


The article titled "10 Trends Shaping Pharma Markets for Clinical Research Leaders" highlights the pivotal trends influencing the pharmaceutical industry, particularly for leaders in clinical research. It identifies critical developments such as:
These trends not only enhance operational efficiency but also improve patient outcomes and ensure compliance with the evolving regulations within the pharma landscape.
The pharmaceutical landscape is experiencing a profound transformation as it adapts to emerging trends that are set to redefine clinical research and market strategies. This evolution presents industry leaders with a unique opportunity to leverage advancements in technology, personalized medicine, and sustainability, ultimately enhancing patient outcomes while streamlining operations. Yet, as they navigate this rapidly evolving terrain, a critical question emerges: how can pharmaceutical companies effectively integrate these trends to not only keep pace with change but also spearhead innovation?
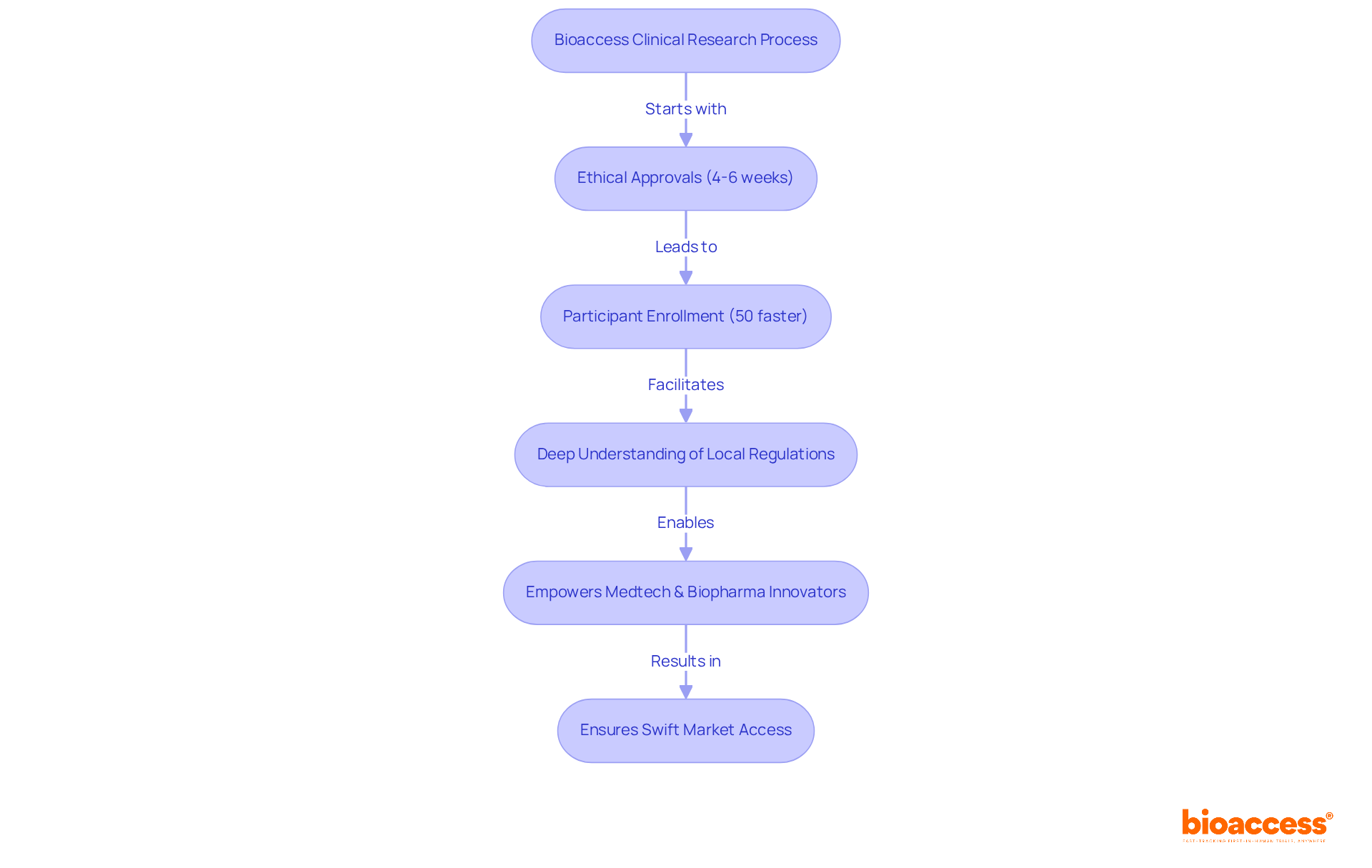
Bioaccess leverages its extensive experience and regional advantages to expedite research within pharma markets. With ethical approvals secured in an impressive 4-6 weeks and participant enrollment occurring 50% faster than in conventional markets, bioaccess distinguishes itself as a leader in early-phase research. This accelerated pace is supported by a deep understanding of local regulatory environments and access to diverse patient populations across Latin America, the Balkans, and Australia. Such capabilities empower Medtech and Biopharma innovators to navigate the complexities of trials more effectively, ensuring that their products reach the market swiftly and efficiently.
As emphasized by industry leaders, including Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, the ability to foster trust and streamline processes is crucial for effective recruitment and retention in research studies, further enhancing bioaccess's pivotal role in advancing medical solutions.
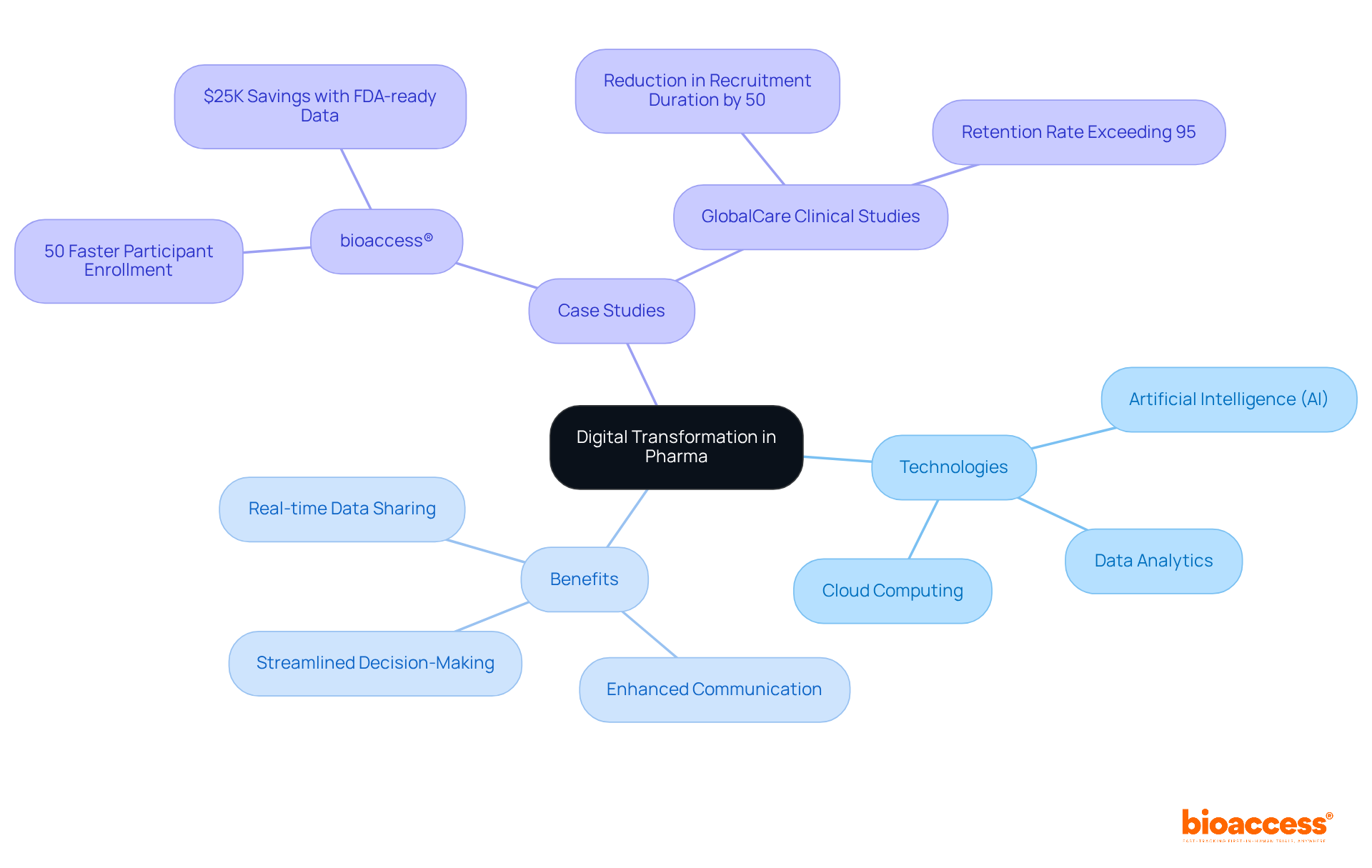
Digital transformation is fundamentally reshaping operations in pharma markets through the integration of advanced technologies such as cloud computing, artificial intelligence (AI), and data analytics. These innovations enable real-time data sharing, enhance communication among stakeholders, and streamline decision-making processes. For instance, electronic data capture systems significantly improve data gathering during research studies, reducing errors and accelerating timelines. This efficiency allows organizations within the pharma markets to adapt swiftly to market changes and regulatory requirements, ultimately enhancing health outcomes and operational performance.
Moreover, bioaccess® is at the forefront of efforts to expedite studies, achieving a 50% faster participant enrollment and realizing $25K in savings with its FDA-ready data, addressing critical challenges in participant recruitment for early-phase research. The collaboration between GlobalCare Clinical Studies and bioaccess™ exemplifies this trend, leading to a reduction in subject recruitment duration by over 50% and an impressive retention rate exceeding 95% in Colombia. Additionally, AI-driven analytics are proving essential in refining study designs and patient recruitment strategies, with research indicating that over 30% of patients withdraw due to non-clinical issues. Furthermore, 68 percent of industry leaders report that the pandemic has significantly accelerated their digital transformation efforts.
By harnessing these technologies, drug firms can not only enhance their research capabilities in pharma markets but also ensure a more patient-focused approach, fostering trust and engagement throughout the research process. As Adam Singfield aptly states, "The only sustainable advantage you can have over others is agility," highlighting the necessity for organizations to remain adaptable in this rapidly evolving landscape.
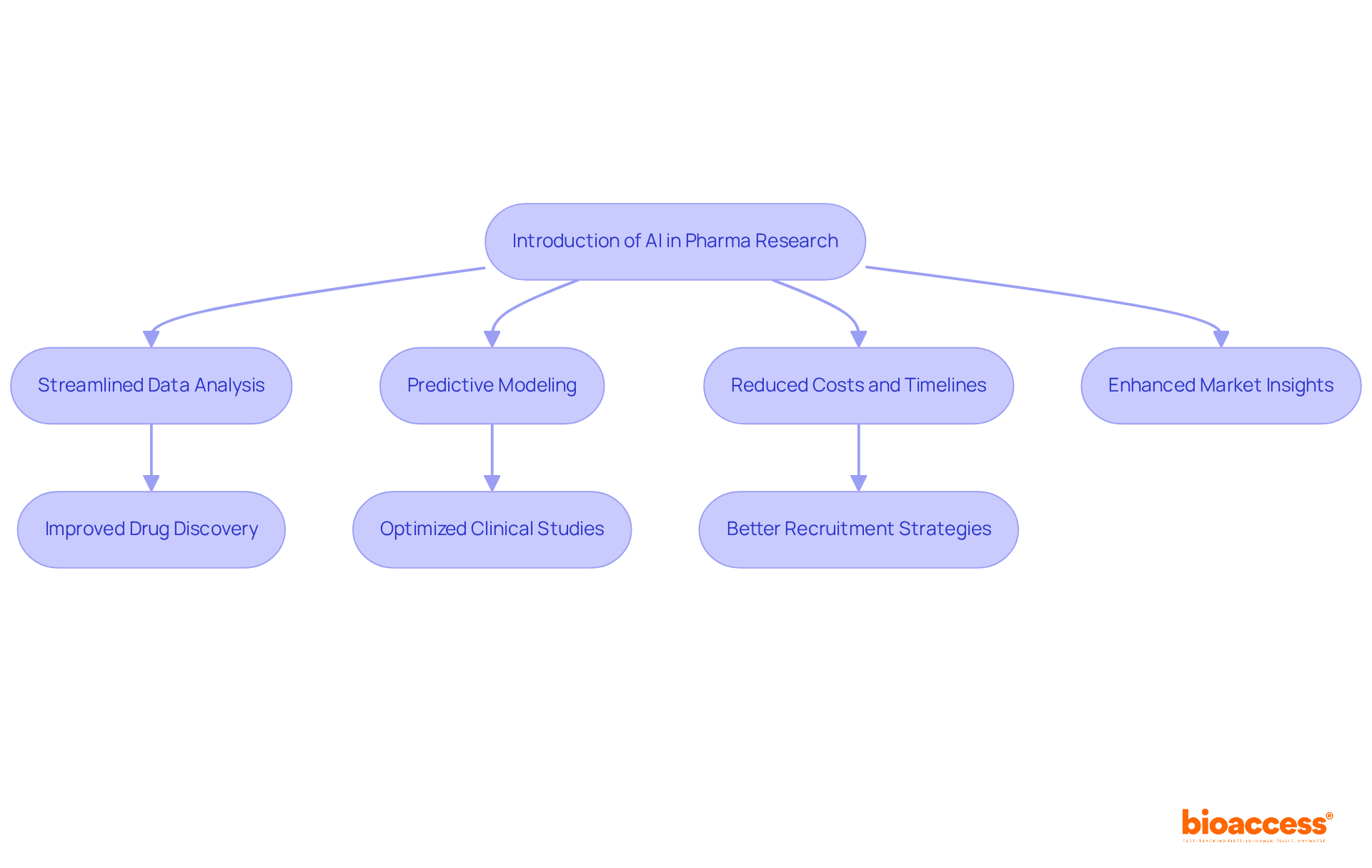
Artificial Intelligence (AI) is set to revolutionize pharma markets research by streamlining data analysis and predictive modeling. By 2025, AI is anticipated to contribute to 30% of new drug discoveries, significantly reducing the costs and timelines traditionally associated with research. AI algorithms excel at analyzing extensive datasets, which enables the identification of trends, optimization of clinical studies, and enhancement of recruitment strategies. This capability not only accelerates the drug development process but also sharpens the precision of market insights within pharma markets, empowering companies to tailor their strategies effectively.
As industry leaders underscore, the incorporation of AI in drug discovery is not merely a trend; it signifies a substantial shift that promises to enhance operational efficiencies and improve outcomes for individuals. For example, Pfizer has reported that automation has markedly reduced errors and optimized their supply chain, illustrating the tangible benefits of AI in real-world applications.
Furthermore, with AI's ability to enhance participant recruitment and refine trial design, companies can expect quicker, more reliable outcomes, ultimately fostering a more adaptable and responsive healthcare environment.
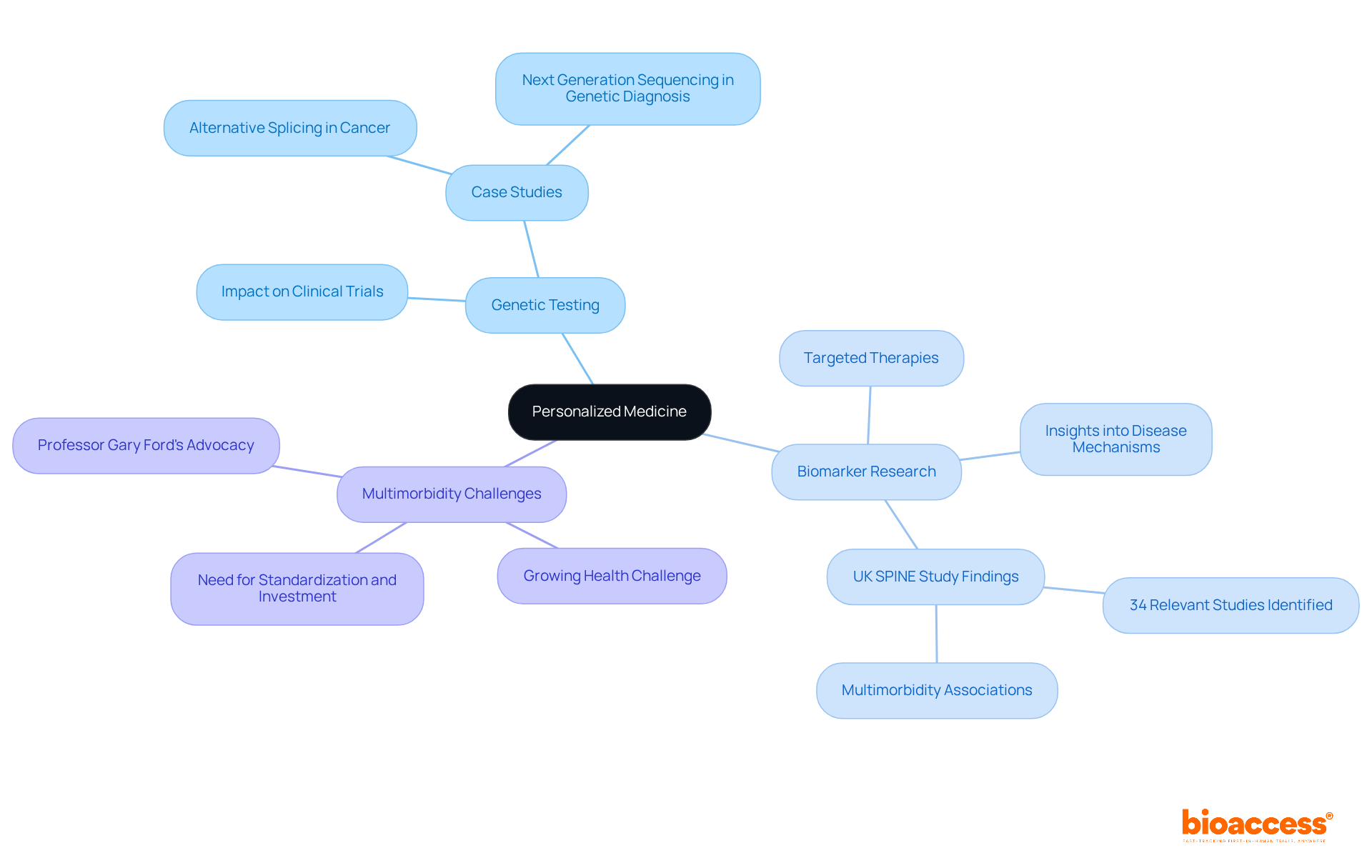
Personalized medicine is revolutionizing the pharmaceutical landscape by tailoring treatments to individual profiles shaped by genetic, environmental, and lifestyle factors. This approach not only enhances treatment effectiveness but also mitigates adverse effects, fostering greater adherence and satisfaction among individuals. As the sector transitions towards more personalized therapies, research must evolve to incorporate biomarker studies and genetic testing into study designs. This evolution ensures that new drugs are developed with a focus on the specific needs of diverse patient populations, ultimately leading to improved health outcomes.
The importance of biomarker research in medical studies is paramount. These studies offer vital insights into disease mechanisms and treatment responses, enabling researchers to pinpoint at-risk populations and refine therapeutic strategies. For example, the UK SPINE study identified 34 relevant studies on biomarkers and multimorbidity from approximately 5,000 hits, highlighting the necessity for innovative methods in comprehending complex health conditions. Furthermore, with one in seven citizens in the UK projected to be over 75 by 2040, the challenge of multimorbidity becomes increasingly pressing, underscoring the relevance of personalized medicine.
Additionally, the impact of genetic testing on medical studies is profound. By identifying specific genetic markers associated with diseases, researchers can more effectively categorize population groups, ensuring that clinical trials are more representative and impactful. This trend is illustrated by case studies examining alternative splicing in cancer, which reveal significant correlations between genetic variations and disease progression, paving the way for targeted therapies.
As the field continues to advance, the integration of biomarker studies and genetic testing will be crucial in shaping the future of drug development, ultimately leading to more effective and personalized treatment options for individuals. Professor Gary Ford advocates for a shift away from singular disease treatment, promoting therapies focused on preventing multimorbidity rather than merely addressing conditions post-emergence. Tackling the complexities of multimorbidity research and emphasizing the need for standardization and investment will be essential in propelling this field forward.
The pharmaceutical industry is currently facing rapid regulatory changes that compel organizations to remain agile and informed. These new compliance landscapes, driven by technological advancements and evolving patient needs, necessitate a proactive approach to regulatory affairs. Companies must invest in regulatory intelligence tools and cultivate robust relationships with regulatory bodies to navigate these changes effectively.
bioaccess offers a comprehensive process for enhancing medical device evaluations, specifically designed to address the regulatory challenges encountered by startups. This includes:
By leveraging these services, organizations can ensure that their clinical trials align with the latest standards, thereby minimizing the risk of delays and increasing the likelihood of successful product approvals.
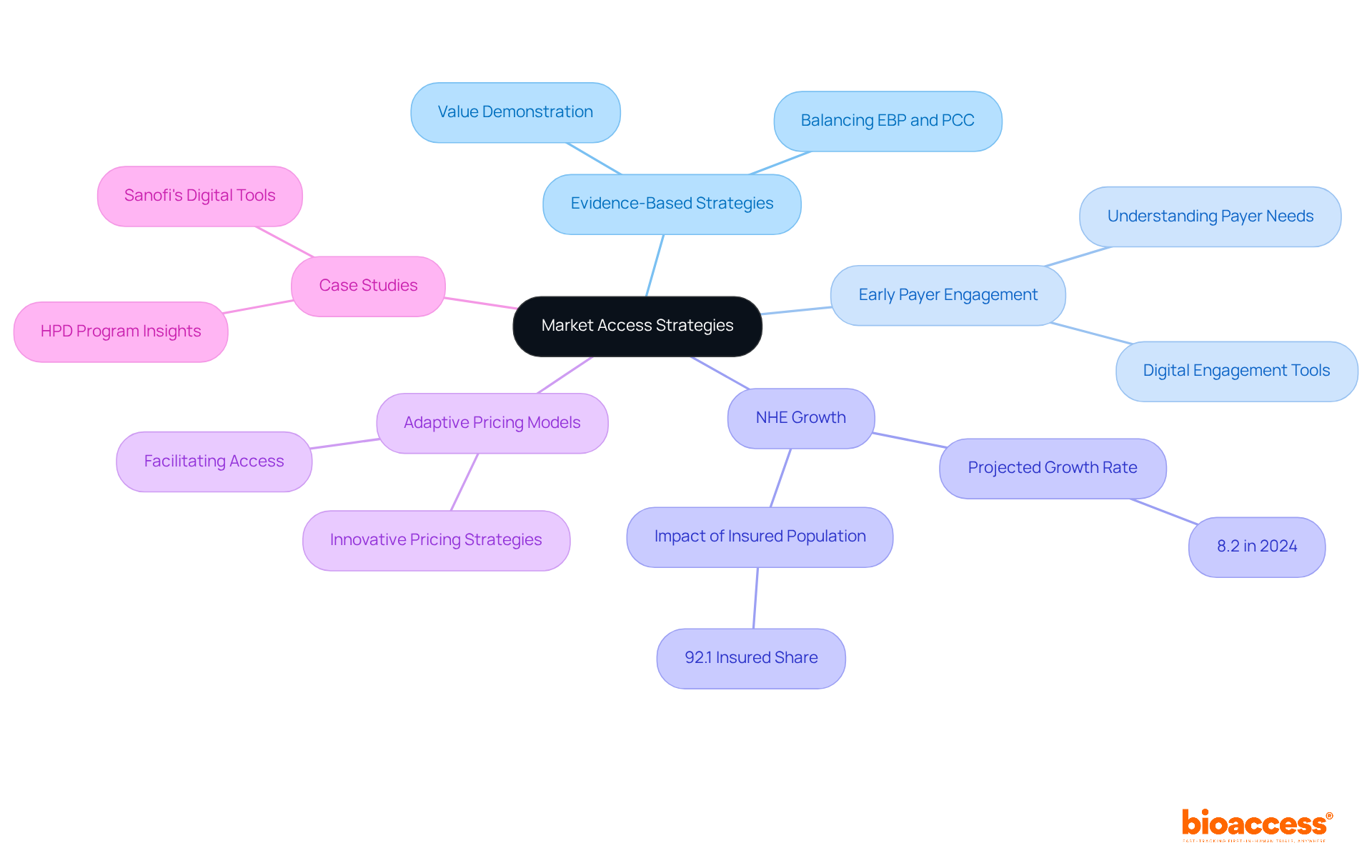
Market access plans are crucial for ensuring that innovative medications reach individuals swiftly. In today's evolving healthcare landscape, companies must adopt evidence-based value demonstration strategies to effectively communicate their products' benefits. Early engagement with payers during the drug development process is essential for grasping their requirements and expectations, which can significantly impact the success of new therapies.
Projections indicate that National Health Expenditure (NHE) growth is anticipated to reach 8.2 percent in 2024, driven by increased service utilization and a high insured population share of 92.1 percent. Moreover, adaptive pricing and reimbursement models are becoming increasingly important, as they facilitate broader access to cutting-edge treatments.
As noted by HCAI, "The final rulemaking action for the HPD Non-Claims Payment Data Collection Regulations was approved, establishing regulations for collecting data to enhance transparency and improve healthcare access." By aligning product value with payer needs, drug companies can bolster their positioning in the pharma markets and ensure that patients benefit from the latest advancements in medicine.
For instance, companies like Sanofi have effectively integrated digital tools for payer engagement, showcasing the efficacy of tailored strategies in navigating the complexities of market access.
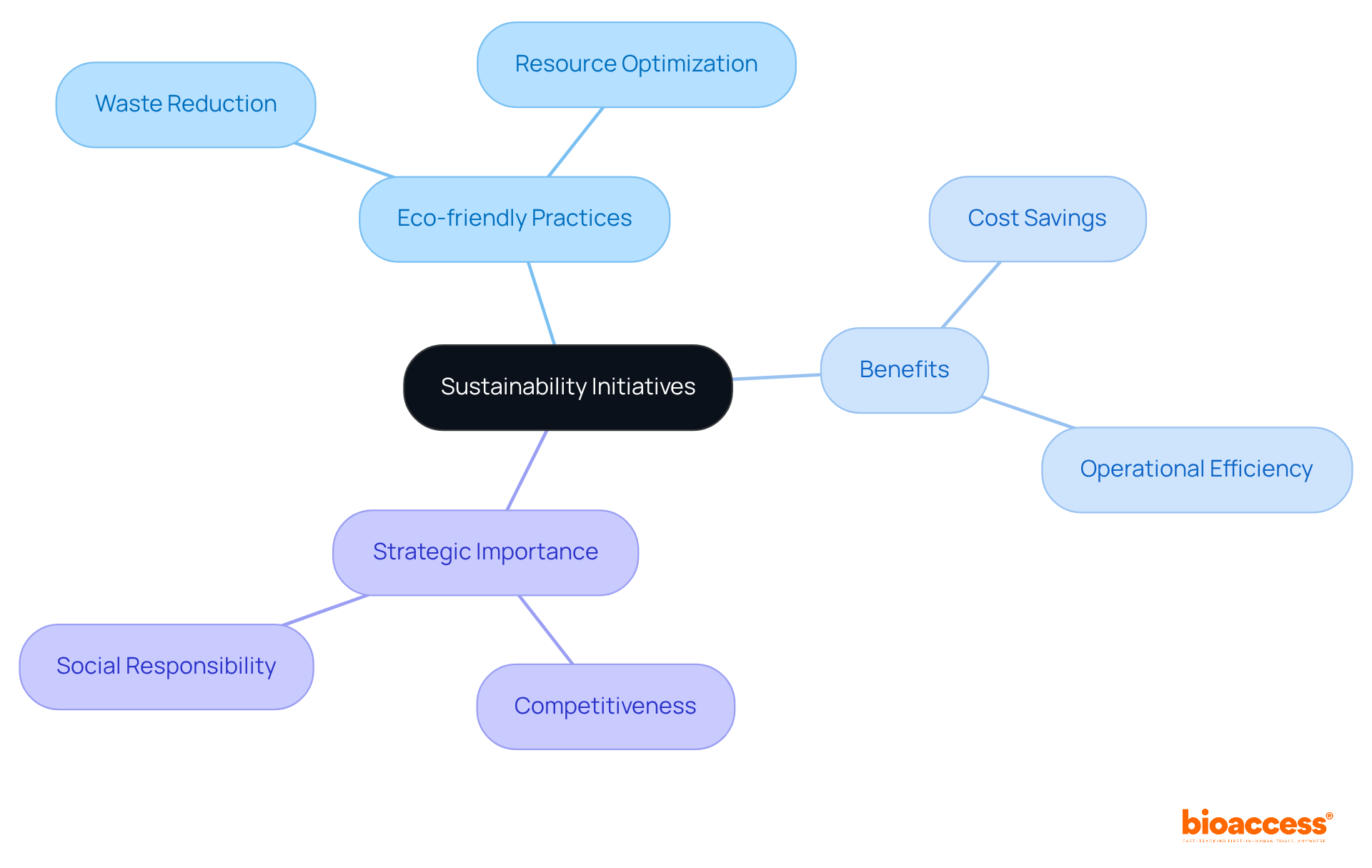
Sustainability initiatives are increasingly vital in the pharma markets, as companies respond to mounting consumer demands and regulatory pressures. By embracing eco-friendly practices—such as waste reduction and resource optimization—organizations not only enhance their reputation but also ensure compliance with evolving regulations.
Moreover, the integration of sustainability into the drug development process is not merely a trend; it can result in significant cost savings and improved operational efficiency. As the industry progresses towards a more environmentally conscious future, it is imperative for research leaders to prioritize sustainability within their strategic frameworks. This commitment is essential for maintaining competitiveness and fulfilling social responsibilities.
Data analytics is revolutionizing the pharma markets by providing actionable insights that significantly enhance decision-making processes. By utilizing advanced analytics tools, organizations can sift through extensive datasets from clinical trials, medical records, and market research to uncover trends and refine strategies. This analytical capability enables companies to make informed decisions concerning study designs, participant recruitment, and resource allocation, ultimately resulting in enhanced operational efficiency.
In this context, bioaccess® allows treatment-naive cardiology or neurology groups to be enrolled 50% faster than conventional Western sites, resulting in significant savings of $25K per individual with FDA-ready data—no rework, no delays.
To fully harness the potential of data analytics, it is crucial to eliminate data silos, which can hinder the extraction of valuable insights from healthcare data. Moreover, predictive analytics plays a crucial role in enhancing outcomes for individuals and decreasing unnecessary hospital stays, highlighting its significance in medical research.
At present, 33.04% of medical facilities are utilizing data analytics in the healthcare sector, reflecting a growing trend in the adoption of data-driven approaches. Healthcare providers leveraging electronic medical records and analytical systems have reported enhanced service delivery and patient care, demonstrating the tangible benefits of these strategies.
Consequently, the overall success rates of clinical research projects are increasing in pharma markets, highlighting the essential role of analytics in influencing the future of drug development. To maximize these benefits, organizations should focus on investing in technology, ensuring data quality, and integrating analytics into their decision-making processes to drive successful outcomes.

Collaborative innovation is increasingly vital in the pharma markets as companies leverage the expertise of technology firms. Strategic partnerships enable organizations in the pharma markets to tap into advanced technologies and innovative solutions, significantly enhancing their research capabilities.
For instance, bioaccess® has demonstrated the ability to enroll treatment-naive cardiology or neurology groups 50% quicker than Western locations, realizing $25K savings per individual with FDA-ready data—no rework, no delays. This capability is essential for Medtech and Biopharma startups within pharma markets facing participant recruitment challenges in early-stage studies.
Furthermore, studies indicate that collaborative data efforts can reduce drug development timelines by up to 30% in pharma markets. These alliances promote enhanced involvement of individuals through customized treatment methods, ultimately resulting in more effective marketing strategies.
The collaboration between bioaccess™ and Caribbean Health Group to position Barranquilla as a leading destination for clinical trials in Latin America, supported by Colombia's Minister of Health, exemplifies how strategic partnerships can enhance clinical trial services within pharma markets.
As the pharma markets continue to evolve, nurturing collaborative relationships will be essential for driving growth and sustaining a competitive advantage in these markets.

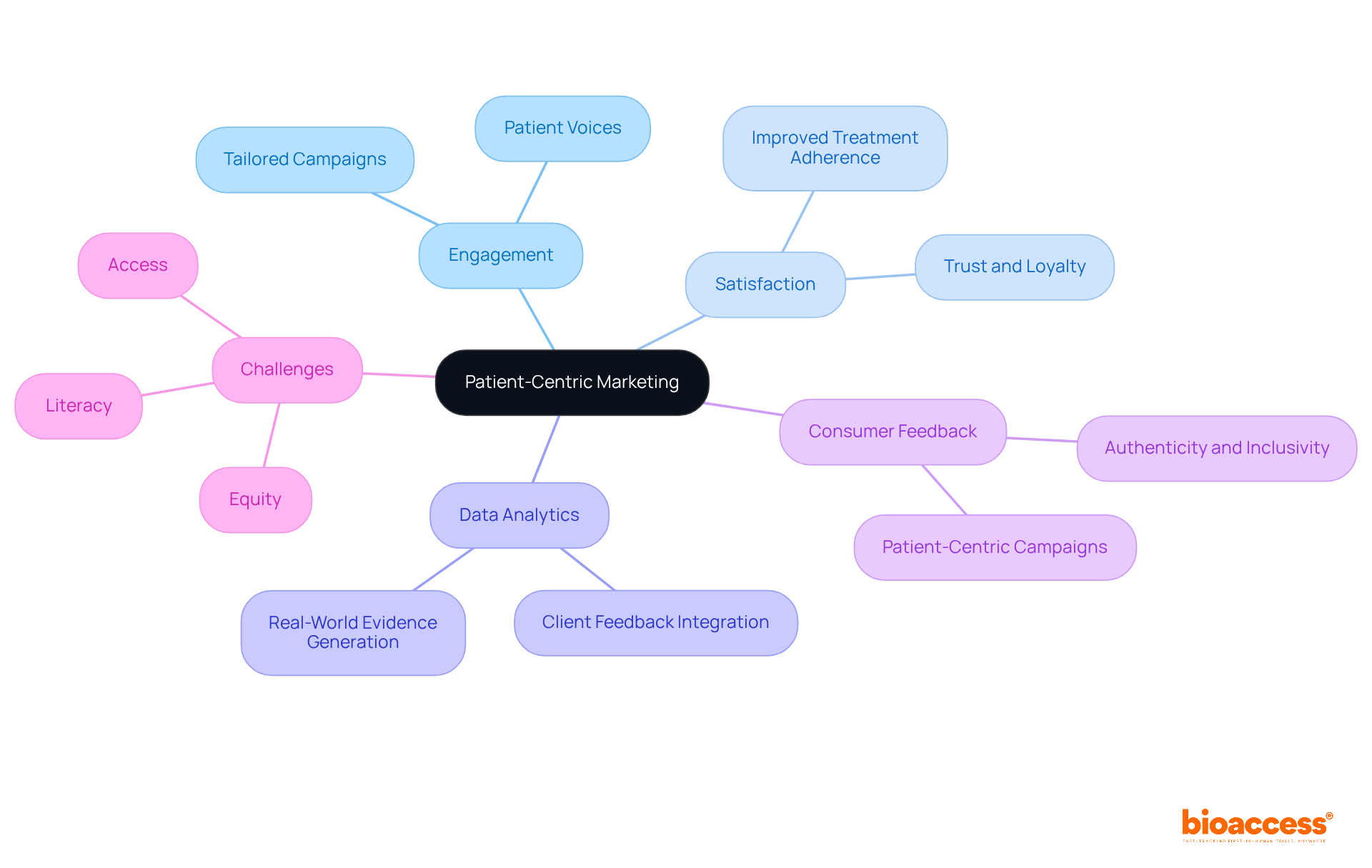
Consumer-focused marketing is revolutionizing the pharmaceutical landscape by prioritizing the needs and preferences of individuals in marketing strategies. By leveraging data analytics and actively incorporating client feedback, companies can create tailored marketing campaigns that resonate with their target audiences. This approach not only boosts engagement but also fosters trust and loyalty, ultimately resulting in improved treatment adherence and satisfaction.
As consumer expectations evolve and regulatory demands for transparency increase, adopting consumer-centric marketing strategies becomes essential for success in the competitive pharma markets. Moreover, integrated support ecosystems are redefining post-trial engagement, ensuring that patient voices are heard throughout the product lifecycle.
Addressing persistent gaps in equity, literacy, and access remains a critical challenge that the industry must confront to fully realize the benefits of patient-centric approaches.
The evolving landscape of pharmaceutical markets is significantly influenced by several key trends reshaping clinical research and operations. The acceleration of research processes through platforms like bioaccess and the transformative impact of digital technologies are crucial for enhancing efficiency and responsiveness in drug development. Furthermore, the integration of artificial intelligence, personalized medicine, and sustainability initiatives underscores the industry's commitment to improving health outcomes and addressing patient needs.
Throughout this discussion, various trends have been explored, including the vital role of regulatory changes that necessitate agile compliance strategies, alongside market access strategies that ensure new treatments reach patients effectively. The emphasis on data analytics illustrates how actionable insights can drive better decision-making, while collaborative innovation highlights the power of partnerships in fostering growth and enhancing research capabilities. Additionally, the importance of patient-centric marketing reinforces the need for pharmaceutical companies to prioritize consumer engagement and satisfaction.
As the pharmaceutical industry continues to navigate these dynamic trends, embracing innovation and adaptability will be essential for success. Stakeholders must remain vigilant in understanding and responding to these shifts, ensuring they leverage technology, foster collaboration, and maintain a focus on patient outcomes. By doing so, the pharmaceutical sector can enhance its operational efficiencies and fulfill its critical role in advancing healthcare and improving patient lives.
What is bioaccess and how does it impact clinical research in pharma markets?
Bioaccess leverages its extensive experience and regional advantages to expedite clinical research in pharma markets. It secures ethical approvals in 4-6 weeks and achieves participant enrollment 50% faster than conventional markets, helping Medtech and Biopharma innovators navigate trial complexities effectively.
What regions does bioaccess operate in?
Bioaccess operates across Latin America, the Balkans, and Australia, providing access to diverse patient populations and a deep understanding of local regulatory environments.
How does digital transformation enhance efficiency in pharma operations?
Digital transformation integrates advanced technologies like cloud computing, AI, and data analytics, enabling real-time data sharing, improving communication, and streamlining decision-making processes in pharma operations.
What are the benefits of electronic data capture systems in research studies?
Electronic data capture systems significantly improve data gathering, reduce errors, and accelerate timelines during research studies, enhancing overall operational performance.
How does bioaccess contribute to participant recruitment in early-phase research?
Bioaccess achieves a 50% faster participant enrollment and realizes $25K in savings with its FDA-ready data, addressing challenges in recruitment for early-phase research.
What role does AI play in pharmaceutical market research?
AI streamlines data analysis and predictive modeling, contributing to 30% of new drug discoveries by 2025, reducing costs and timelines traditionally associated with research.
How does AI enhance clinical studies and recruitment strategies?
AI algorithms analyze extensive datasets to identify trends, optimize clinical studies, and enhance recruitment strategies, leading to quicker and more reliable outcomes.
What impact has the pandemic had on digital transformation in the pharma industry?
The pandemic has significantly accelerated digital transformation efforts, with 68 percent of industry leaders reporting an increased focus on adopting advanced technologies to enhance research capabilities.
What are the expected outcomes of incorporating AI in drug discovery?
Incorporating AI in drug discovery is expected to enhance operational efficiencies, reduce errors, and foster a more adaptable healthcare environment, ultimately improving outcomes for individuals.