


The primary objective of this article is to delineate effective strategies for mastering clinical trial supply logistics. It asserts that a comprehensive approach—encompassing procurement, transportation, demand planning, and stakeholder collaboration—is vital for optimizing efficiency and ensuring successful outcomes in clinical research. This is underscored by evidence of enhanced communication and technology integration, which have led to expedited study completions.
Mastering clinical trial supply logistics stands as a pivotal factor in the success of medical research, influencing critical aspects such as patient recruitment and the timely delivery of essential materials. By implementing effective strategies, organizations can significantly enhance their operational efficiency, ensuring that groundbreaking therapies reach the market faster.
However, the complexities of procurement, transportation, and stakeholder collaboration pose significant challenges. How can research teams navigate these intricacies to achieve optimal outcomes?
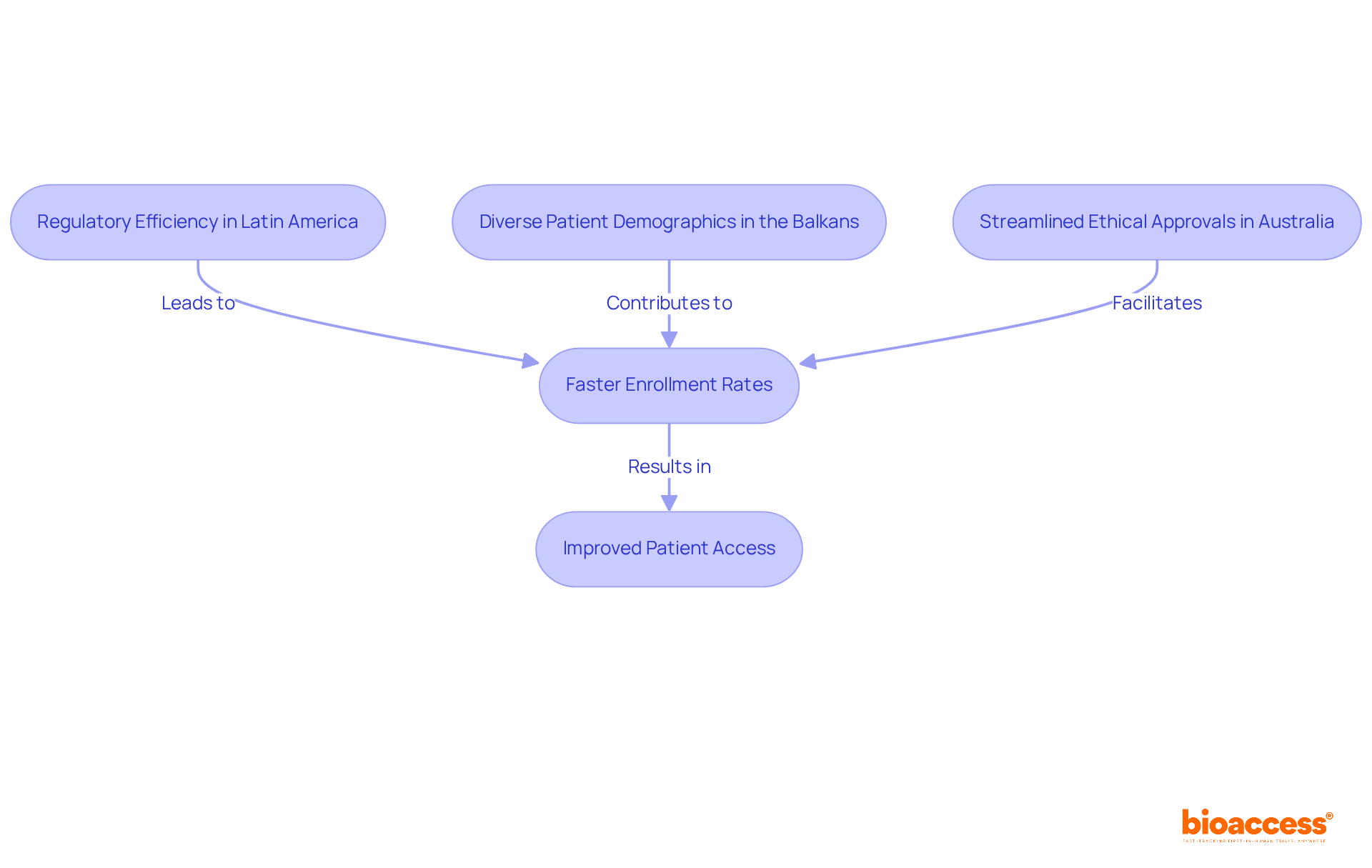
bioaccess® harnesses the regulatory efficiency of Latin America, the diverse patient demographics in the Balkans, and Australia's streamlined ethical approval procedures to significantly enhance research logistics. Ethical approvals are secured in just 4-6 weeks, with enrollment rates 50% faster than traditional markets. This enables Medtech, Biopharma, and Radiopharma innovators to adeptly navigate the complexities of research studies. Such a strategic advantage not only accelerates the journey to market for groundbreaking innovations but also improves patient access to new therapies, ultimately benefiting healthcare systems worldwide.
The importance of regulatory pace in medical studies cannot be overstated; it serves as a critical indicator of a nation's attractiveness for conducting research. By optimizing these processes, bioaccess® plays an essential role in advancing medical technology and enhancing patient outcomes.
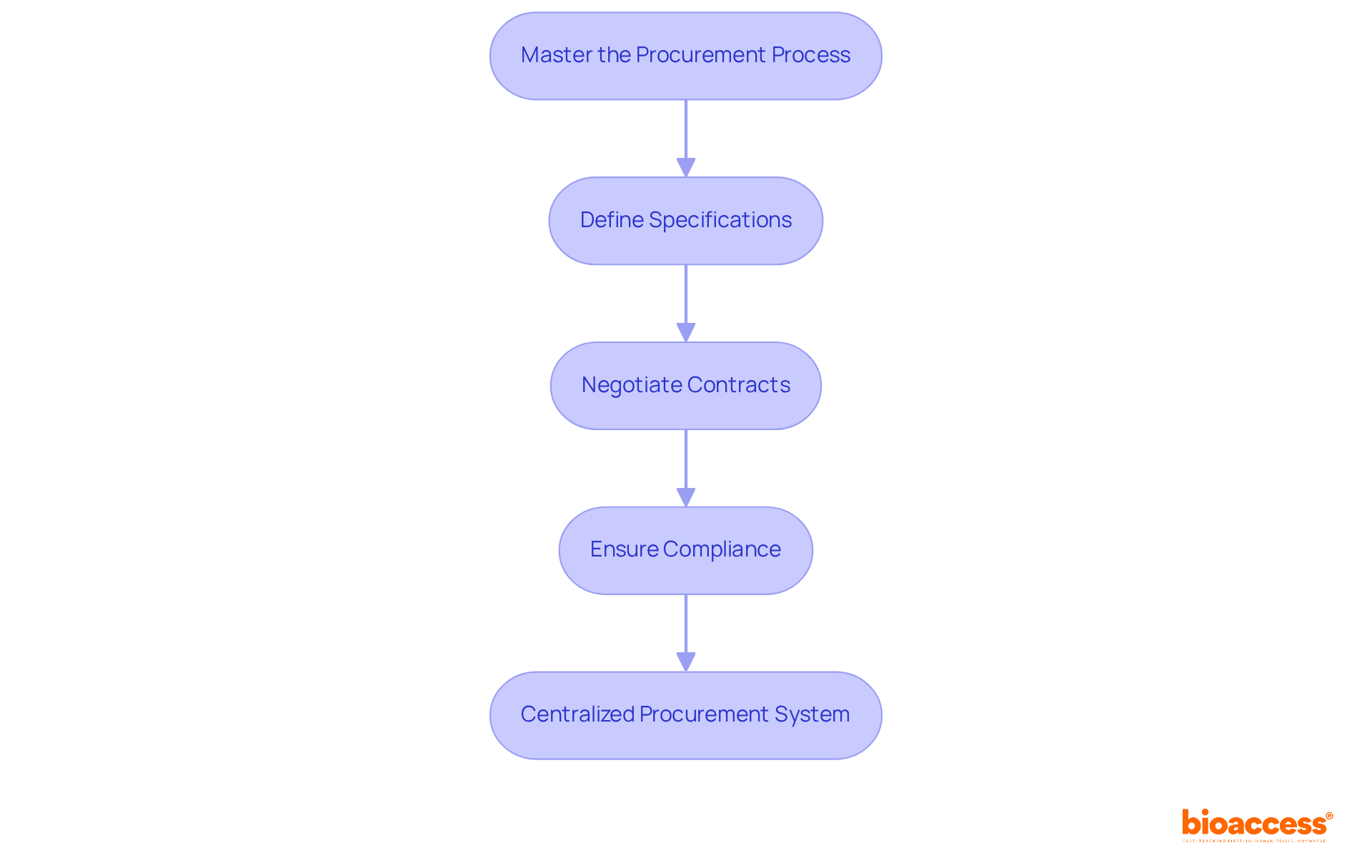
Mastering the procurement process for research materials is essential for the success of clinical trial supply logistics in clinical research. Establishing strong communication channels with providers and stakeholders is crucial in this endeavor. It involves:
A centralized procurement system can significantly streamline the ordering process within clinical trial supply logistics, enabling real-time tracking of inventory levels and facilitating timely deliveries. Frequent assessments of vendors, coupled with the development of robust partnerships, further enhance procurement efficiency, mitigating the risk of shortages that could impede project advancement.
As Tim Holmes, Manager of Oversight of Supplies, emphasizes, being realistic and clear in communication with medical teams is vital, as they may lack expertise in supply management. Encouraging open communication and teamwork allows organizations to navigate the complexities of procurement more effectively, ultimately resulting in smoother research operations.
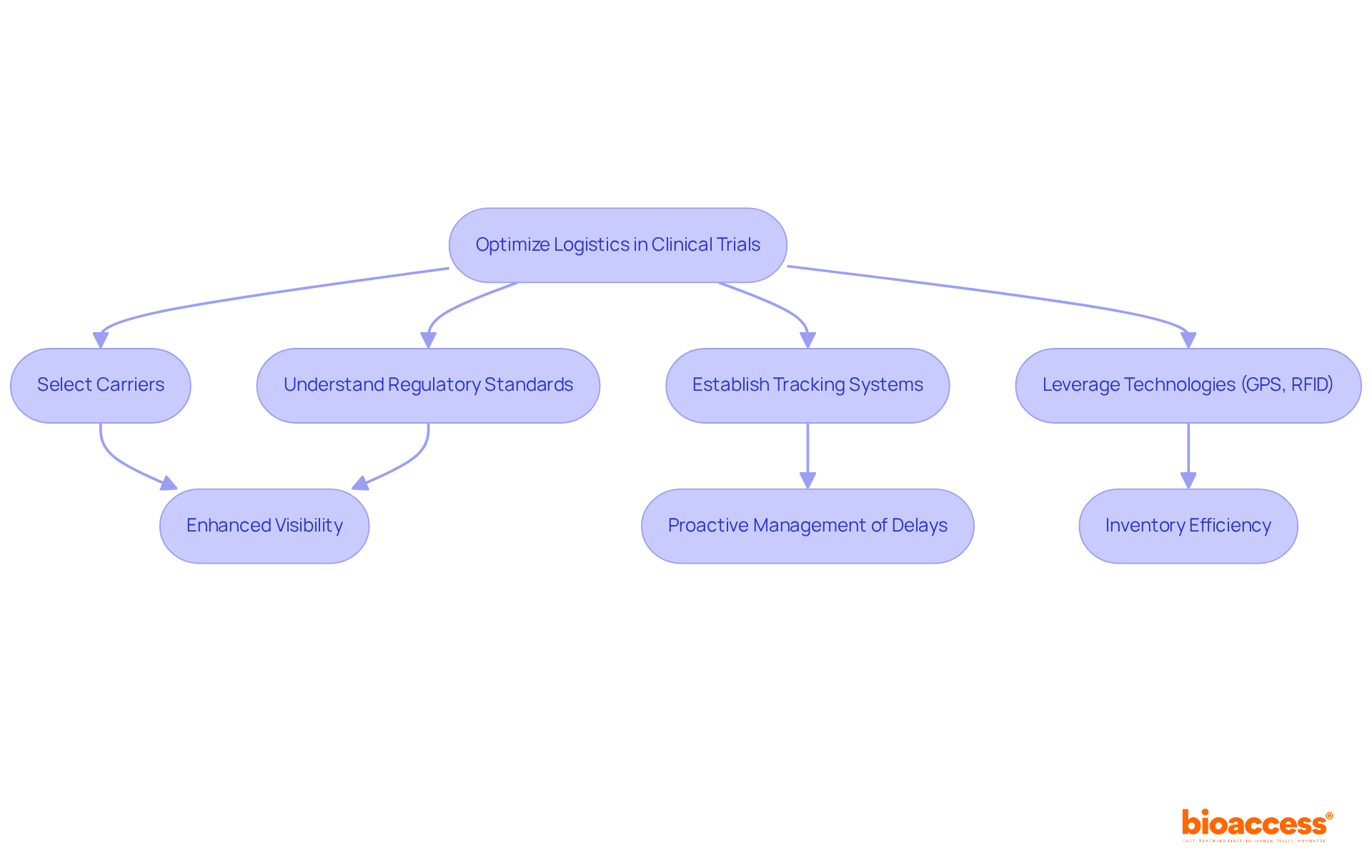
Efficient clinical trial supply logistics and transportation in medical studies are paramount, relying on the strategic selection of carriers, a thorough understanding of regulatory shipping standards, and the establishment of robust tracking systems. Technologies such as GPS and RFID play a pivotal role in enhancing visibility across the supply chain, enabling real-time monitoring of shipments and facilitating proactive management of potential delays.
For instance, studies indicate that nearly 70% of clinical experiments utilize Interactive Response Technologies (IRT), significantly enhancing inventory visibility and operational efficiency. Furthermore, creating contingency plans for unexpected disruptions is crucial to uphold schedules amid logistical challenges. A recent analysis revealed that 3% of studies faced product impact due to shipping errors, underscoring the necessity for meticulous logistics management.
As Kirk Randall, Sales Director at Cryoport, aptly states, "The success of these medical studies significantly relies on attaining and sustaining ideal performance conditions for temperature-sensitive products during the study." By leveraging advanced technologies and maintaining a focus on tracking and monitoring, research sponsors can navigate the complexities of logistics with greater efficacy.
Efficient demand planning and forecasting methods are crucial for anticipating resource requirements in medical studies, particularly regarding clinical trial supply logistics to address patient recruitment issues encountered by Medtech and Biopharma startups, which frequently contend with restricted financial assets and infrastructure. By examining historical information and trends, organizations can make informed forecasts about future resource requirements. Cooperation with medical teams is essential to understand study timelines and patient enrollment rates, ensuring that the availability of resources matches anticipated demand.
Utilizing advanced forecasting software, such as AI-powered tools, enhances the accuracy of these predictions, allowing for better resource allocation. These tools can assist in recognizing participants most inclined to register and finalize studies, ultimately resulting in more effective research operations. This strategic alignment not only aids in optimizing inventory levels but also greatly minimizes the risk of delays due to shortages.
Moreover, efficient inventory management within clinical trial supply logistics is essential for maximizing resource utilization and guaranteeing the accessibility of study materials, thus speeding up research processes in the Medtech and Biopharma industries.

Creating efficient sourcing strategies for research project materials requires a comprehensive approach that involves:
Expanding the supplier network is crucial to mitigate risks associated with dependence on a single source, particularly in the dynamic landscape of clinical trial supply logistics. Establishing enduring partnerships with key vendors not only enhances collaboration but also ensures preferential access to critical resources during peak demand periods, which is essential for clinical trial supply logistics and significantly impacts testing timelines.
Regular assessment of supplier performance is vital, as it enables organizations to adapt to evolving market conditions and foster continuous improvement. For instance, companies that conduct structured supplier quality audits can systematically evaluate operational areas that influence product quality, leading to informed sourcing decisions. Moreover, integrating supplier diversity into sourcing strategies can bolster innovation and resilience, as diverse suppliers frequently offer unique perspectives and solutions. By fostering an inclusive sourcing process, organizations can not only comply with regulatory requirements but also enhance overall efficiency and outcomes.

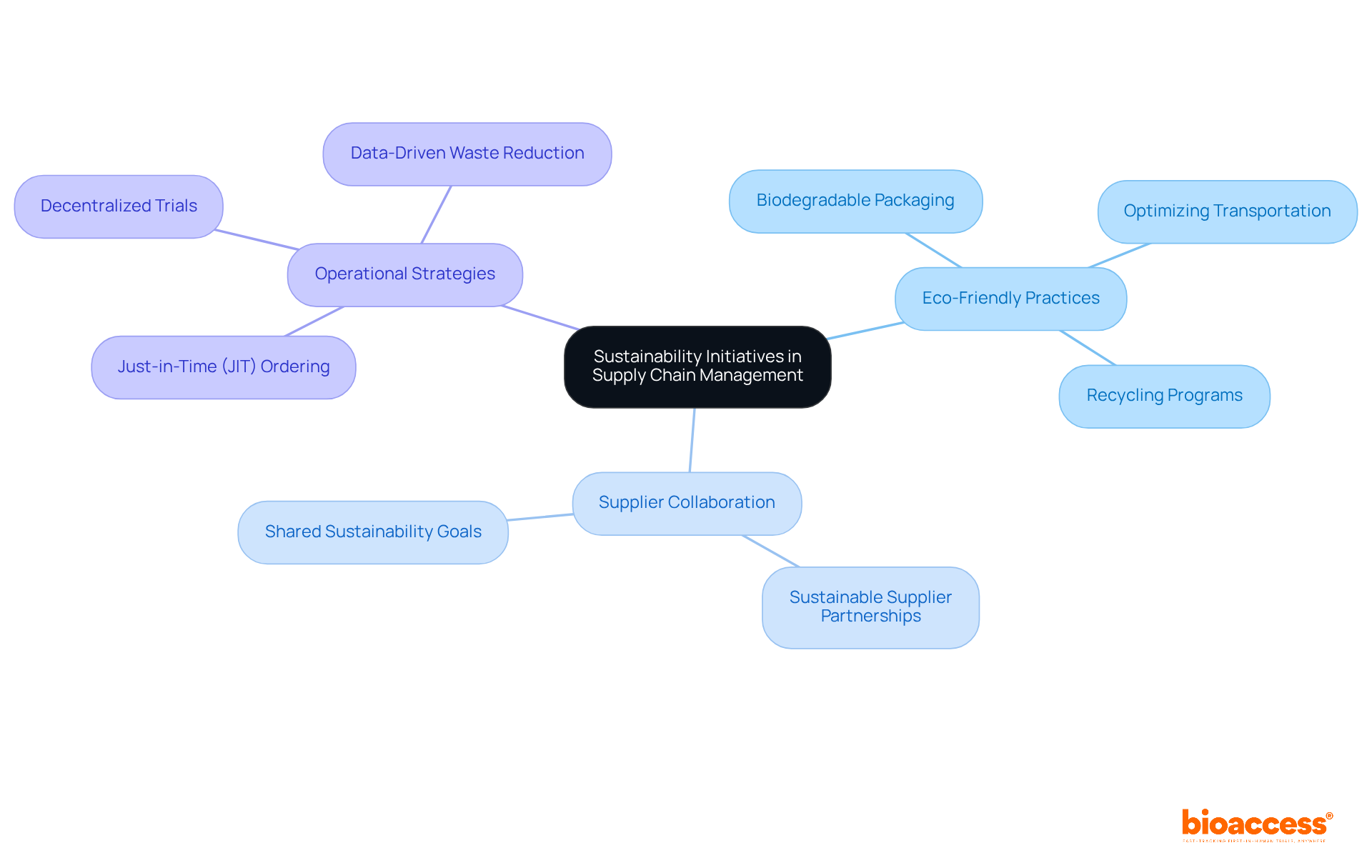
Integrating sustainability initiatives into supply chain management is essential for assessing the environmental impact linked to sourcing, transportation, and waste management. Organizations can implement various eco-friendly practices, including:
Collaborating with suppliers who prioritize sustainability not only enhances the environmental performance of research studies but also aligns with the increasing demand for corporate social responsibility within the healthcare sector. For instance, the implementation of just-in-time (JIT) ordering significantly reduces overstocking and waste, while decentralized experiments offer innovative direct-to-patient delivery models that further decrease unnecessary drug waste. By focusing on these strategies, organizations can enhance their operational efficiency while contributing to a more sustainable future in medical research.
Identifying key participants in the clinical trial supply logistics is crucial, as it involves recognizing:
Establishing clear lines of communication among these stakeholders is essential for aligning timelines, requirements, and expectations. Frequent meetings and updates foster collaboration, enabling teams to proactively address potential issues that could significantly impact progress. For instance, effective stakeholder engagement can lead to a retention rate exceeding 95%, as evidenced by bioaccess's patient-centric strategies. By forging robust connections with these vital individuals, the effectiveness of clinical trial supply logistics is enhanced, ensuring that all parties remain informed and actively involved throughout the process. Indeed, research indicates that regular interaction among stakeholders can improve overall study results, underscoring its critical role in successful medical research.
In the realm of clinical research, establishing efficient clinical trial supply logistics for warehousing and storage options is paramount. It is essential to confirm that facilities adhere to regulatory standards concerning temperature control, security, and inventory oversight. Our extensive healthcare study coordination services encompass feasibility assessments and site selection, which are crucial for identifying appropriate storage facilities that comply with these regulatory standards. Regulatory compliance mandates the preservation of essential documents for 15 years, including protocols and financial records associated with research studies. This requirement is vital for ensuring transparency and accountability.
Implementing robust inventory management systems significantly enhances the ability to track stock levels and expiration dates, thereby reducing the risk of waste. Regular audits of storage facilities not only ensure adherence to regulations but also help identify areas for improvement, fostering a culture of continuous enhancement. Moreover, educating personnel on appropriate handling and storage practices is crucial for preserving the integrity and quality of research materials, ultimately aiding the success of the studies.
The consequences of non-compliance can be severe, including legal repercussions such as fines and suspension of approval processes. This highlights the significance of following these standards meticulously. By incorporating these thorough services, we guarantee that all facets of clinical trial supply logistics are efficiently managed, paving the way for successful clinical outcomes.
Mastering supply logistics for research necessitates a comprehensive strategy that encompasses procurement, transportation, demand planning, and collaboration with stakeholders. This thorough approach not only optimizes procedures but also enhances the overall efficiency and effectiveness of medical studies. For instance, organizations that implement continuous monitoring and evaluation of their logistics processes can uncover opportunities for improvement, fostering innovation and adaptability in an ever-evolving landscape.
The impact of logistics oversight on clinical study outcomes is significant. A well-organized clinical trial supply logistics chain is essential for ensuring the timely delivery of materials, directly influencing the speed and success of trials. Best practices such as establishing clear communication pathways among stakeholders, leveraging technology for real-time data monitoring, and employing robust risk oversight strategies are critical for achieving optimal outcomes.
Examples of effective comprehensive strategies include the integration of advanced logistics systems that facilitate seamless communication and collaboration among all participants. Such systems have proven to significantly reduce delays and enhance patient recruitment efforts, ultimately leading to quicker study completion and more reliable data.
By incorporating insights from industry experts, it becomes evident that a strategic focus on logistics management can provide a competitive advantage in the research sector. With over 20 years of experience in Medtech, bioaccess® is adept at navigating the complexities of research logistics. As the pharmaceutical landscape continues to evolve, organizations that prioritize a holistic approach to clinical trial supply logistics will be better equipped to tackle challenges and seize emerging opportunities, ultimately contributing to job creation, economic growth, and healthcare improvement within local economies.
Mastering clinical trial supply logistics is fundamental to the success of medical research and the timely introduction of innovative therapies. Effective strategies that encompass procurement, transportation, demand planning, and collaboration among stakeholders significantly enhance the efficiency and effectiveness of clinical studies. This comprehensive approach streamlines processes and equips research teams to navigate the complexities of the supply chain.
Key insights highlight the importance of:
Furthermore, identifying key players in the clinical research supply chain and establishing robust communication channels among them are essential for aligning expectations and timelines. Collectively, these strategies contribute to reducing delays and improving patient access to new therapies, ultimately benefiting healthcare systems globally.
In light of these insights, it is crucial for organizations engaged in clinical research to prioritize a holistic approach to supply logistics. By embracing best practices and innovative solutions, stakeholders can foster a more agile and responsive supply chain that meets regulatory requirements and adapts to the evolving landscape of medical research. A commitment to mastering clinical trial logistics will enhance study outcomes and pave the way for advancements in healthcare that can transform lives.
What is bioaccess® and how does it enhance clinical trial logistics?
bioaccess® enhances clinical trial logistics by leveraging the regulatory efficiency of Latin America, diverse patient demographics in the Balkans, and streamlined ethical approval procedures in Australia. It secures ethical approvals in 4-6 weeks and achieves enrollment rates that are 50% faster than traditional markets, benefiting Medtech, Biopharma, and Radiopharma innovators.
How does bioaccess® impact the journey to market for medical innovations?
By optimizing research study processes, bioaccess® accelerates the journey to market for groundbreaking innovations and improves patient access to new therapies, ultimately benefiting healthcare systems worldwide.
Why is regulatory pace important in medical studies?
Regulatory pace is a critical indicator of a nation's attractiveness for conducting research. It significantly influences the efficiency and speed of clinical trials.
What is essential for mastering the procurement process for clinical trial supplies?
Mastering the procurement process involves clearly defining specifications for medical research materials, negotiating contracts, ensuring compliance with regulatory requirements, and establishing strong communication channels with providers and stakeholders.
How can a centralized procurement system benefit clinical trial logistics?
A centralized procurement system streamlines the ordering process, enables real-time tracking of inventory levels, and facilitates timely deliveries, thereby enhancing overall efficiency in clinical trial supply logistics.
What role does communication play in the procurement process?
Clear and realistic communication with medical teams is vital, as they may lack expertise in supply management. Encouraging open communication and teamwork helps navigate the complexities of procurement effectively.
What are the key components for optimizing logistics and transportation in clinical trials?
Key components include strategic selection of carriers, understanding regulatory shipping standards, and establishing robust tracking systems to enhance visibility across the supply chain.
How do technologies like GPS and RFID contribute to clinical trial logistics?
Technologies such as GPS and RFID enhance visibility by enabling real-time monitoring of shipments, which facilitates proactive management of potential delays.
What percentage of clinical experiments utilize Interactive Response Technologies (IRT)?
Nearly 70% of clinical experiments utilize Interactive Response Technologies (IRT), which significantly enhance inventory visibility and operational efficiency.
Why are contingency plans important in clinical trial logistics?
Contingency plans are crucial for managing unexpected disruptions and upholding schedules amid logistical challenges, as shipping errors can impact the success of studies.