


The article emphasizes strategies to enhance patient diversity in clinical trials, underscoring the critical role of community engagement, cultural competency, and tailored recruitment efforts. It outlines specific approaches, including:
These strategies are designed to ensure that clinical research accurately reflects the diverse populations it serves, highlighting the necessity of collaboration and targeted efforts in the Medtech landscape.
Enhancing patient diversity in clinical trials transcends being a mere regulatory checkbox; it represents a crucial element that can pave the way for more effective and inclusive healthcare solutions. By adopting innovative strategies that prioritize the recruitment of diverse participants, clinical research can more accurately mirror the populations it seeks to serve, ultimately leading to improved health outcomes for all. Nevertheless, a significant challenge persists: how can clinical trial sponsors effectively bridge the divide between diverse communities and research participation? This article delves into ten actionable strategies aimed at enhancing patient diversity, ensuring that clinical trials are not only representative but also equitable in their approach.
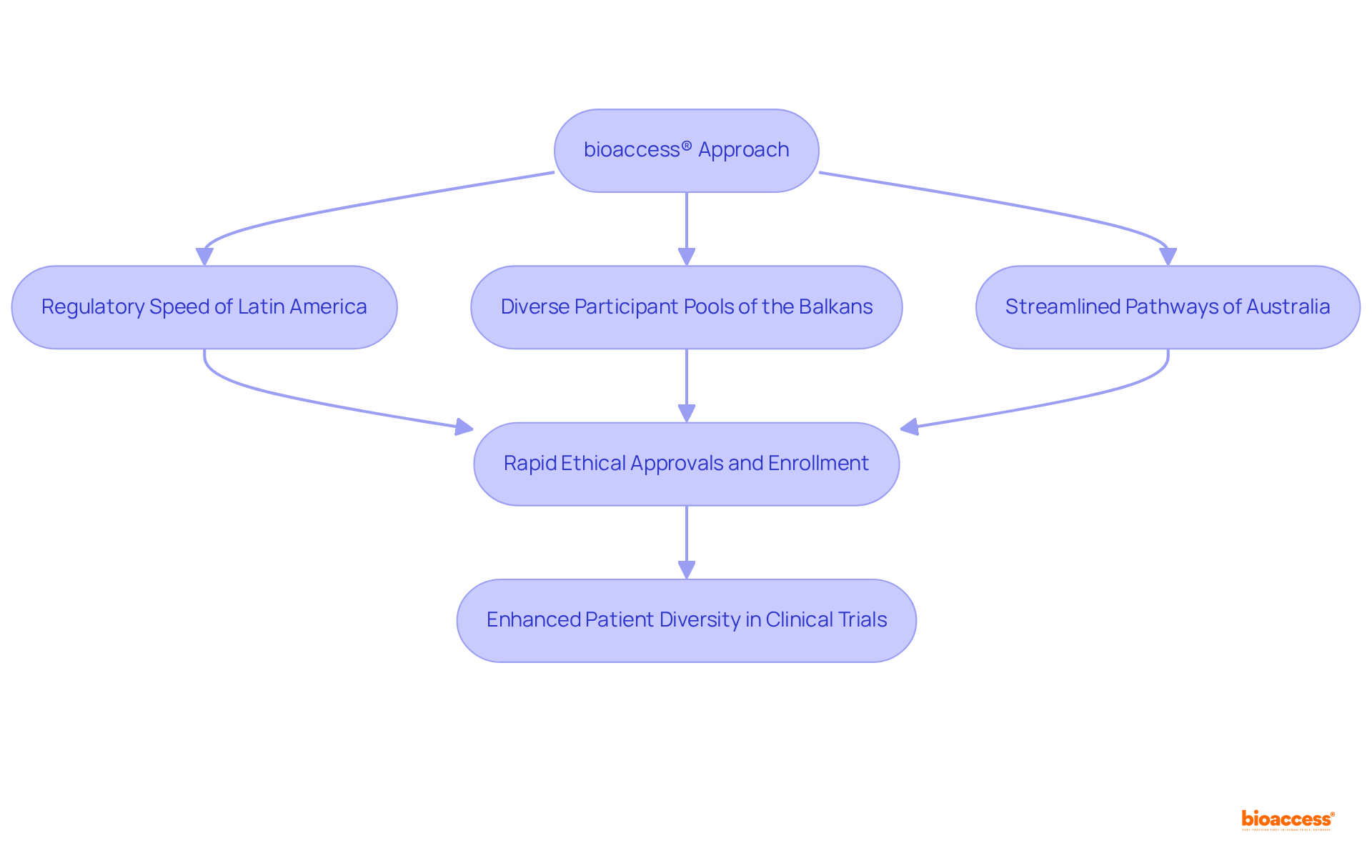
bioaccess® effectively integrates the regulatory speed of Latin America, the diverse participant pools of the Balkans, and Australia's streamlined pathways to facilitate rapid ethical approvals and enrollment. This distinctive approach broadens the participant base for patient diversity clinical trials, ensuring that research studies accurately reflect the communities they aim to serve. By securing ethical approvals in just 4-6 weeks and achieving enrollment rates that are 50% faster than traditional markets, bioaccess® sets a new standard for clinical flexibility, significantly enhancing patient diversity in clinical trials.
Moreover, the organization’s unwavering commitment to ethical practices ensures that patient diversity clinical trials not only include various populations but also treat them with the utmost respect and care throughout the process. This dedication fosters trust and engagement among participants from diverse backgrounds, reinforcing the importance of collaboration in advancing clinical research.
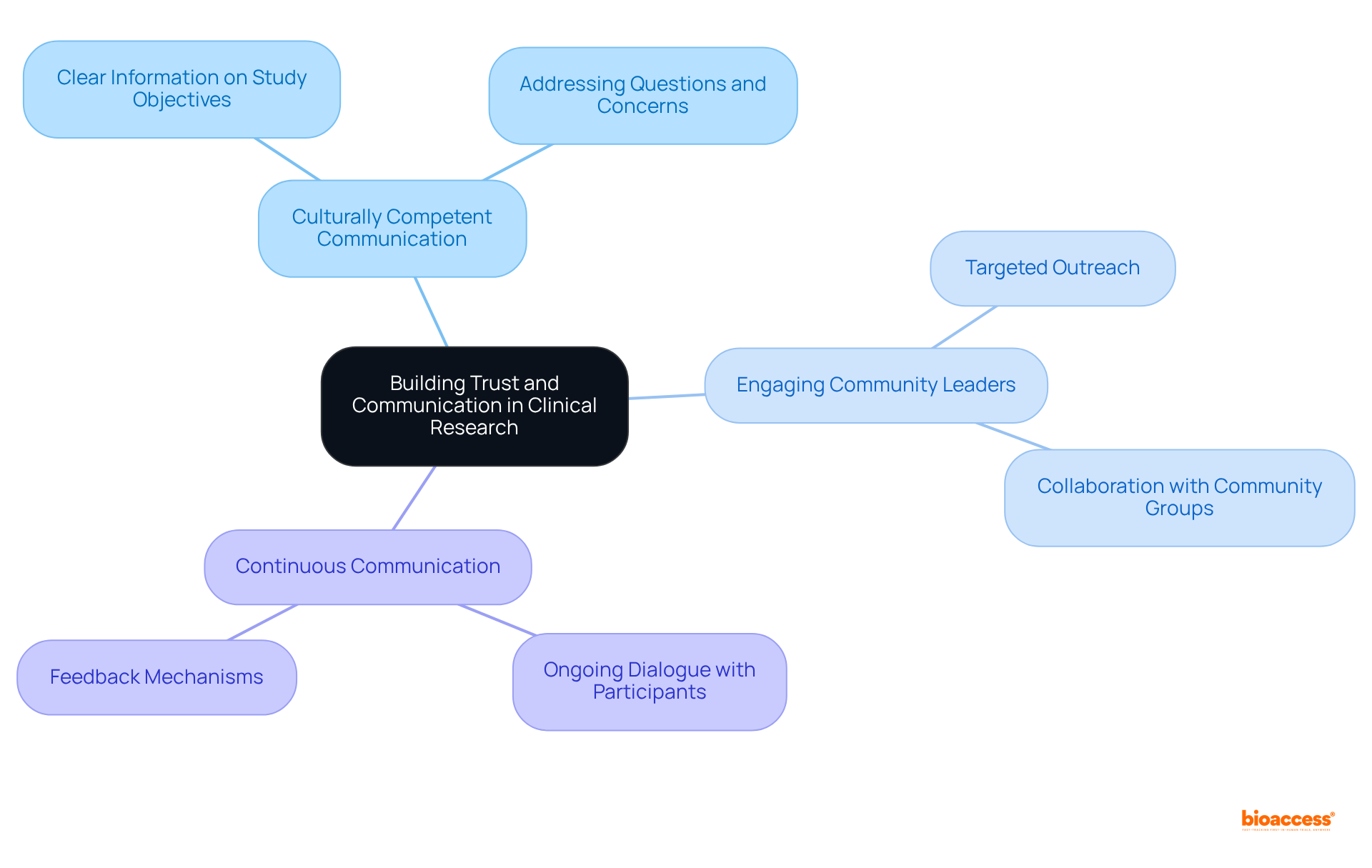
Establishing trust with diverse client groups is essential in clinical research, particularly in patient diversity clinical trials, necessitating clear communication and a genuine commitment to resolving their concerns. Clinical study sponsors must prioritize culturally competent communication strategies that resonate with varied communities. This includes providing clear information regarding the study's objectives, methods, and potential benefits, while also attentively addressing individuals' questions and concerns. Engaging community leaders and advocates can further bridge understanding gaps and foster trust, facilitating participation in research studies. A recent initiative by COUCH Health exemplifies this, achieving 78% of screened patients from diverse backgrounds, underscoring the effectiveness of targeted outreach.
Moreover, maintaining continuous communication throughout the testing process can significantly enhance trust and encourage ongoing involvement, ultimately leading to improved outcomes and greater diversity in medical research. Effective community engagement strategies have been shown to reduce screen failure rates by 21%, highlighting the tangible benefits of sustaining dialogue with participants. By implementing these strategies, research sponsors can enhance patient diversity clinical trials and inclusion in their studies, ensuring that the benefits of medical advancements are accessible to all groups.
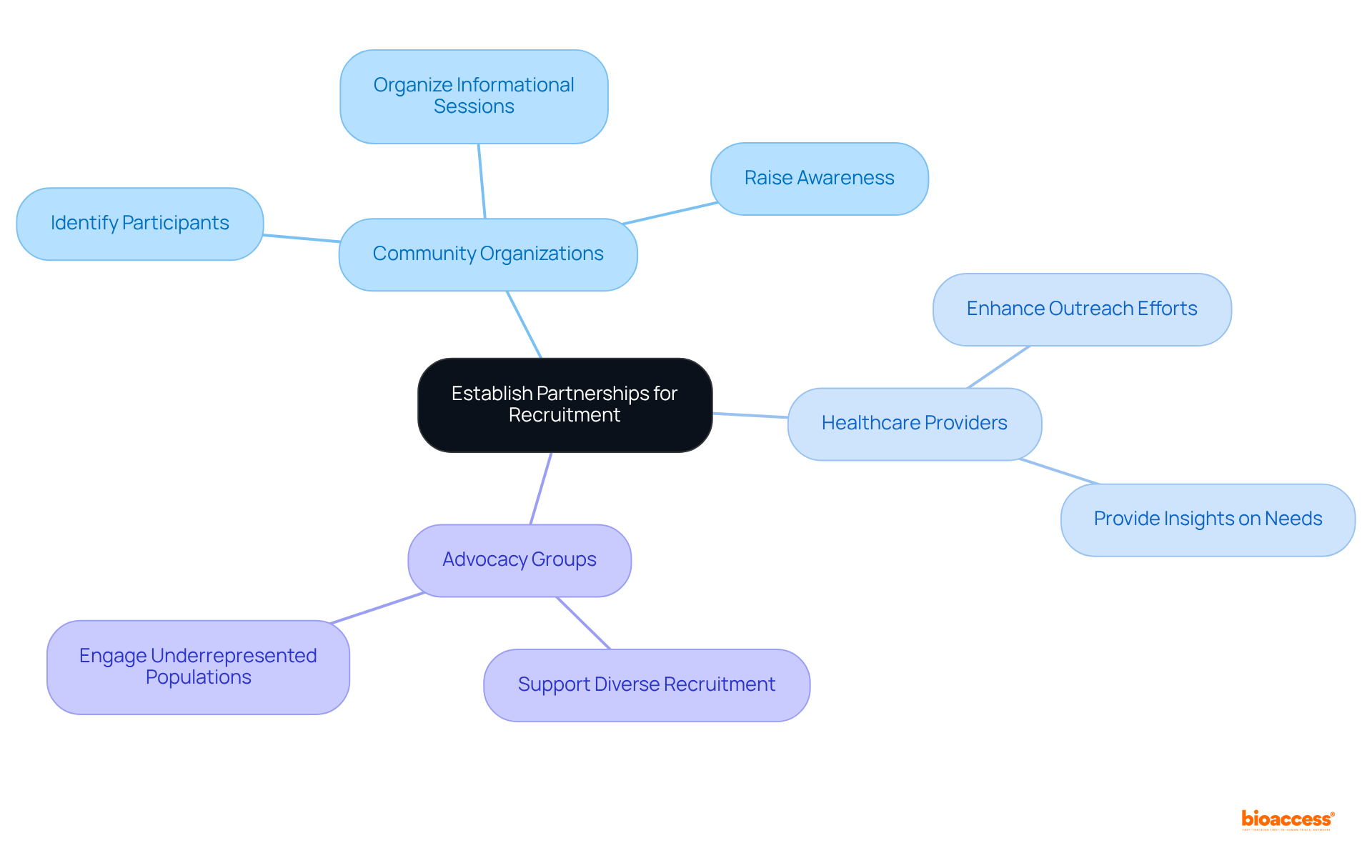
Establishing collaborations with local community organizations, healthcare providers, and advocacy groups is essential for enhancing recruitment initiatives in research studies. These partnerships can help identify potential participants for patient diversity clinical trials and provide valuable insights into the specific needs and concerns of diverse populations. By aligning with trusted community leaders, research sponsors can significantly enhance their outreach efforts, ensuring that information about patient diversity clinical trials effectively reaches underrepresented populations.
Moreover, community groups play a vital role in organizing informational sessions, workshops, and health fairs that educate potential participants about the benefits of research involvement. This proactive engagement not only raises awareness but also stimulates interest in strategies for patient diversity clinical trials and diverse participant recruitment.
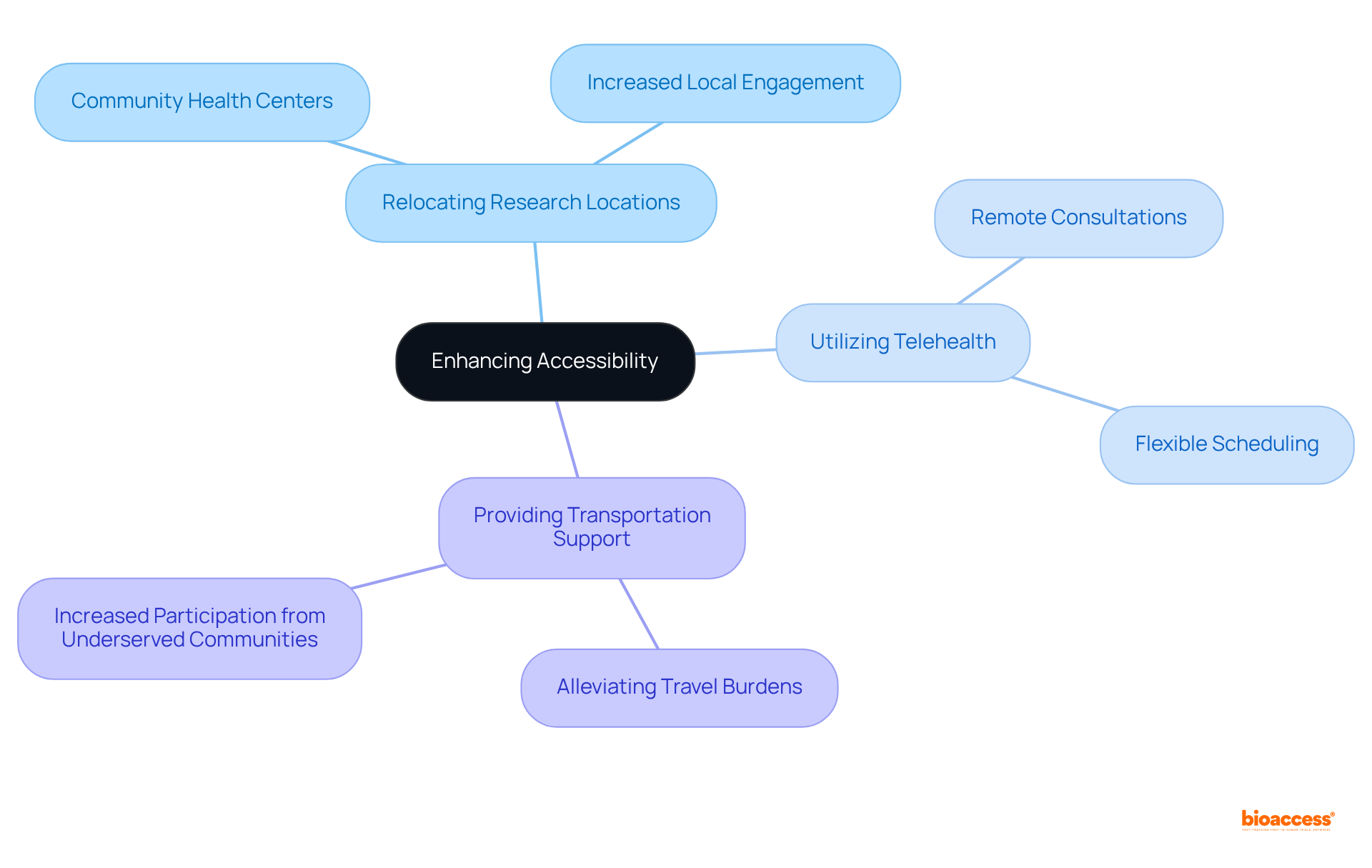
To improve accessibility, research sponsors must prioritize engaging individuals within their communities. This approach can encompass:
By alleviating travel burdens and enhancing convenience, sponsors can attract a broader spectrum of participants, particularly from underserved communities. As Cathy Vasquez, a Clinical Trial Manager, asserts, 'When people from different backgrounds participate in patient diversity clinical trials, the information gathered is richer and more representative of the entire population.'
Flexible scheduling and online participation further accommodate diverse individual needs, ensuring that research studies remain accessible to all, regardless of geographical location or socioeconomic status. This dedication to inclusivity not only elevates the quality of research but also paves the way for patient diversity clinical trials, fostering a healthier and more equitable future in healthcare. Moreover, available studies can contribute to reducing health inequalities among various demographic groups, ultimately leading to improved health outcomes. Additionally, participation in research studies grants patients access to innovative therapies before their general release, allowing them to receive more personalized and dedicated attention from their healthcare providers. This comprehensive approach fosters trust within diverse populations, as highlighted in the case study 'Enhanced Trust in Medical Research,' which underscores the significance of addressing specific needs to encourage participation.
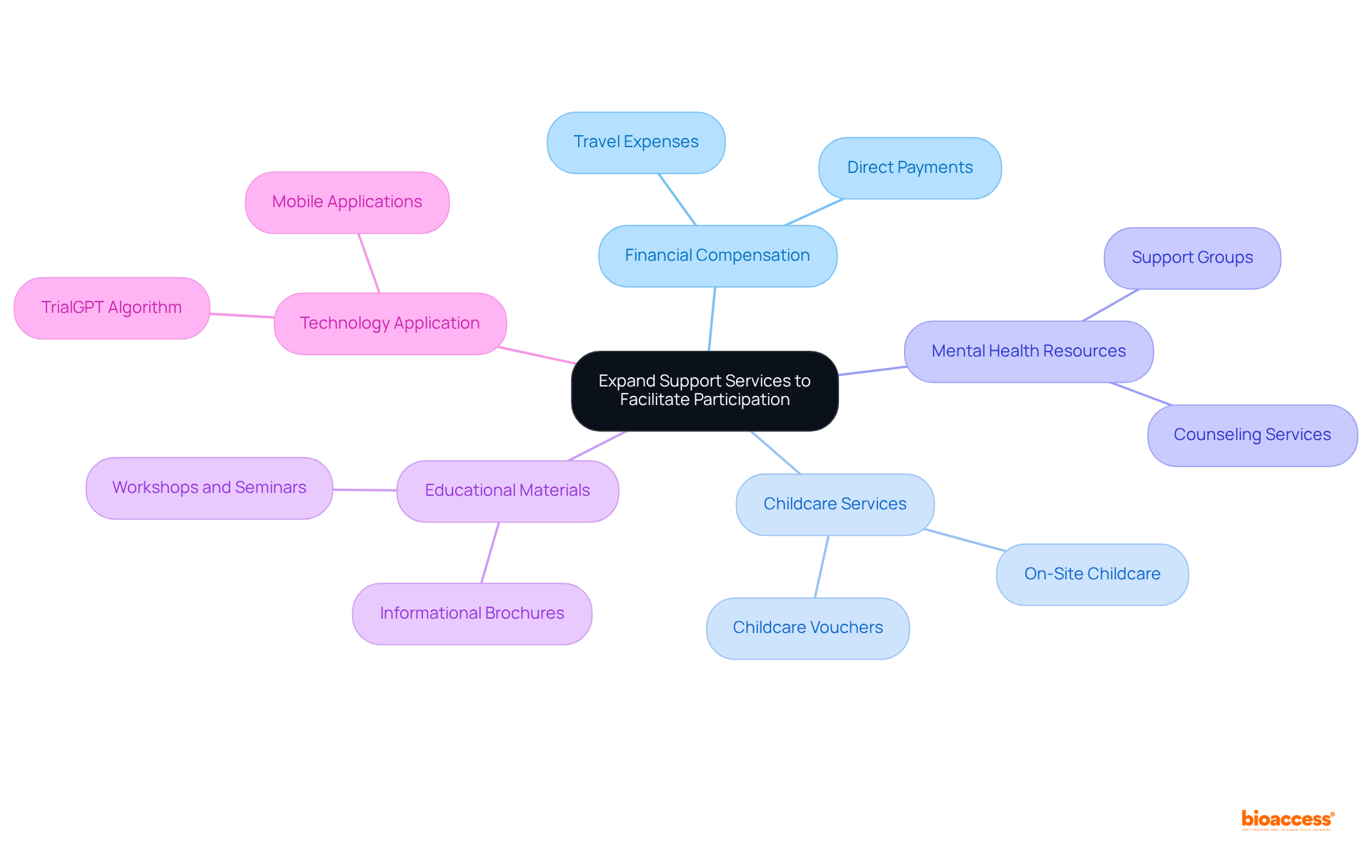
Enhancing support services is essential for promoting involvement in patient diversity clinical trials, especially among diverse populations. This enhancement can include:
By addressing the logistical and emotional obstacles that potential participants may encounter, sponsors can cultivate a more welcoming atmosphere for a variety of individuals.
Moreover, providing educational materials and tailored support during the testing process can help participants feel more at ease and knowledgeable, ultimately resulting in increased enrollment rates and improved retention among various groups in patient diversity clinical trials. For instance, navigators have reported an 88% resolution rate of patient barriers, demonstrating the effectiveness of targeted support services.
Additionally, the application of technology, such as the TrialGPT algorithm, can streamline the recruitment process for patient diversity clinical trials, facilitating easier connections for diverse populations with suitable research opportunities. This comprehensive approach not only enhances participation but also contributes to more equitable research outcomes.
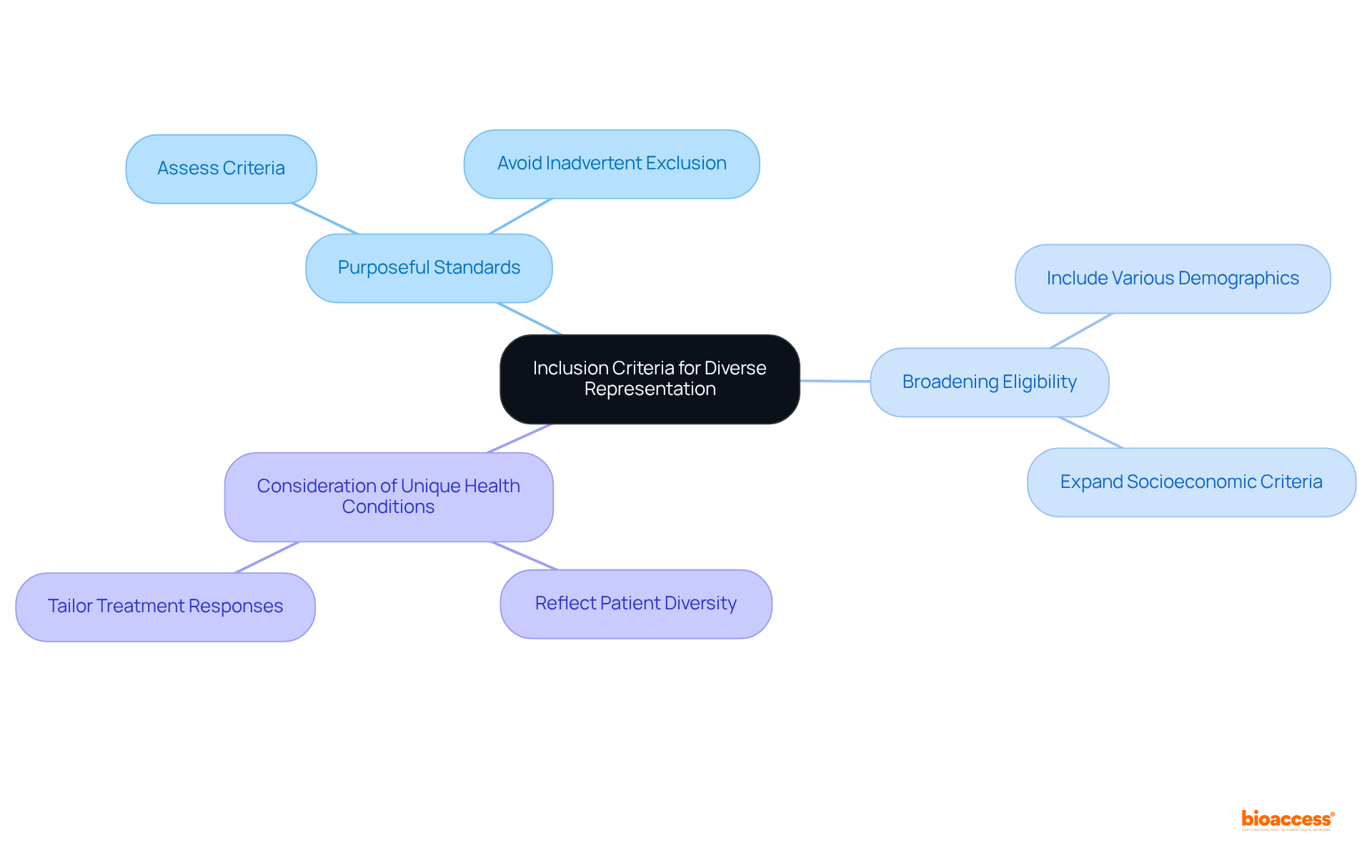
Purposeful inclusion standards are essential for achieving patient diversity in clinical trials. Researchers must critically assess their criteria to avoid the inadvertent exclusion of specific populations. This may require broadening eligibility requirements to encompass individuals from various racial, ethnic, and socioeconomic backgrounds.
Moreover, it is vital for researchers conducting patient diversity clinical trials to consider the unique health conditions and treatment responses of different demographic groups. This ensures that the study design accurately reflects the patient diversity in clinical trials that it aims to benefit. By adopting this approach, medical studies can yield more comprehensive and relevant outcomes, ultimately benefiting all patients.
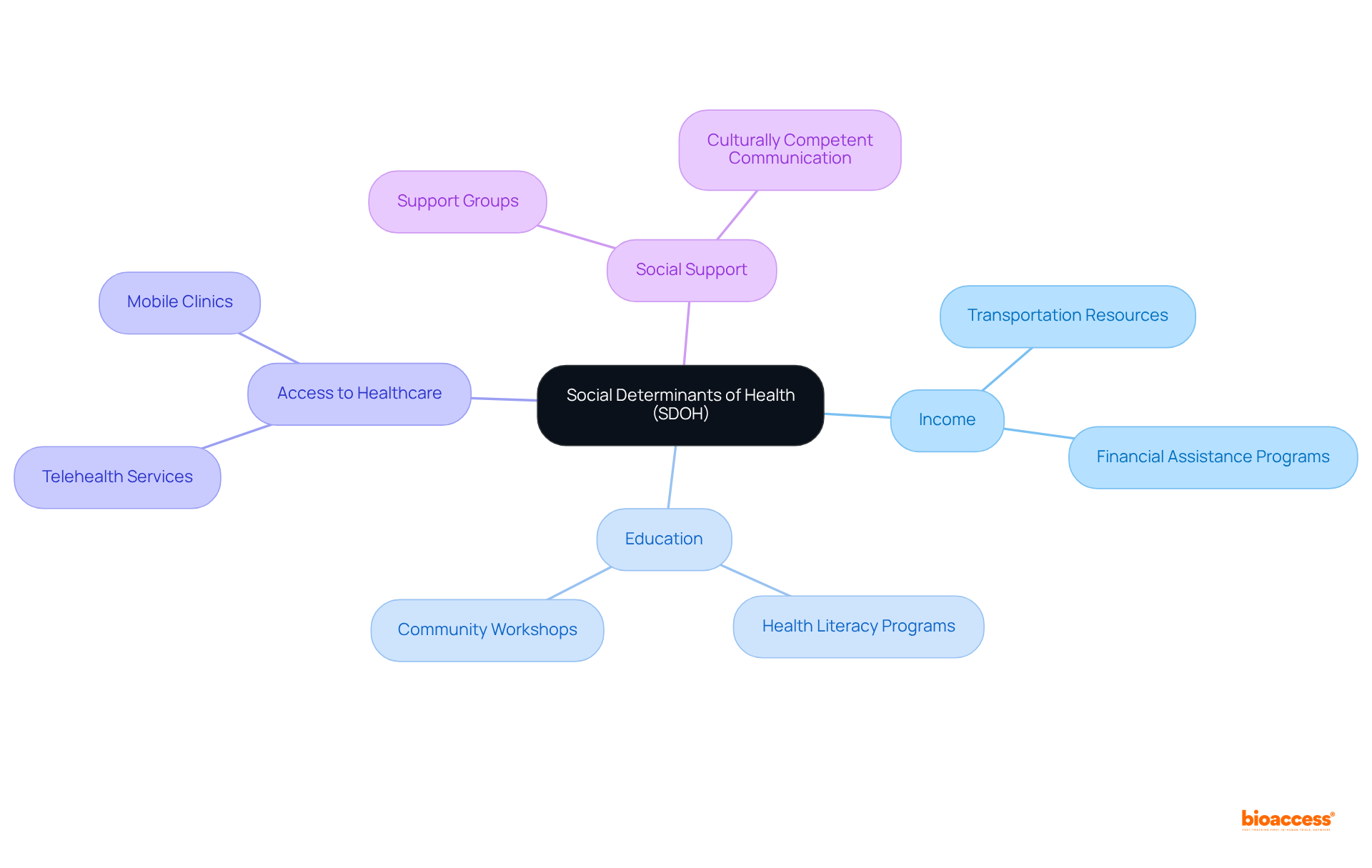
Understanding social determinants of health (SDOH) is crucial for enhancing patient diversity in clinical trials. Factors such as income, education, access to healthcare, and social support significantly influence an individual's willingness and ability to participate in research. By identifying and addressing these determinants, clinical study sponsors can develop more inclusive recruitment strategies that resonate with patient diversity clinical trials.
For example, providing resources to overcome transportation barriers, offering flexible scheduling, and ensuring culturally competent communication can effectively mitigate the impact of SDOH on trial participation.
With over 15 years of experience, bioaccess® is adept at leveraging its knowledge of these factors to enhance participant recruitment. By combining the regulatory efficiency of Latin America with the diverse patient pools of the Balkans, bioaccess® can implement tailored strategies that address the unique challenges faced by diverse populations.
Adopting a comprehensive recruitment strategy allows sponsors to enhance patient diversity in clinical trials and ensure that studies accurately represent the communities they aim to support.
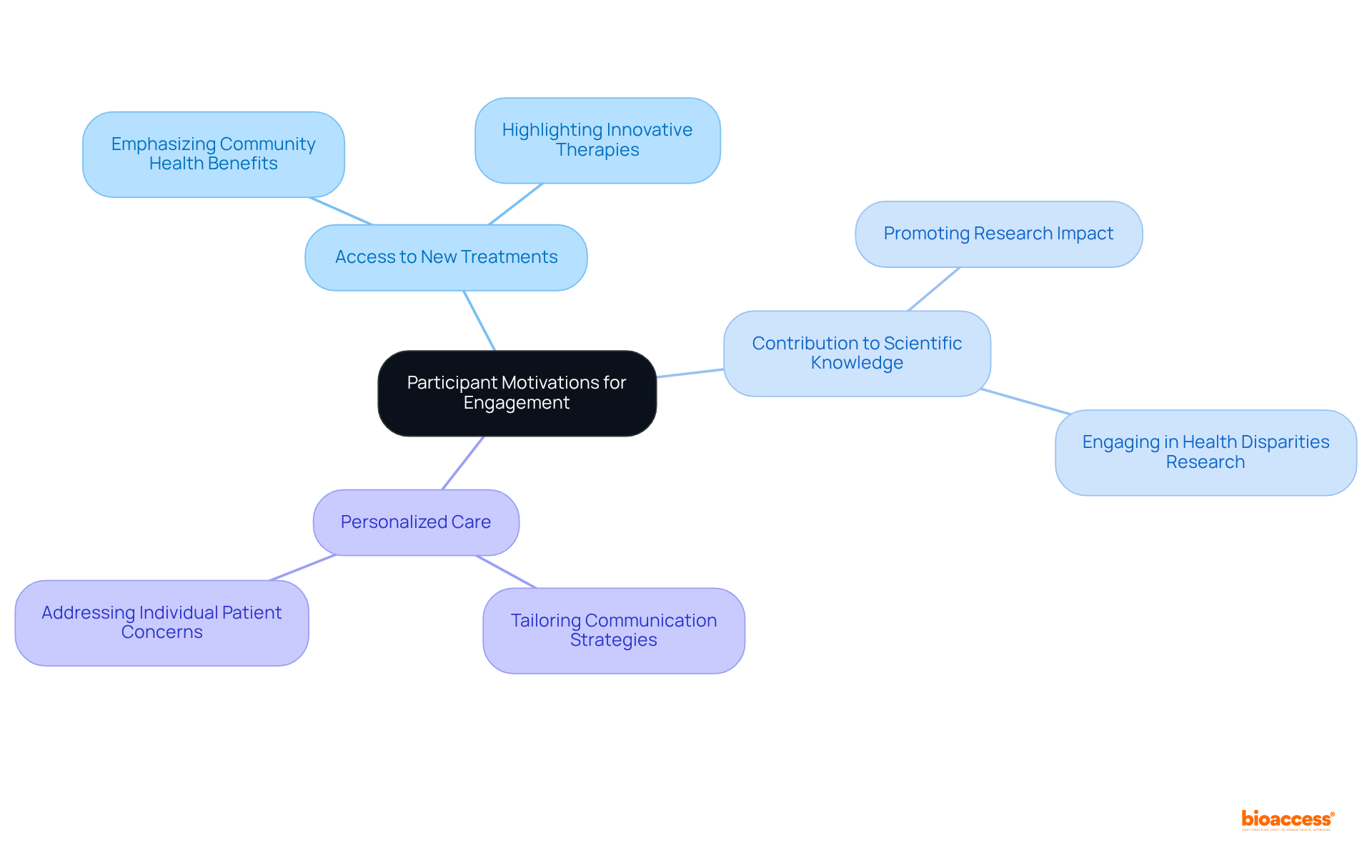
Tackling participant motivations is crucial for enhancing involvement in research studies. Many individuals are motivated by the potential to access new treatments, contribute to scientific knowledge, or receive personalized care. By clearly conveying these advantages and aligning study objectives with participant interests, sponsors can improve recruitment efforts. Significantly, merely 5% of qualified individuals engage in clinical trials, highlighting the essential need to comprehend these motivations.
Additionally, understanding the unique motivations of diverse populations can help tailor outreach strategies for patient diversity clinical trials. For example, emphasizing community health benefits or the opportunity to contribute to research that addresses specific health disparities can resonate more deeply with underrepresented groups. With 80% of physicians most likely to refer their patients to familiar colleagues and research centers, targeted outreach becomes even more vital.
As Noah Nasser observed, bridging the divide between controlled study outcomes and real-world implementation is essential. Furthermore, bioaccess® delivers ethical approvals in just 4-6 weeks, making the trial process more appealing to potential participants. To effectively enhance engagement, research directors should create outreach strategies that not only emphasize the advantages of participation in patient diversity clinical trials but also address the specific interests and concerns of various patient groups.
Highlighting significant industry and regulatory progress in patient diversity clinical trials plays a crucial role in encouraging participation from diverse populations. Recent initiatives by regulatory agencies, such as the FDA's focus on patient diversity clinical trials, demonstrate a commitment to inclusivity and fairness in investigations. By conveying these improvements to potential participants, sponsors can establish trust and assurance in the research process. This includes sharing success stories of diverse participants and showcasing how their involvement has led to meaningful changes in treatment protocols and healthcare practices. Such openness inspires individuals from underrepresented groups to participate in patient diversity clinical trials, recognizing that their contributions are both appreciated and significant.
Assessing and adjusting approaches for continuous enhancement in diversity is essential for the success of medical studies. Sponsors must regularly evaluate their recruitment and engagement efforts, gathering feedback from participants and community partners to pinpoint areas for improvement. This iterative approach facilitates the refinement of strategies based on real-world experiences and outcomes.
Moreover, leveraging data analytics to track diversity metrics provides valuable insights into recruitment effectiveness and participant demographics. For instance, the representation of white participants in research studies has been observed to fluctuate between 14.4 percent and 81 percent, while Black/African American involvement has dipped below 5 percent in certain years. By consistently monitoring progress and implementing necessary modifications, research sponsors can ensure that their initiatives to enhance diversity are not only effective but also sustainable over time.
Incorporating case studies, such as Walgreens' initiative to boost racial and ethnic diversity in clinical trials, offers practical examples of successful strategies in action. Additionally, quotes from authoritative figures like Arthur L. Caplan, who contends that traditional recruitment categories are outdated, lend credibility to the discussion. Finally, actionable tips for adapting recruitment based on participant feedback—such as employing community engagement strategies and providing information in multiple languages—can empower the target audience to apply the information more effectively.
Enhancing patient diversity in clinical trials is essential for ensuring that research accurately reflects the diverse populations it aims to serve. By integrating innovative strategies—such as building trust through effective communication, establishing community partnerships, and improving accessibility—clinical trial sponsors can significantly broaden participant recruitment. These approaches not only foster inclusivity but also lead to more representative and meaningful research outcomes, ultimately benefiting healthcare advancements for all.
Key insights from the article underscore the importance of:
By actively engaging with various communities and continuously evaluating recruitment strategies, sponsors can overcome barriers that have historically limited participation from underrepresented groups. This proactive stance is crucial for creating a more equitable and effective clinical trial landscape.
In conclusion, the journey towards enhancing patient diversity in clinical trials necessitates a commitment to collaboration, inclusivity, and continuous improvement. As the industry progresses, it is imperative for stakeholders to prioritize these strategies, recognizing that diverse representation not only enriches research but also fosters trust and engagement among participants. Embracing these principles will pave the way for a healthier future, where the benefits of medical advancements are accessible to all communities.
What is bioaccess® and how does it enhance patient diversity in clinical trials?
bioaccess® integrates the regulatory speed of Latin America, diverse participant pools from the Balkans, and Australia's streamlined pathways to facilitate rapid ethical approvals and enrollment. This approach broadens the participant base for patient diversity clinical trials, achieving ethical approvals in 4-6 weeks and enrollment rates that are 50% faster than traditional markets.
Why is trust important in engaging diverse patient populations for clinical trials?
Establishing trust is essential for participation in clinical research, particularly in patient diversity trials. Clear communication and a genuine commitment to addressing concerns help build this trust, making it easier to engage diverse communities.
What strategies can clinical study sponsors use to build trust with diverse populations?
Sponsors should prioritize culturally competent communication, provide clear information about study objectives and benefits, and actively address participants' questions. Engaging community leaders and advocates can also bridge understanding gaps and foster trust.
How can community engagement impact patient diversity in clinical trials?
Effective community engagement strategies can enhance trust and encourage ongoing involvement, leading to improved outcomes and greater diversity. Such strategies have been shown to reduce screen failure rates by 21%.
What role do local community partnerships play in recruitment for patient diversity clinical trials?
Collaborating with local organizations, healthcare providers, and advocacy groups helps identify potential participants and understand the specific needs of diverse populations. These partnerships enhance outreach and ensure information about trials reaches underrepresented groups.
How can community organizations help in the recruitment process for clinical trials?
Community groups can organize informational sessions, workshops, and health fairs to educate potential participants about the benefits of research involvement, thereby raising awareness and stimulating interest in patient diversity clinical trials.