate communication channels and conflicting priorities, Clinical Study Directors can unlock their teams' full potential, leading to remarkable accomplishments in human trials.
Effective market access strategies are paramount for ensuring that innovative products successfully reach their intended audience. Clinical Study Directors must collaborate with market access teams early in the investigation process to identify potential obstacles and opportunities. This proactive collaboration fosters a deeper understanding of the healthcare landscape and stakeholder dynamics, facilitating smoother pathways to commercialization. For instance, companies like Novo Nordisk and Eli Lilly have successfully implemented market access strategies involving real-world studies to demonstrate the broader benefits of their therapies, thereby influencing reimbursement decisions and care pathways.
As we look towards 2025, the emphasis on early stakeholder engagement has become increasingly critical. Successful pharmaceutical and life sciences organizations excel at navigating the complexities of market access by nurturing connections with key opinion leaders (KOLs), healthcare professionals (HCPs), and patient advocacy groups. This approach allows Clinical Directors to enhance their strategies and overcome commercialization obstacles. A market access professional aptly noted, 'Investing time in building relationships with stakeholders is essential; don’t let your first interaction be when you need something.' This method not only maximizes the impact of studies but also aligns with the evolving demands of the healthcare market, ultimately driving successful product commercialization.
Ongoing education is essential for Clinical Research Directors to navigate the ever-evolving landscape of clinical studies. Engaging in professional development opportunities—such as attending industry conferences and participating in specialized training—significantly enhances both knowledge and skills.
Statistics reveal that:
By prioritizing ongoing education, directors can ensure their practices remain innovative and effective, ultimately leading to improved outcomes in human trials. For instance, organizations that emphasize learning and development are 92% more likely to innovate, which is vital in a field characterized by rapid and impactful advancements.
Additionally, SAP encourages employees to dedicate 15% of their time to personal development, exemplifying how organizations can cultivate a culture of learning. Embracing a growth mindset not only benefits individual professionals but also enhances the overall performance of clinical research teams.
As Albert Einstein aptly noted, 'Intellectual growth should commence at birth and cease only at death,' reinforcing the notion that learning is a lifelong journey.
In the competitive landscape of clinical research, enhancing human trials is paramount for Clinical Research Directors. The strategies outlined here emphasize the necessity of regulatory speed, diverse patient recruitment, ethical integrity, and effective project management—elements that are vital for the successful execution of clinical trials. By adopting these approaches, organizations can streamline their processes and significantly improve the outcomes of their studies.
Key insights reveal that global-first agility, exemplified by partnerships with platforms like bioaccess®, can drastically reduce approval times and facilitate access to diverse patient populations. Moreover, the importance of early-phase studies in laying a solid foundation for subsequent trials cannot be overstated, as they play a crucial role in validating treatment safety and efficacy. Fostering effective communication within research teams and engaging in continuous learning are also essential for navigating the complexities of modern clinical investigations.
As the clinical research environment continues to evolve, embracing these strategies becomes increasingly critical. By prioritizing regulatory compliance, inclusivity in patient recruitment, and ongoing professional development, Clinical Research Directors can enhance the integrity of their trials and drive innovation in medical solutions. The call to action is clear: invest in these strategies to ensure the successful delivery of groundbreaking therapies that can transform patient care and improve health outcomes for diverse populations.
What is bioaccess® and how does it benefit clinical trials?
bioaccess® is a platform that accelerates clinical trials by achieving ethical approvals in 4-6 weeks through regulatory speed, diverse patient populations, and streamlined pathways. It enables Clinical Research Directors to initiate studies more quickly, enhancing operational efficiency and shortening the time-to-market for medical solutions.
Why is regulatory speed important in clinical trials?
Regulatory speed is crucial for Clinical Research Directors as it allows for faster approvals and the ability to adapt to changing market needs and regulatory frameworks. Regions like Colombia offer quicker approval times (90-120 days), which can significantly streamline the clinical trial process and improve patient access to innovative therapies.
What advantages does Colombia offer for clinical trials?
Colombia has a large population of over 50 million, with 95% covered by universal healthcare, facilitating patient recruitment. The country's healthcare system is highly regarded, and it offers significant R&D tax benefits, including a 100% tax deduction for investments in science and technology.
How can Clinical Study Directors enhance patient recruitment?
Directors can enhance recruitment by implementing inclusive strategies that reflect the demographics of the target market. This includes engaging with diverse communities, utilizing local networks, and addressing barriers to participation, such as transportation costs and educational resources.
What role does diversity play in clinical studies?
Utilizing diverse patient groups improves the reliability of clinical studies, ensuring results are applicable to a broader population. Targeted outreach efforts have been shown to increase participation among underrepresented groups, thereby enhancing study validity and outcomes.
What strategies can be employed to improve enrollment of underrepresented groups?
Effective strategies include partnering with community organizations, using culturally relevant communication, and providing incentives for participation. These approaches help to address disparities in enrollment and ensure that study outcomes reflect the intended patient demographics.
What does the FDA's 2020 guidance emphasize regarding clinical trials?
The FDA's 2020 guidance emphasizes the need to broaden eligibility criteria and enhance recruitment strategies to mirror the population likely to use the drug. This is important to address disparities in enrollment among different demographic groups.