


This article addresses the pivotal role of Interactive Response Technology (IRT) in enhancing research efficiency within clinical trials, primarily through automation and real-time data access. By streamlining processes such as patient randomization and drug supply management, IRT markedly reduces enrollment times and bolsters data integrity. The result is a significant acceleration in approval timelines, ultimately leading to improved patient outcomes. In the evolving landscape of Medtech, the integration of IRT stands as a testament to innovation, addressing critical challenges faced in clinical research. Collaboration among stakeholders will be essential moving forward, ensuring that the full potential of IRT is realized in advancing healthcare solutions.
The landscape of clinical research is rapidly evolving, driven by the pressing need for efficiency and accuracy in trial management. As Interactive Response Technology (IRT) emerges as a transformative force, it offers a multitude of advantages that streamline processes from patient enrollment to data management. However, with the promise of enhanced productivity comes the challenge of navigating the complexities of implementation and cost.
How can clinical trial stakeholders leverage IRT to not only accelerate research timelines but also ensure data integrity and compliance? This article explores ten compelling ways that clinical trial IRT enhances research efficiency, providing insights into its critical role in shaping the future of clinical studies.
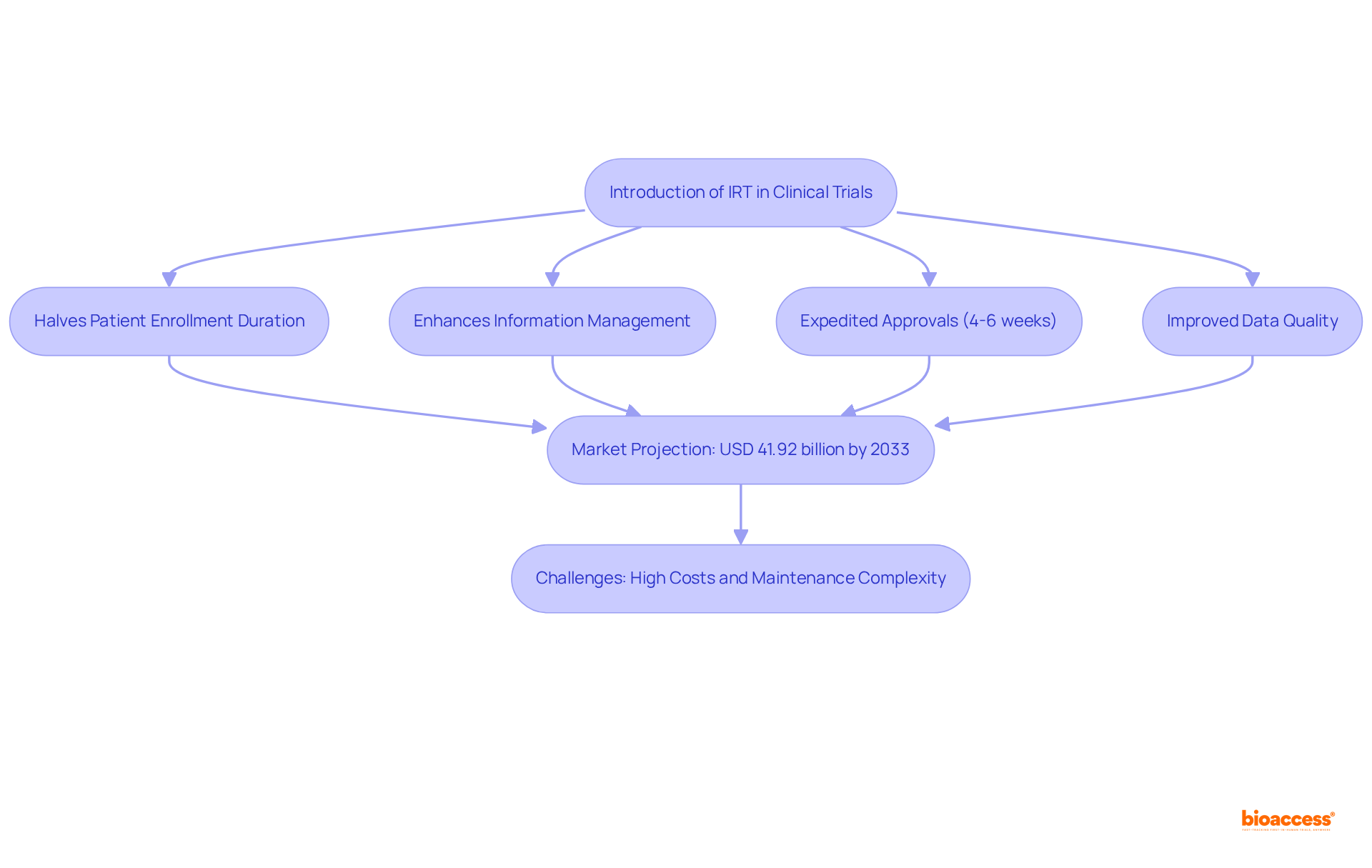
bioaccess® leverages Interactive Response Technology (IRT) as part of its clinical trial IRT to revolutionize research processes, effectively halving patient enrollment durations compared to traditional methods and enhancing information management. By automating critical functions such as randomization and drug supply management, bioaccess® ensures efficient execution, resulting in expedited approvals within 4-6 weeks and faster access to groundbreaking treatments. This technology not only boosts operational efficiency but also elevates the quality of medical data collected, guaranteeing that studies adhere to the highest standards of integrity and reliability.
With the clinical trial IRT market projected to reach approximately USD 41.92 billion by 2033, the integration of clinical trial IRT is becoming increasingly essential for Medtech innovators seeking to navigate the complexities of contemporary clinical research. Industry experts emphasize that the effective application of clinical trial IRT can markedly enhance study efficiency; however, challenges such as high costs and maintenance complexity must be addressed.
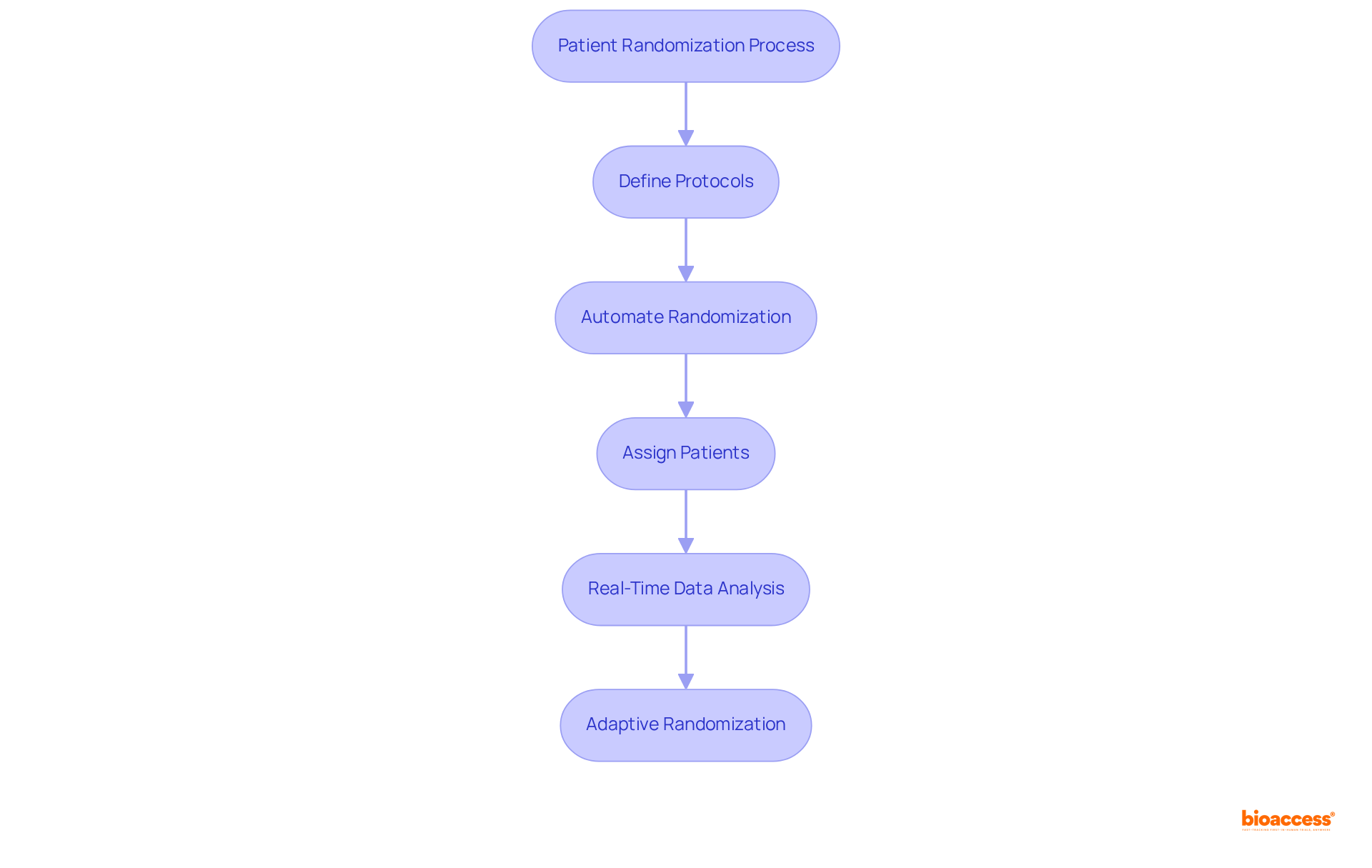
Clinical trial IRT systems are essential for automating the patient randomization process, ensuring that participants are assigned to treatment groups according to predefined protocols. This automation significantly mitigates human error and bias, resulting in more reliable outcomes. Additionally, IRT facilitates adaptive randomization, allowing for real-time adjustments in patient allocations based on ongoing data analysis. This capability not only boosts the efficiency of the testing process but also enhances patient outcomes by directing more participants to superior treatment arms.
For instance, simulations from extensive cardiovascular studies indicate that real-time adaptive randomization (RTAR) can save lives by assigning more patients to the superior treatment group, thereby reducing mortality rates. As Gui Liberali states, "Real-time assignment enhances patient results within the study and narrows the CI for the superior arm." Furthermore, the difference in health check uptake between study groups was 1.4%, underscoring the impact of IRT on study efficiency. As clinical studies grow in complexity, the integration of clinical trial IRT and adaptive randomization becomes increasingly crucial for achieving successful and timely outcomes.
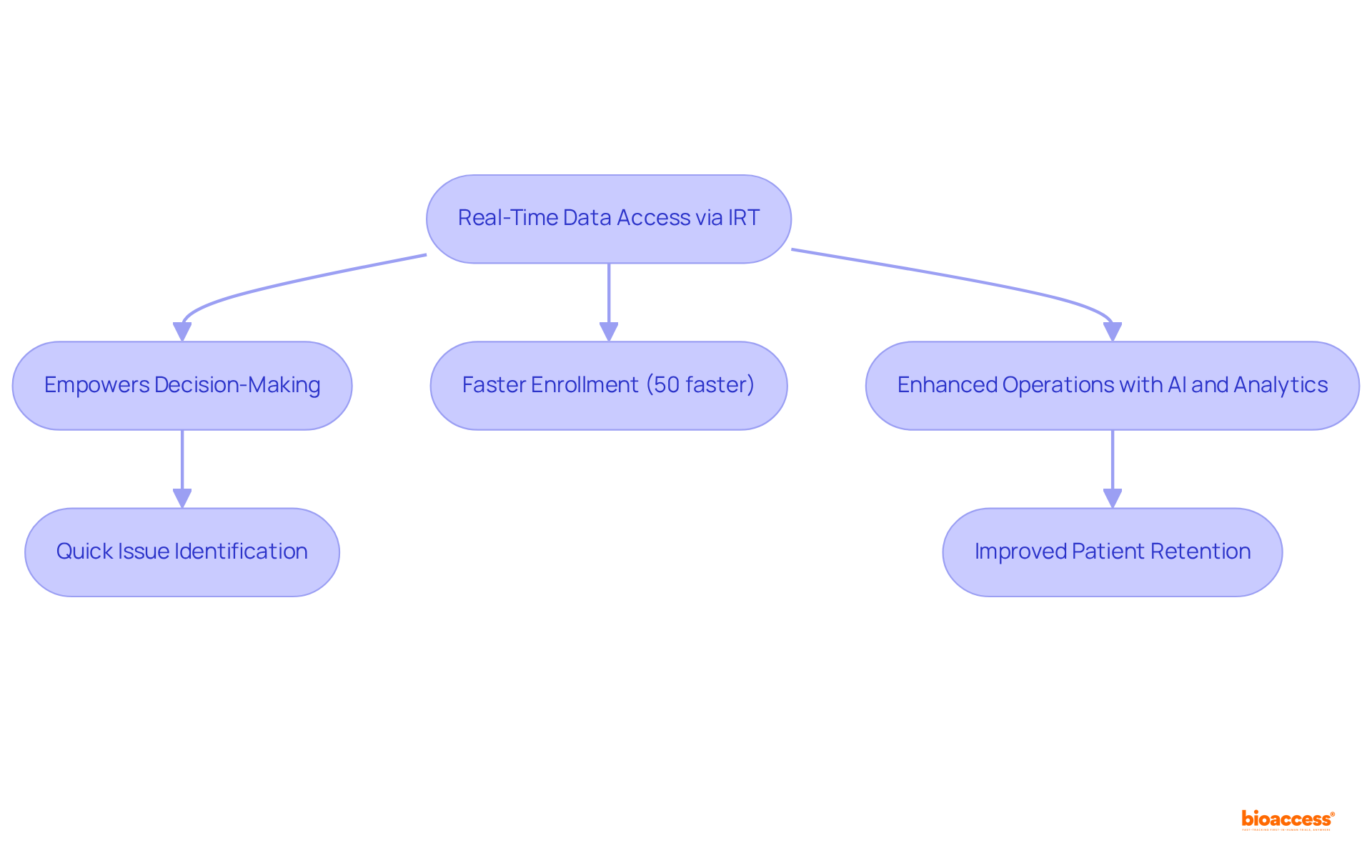
Clinical trial IRT provides immediate access to essential study information, which empowers sponsors and researchers to track progress and make informed decisions swiftly. This capability allows for the prompt identification of issues, such as patient dropouts or information discrepancies, facilitating timely interventions that keep the study on track and in compliance.
As medical trials become increasingly complex and data-intensive, the ability to access and analyze real-time information through clinical trial IRT is evolving into a critical expectation for sponsors. Notably, enrollment is 50% faster than in traditional markets, underscoring the effectiveness achieved through real-time data access.
Furthermore, the integration of AI and analytics is emerging as a strategic approach to streamline operations, particularly in clinical trial IRT, enhancing the overall responsiveness of clinical research. By addressing the challenges posed by dispersed information from multiple sources, IRT enables sponsors to leverage automated visualizations and analytics, ultimately improving patient retention and study outcomes.
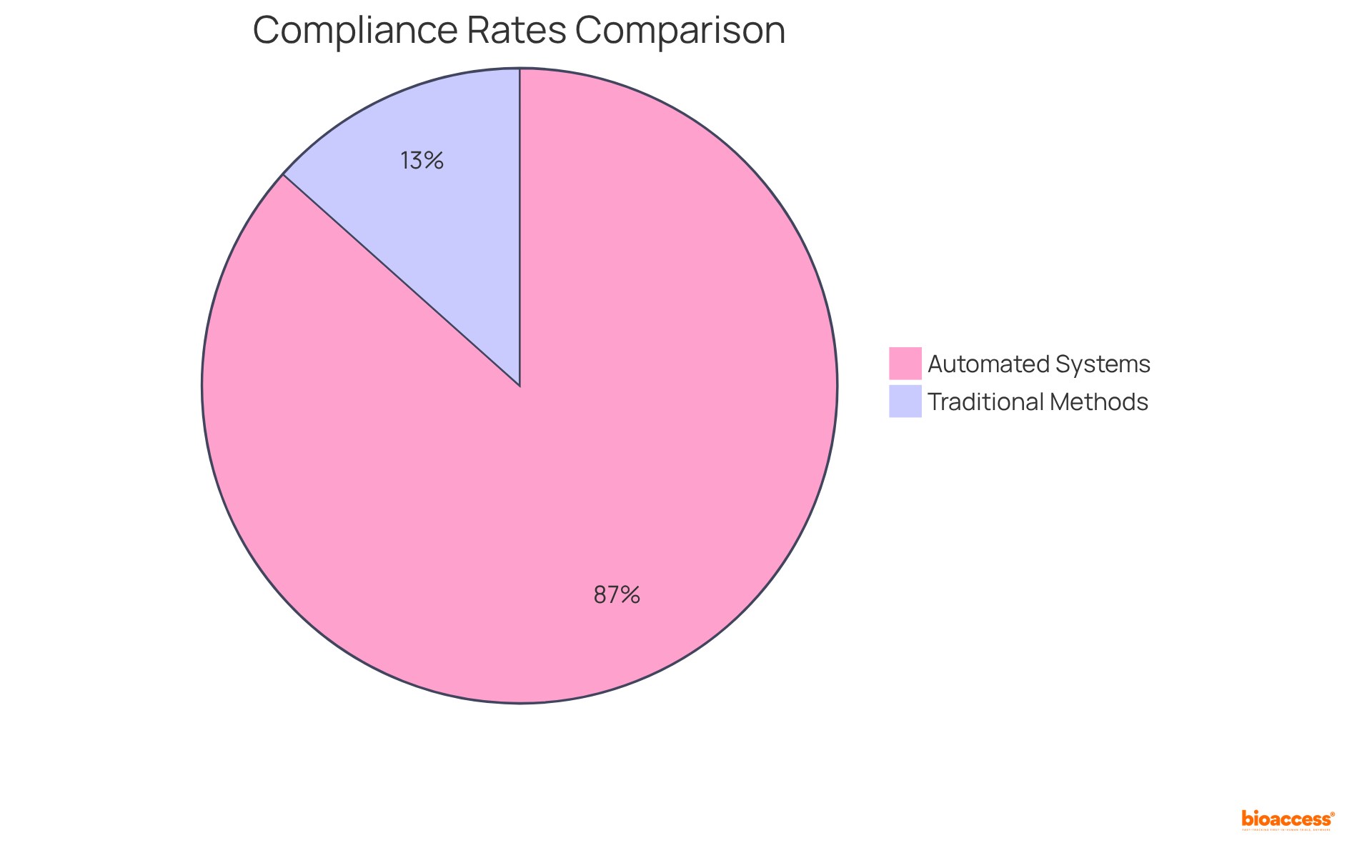
IRT systems significantly enhance compliance by automating documentation and reporting processes, which are essential for adhering to regulatory standards. These systems maintain comprehensive audit trails and generate necessary reports, enabling sponsors to effectively demonstrate compliance to regulatory bodies. This capability not only reduces the likelihood of penalties but also strengthens the integrity of the examination.
For instance, automated medical study reporting can shorten report creation time from three to four weeks to just days or even hours, thereby enhancing workflows and increasing data precision. Furthermore, compliance rates for subjects using electronic patient-reported outcomes (PRO) can soar to as high as 97%, in stark contrast to only 15% for traditional paper methods. This dramatic improvement in compliance translates to fewer replacement subjects, ultimately reducing study completion time and overall costs.
The integration of automated alerts and notifications further alleviates the workload on site personnel, enhancing participant engagement. By ensuring that all study activities are documented accurately and efficiently, automated systems foster a culture of regulatory adherence that is increasingly vital in today's clinical trial IRT landscape.
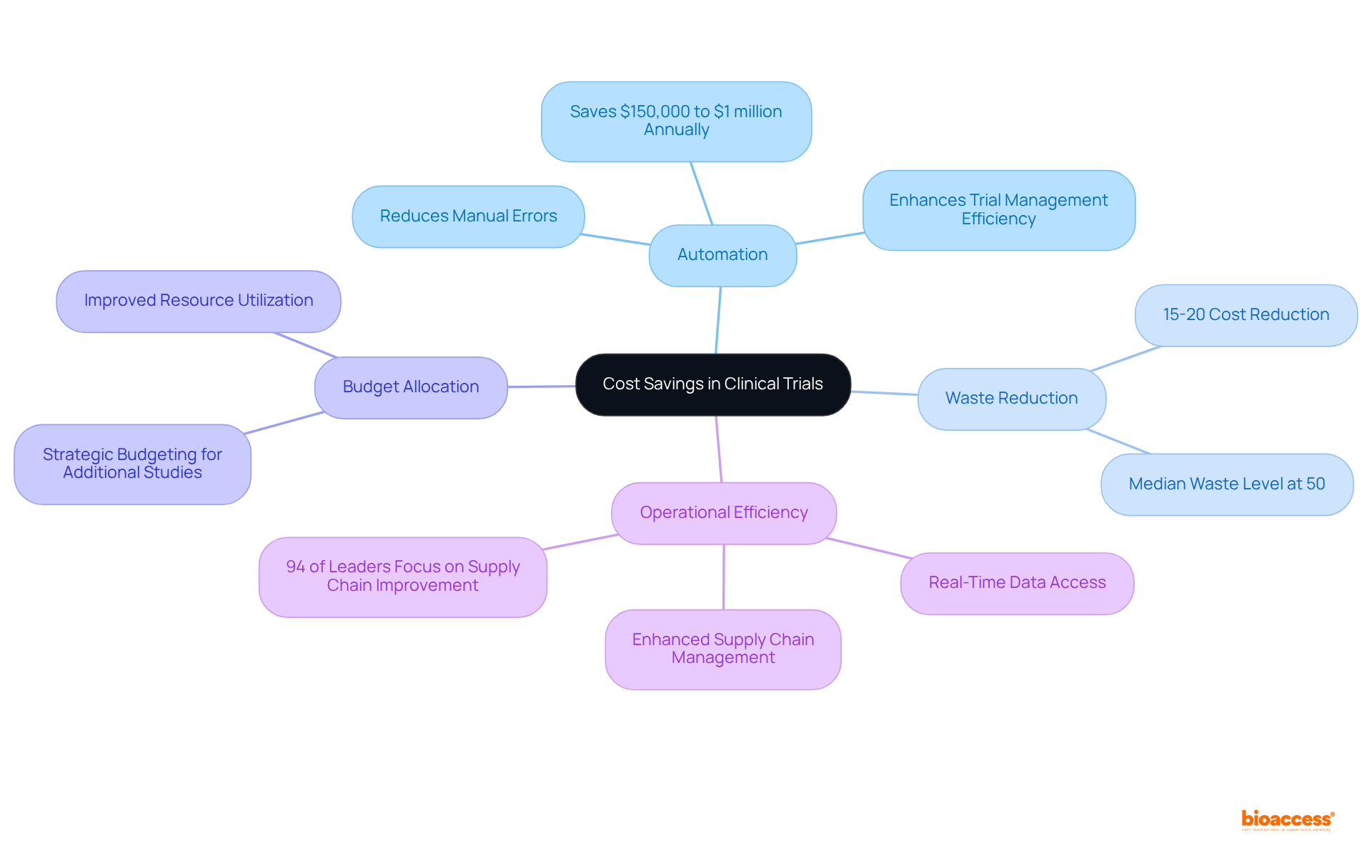
Automating various testing processes through clinical trial IRT significantly decreases dependence on manual labor, thereby reducing errors and resulting in considerable cost savings. Clinical trial IRT solutions can reduce waste and associated costs by an estimated 15-20%, while ensuring efficient patient management and drug supply tracking to prevent wastage and optimize resource utilization. This financial efficiency enables sponsors to allocate their budgets more strategically, potentially facilitating the implementation of additional studies or further investment in research initiatives.
For instance, organizations utilizing IRT have reported savings ranging from $150,000 to $1 million annually in drug and shipping costs, demonstrating the tangible financial impact of these systems. As the intricacy of medical studies continues to grow, especially in 2025 with the heightened emphasis on personalized medicine and uncommon illnesses, the function of clinical trial IRT in enhancing budget efficiency becomes even more essential.
Specialists in the area stress that automation not only lowers expenses but also improves overall management efficiency, allowing sponsors to maneuver through the financial landscape of research with increased agility and foresight. Moreover, with 94% of life sciences leaders recognizing supply chain enhancement as a primary focus, the incorporation of clinical trial IRT alongside other research systems, such as electronic information capture and management systems for studies, further highlights its significance in preserving information integrity and efficiency in patient care.
As Suvoda aptly states, 'Missed patient visits = missed data points,' highlighting the critical role of clinical trial IRT in effectively managing every aspect of the study.
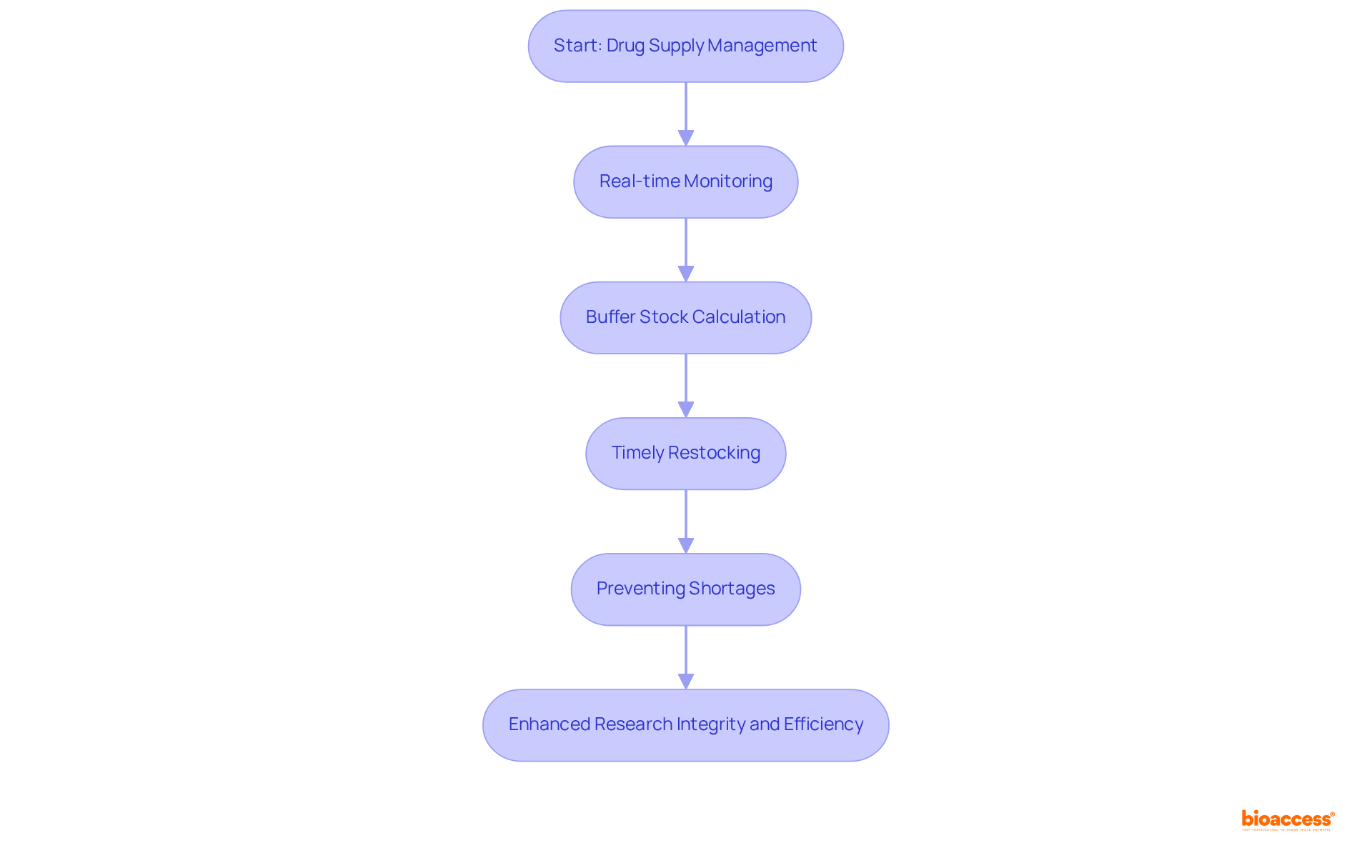
IRT systems are essential for the efficient oversight of drug supplies throughout the research lifecycle. They facilitate real-time monitoring of inventory levels, ensuring that investigational products are available when needed. By accurately calculating buffer stocks and initiating timely restocking, IRT systems are pivotal in preventing shortages, thus preserving the integrity of research studies. This proactive approach not only safeguards project timelines but also enhances overall research efficiency, enabling smoother operations and superior outcomes.
With the medical study supplies market projected to reach approximately USD 5.56 billion by 2034, the significance of IRT systems becomes increasingly apparent. Alan Lahaise emphasizes, "Ensuring drugs reach patients at the right time and place is crucial," underscoring the vital role of IRT in fulfilling this objective. Furthermore, numerous instances demonstrate how clinical trial IRT systems have effectively mitigated drug shortages, ensuring that studies proceed without interruption.
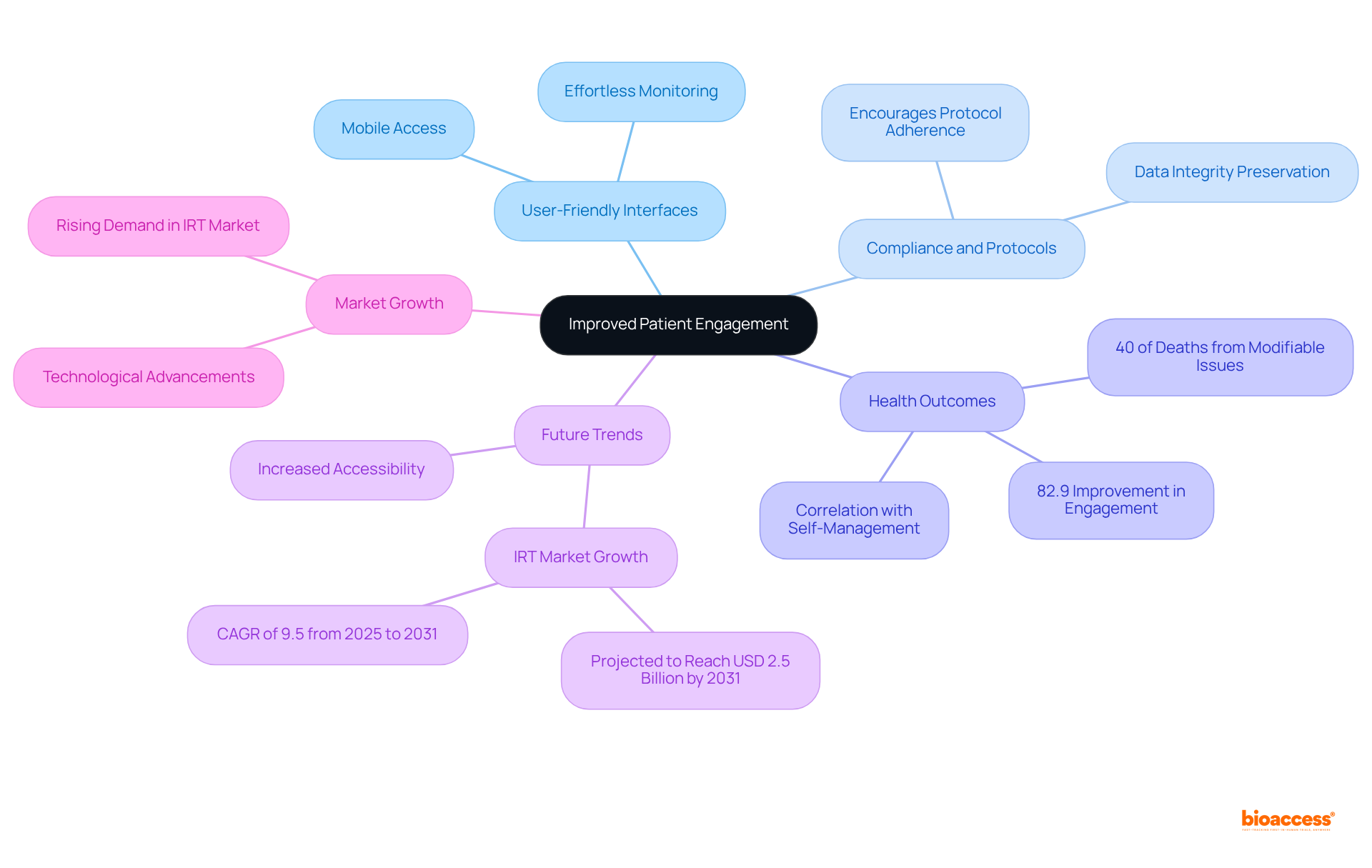
Clinical trial IRT significantly enhances patient engagement by providing a seamless experience for participants. With user-friendly interfaces and mobile access, patients can effortlessly monitor their involvement, schedule visits, and communicate with study personnel. This streamlined involvement not only fosters a positive experience but also encourages compliance with protocols, essential for preserving data integrity and attaining favorable results.
In fact, studies indicate that higher patient participation in self-management correlates with improved health outcomes. Notably, 40% of deaths in the United States are caused by modifiable behavioral issues, underscoring the importance of effective engagement strategies.
As we progress into 2025, the incorporation of clinical trial IRT is anticipated to further transform the research landscape, making it more accessible and efficient for patients. By utilizing technology, research studies can promote a more inclusive environment, ultimately resulting in more thorough and applicable findings that better serve diverse patient groups.
Trials designed with authentic patient input achieve higher recruitment rates, better retention, and more meaningful results. Furthermore, 82.9% of studies reported high levels of improvement in patient engagement.
The Interactive Response Technology (IRT) market is expected to reach USD 2.5 billion by 2031, emphasizing the growing significance of clinical trial IRT in clinical studies.
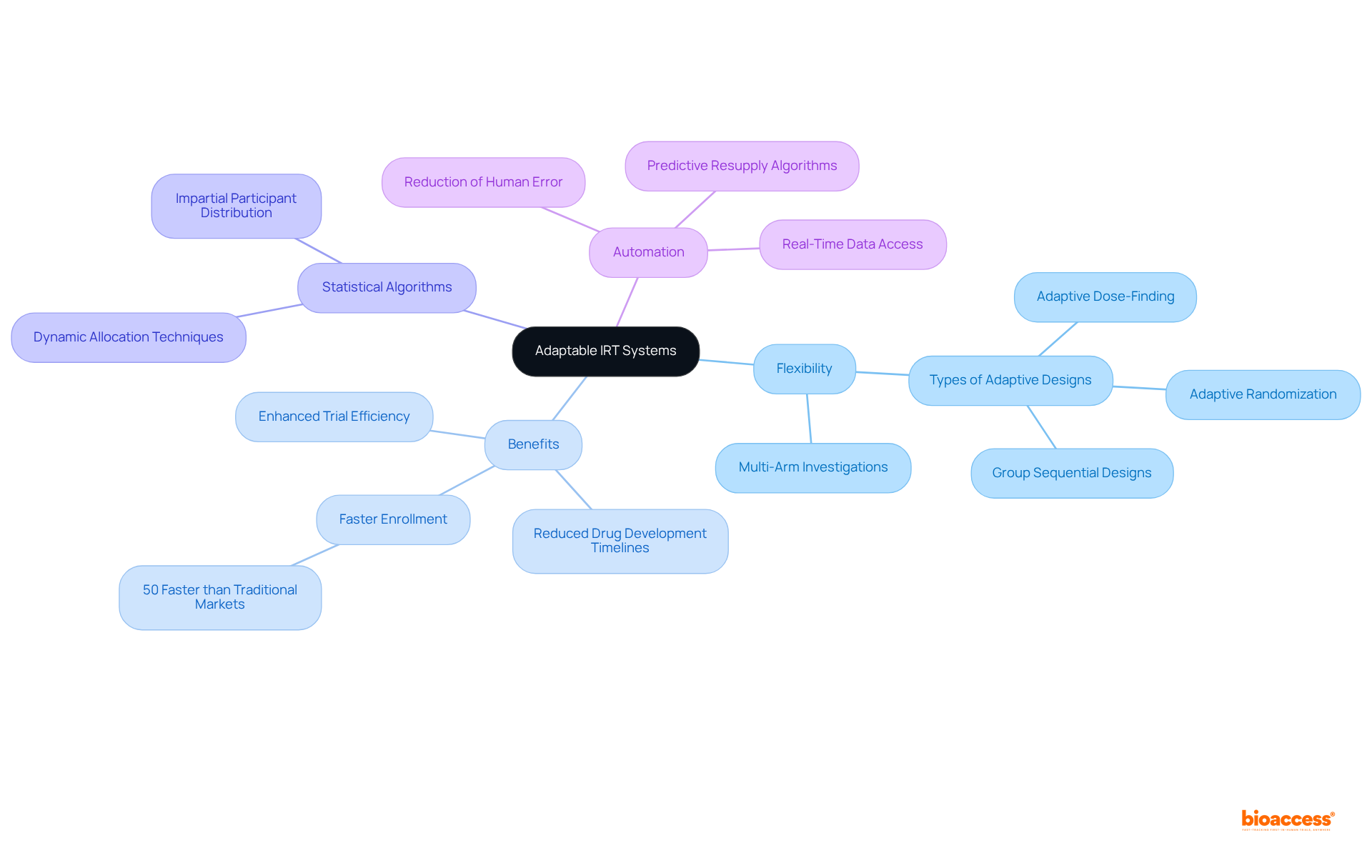
Contemporary Interactive Response Technology (IRT) systems are pivotal in the realm of clinical trial IRT, being designed for flexibility to efficiently accommodate a variety of experimental designs, including intricate adaptive studies and multi-arm investigations. This inherent flexibility empowers sponsors to adopt [innovative methodologies](https://medidata.com/en/life-science-resources/medidata-blog/revolutionizing-clinical-studies-with-adaptive-trial-designs-flexibility-mid-study-changes-and- expert-teams-for-optimal-results), significantly enhancing the efficiency and effectiveness of their studies.
By addressing the specific requirements of diverse patient populations and treatment protocols, IRT systems facilitate the seamless integration of adaptive designs, which are increasingly acknowledged for their capacity to optimize outcomes while minimizing risks. For instance, adaptive study designs can shorten drug development schedules by enabling multiple investigations to occur concurrently, with at least 20% of research studies employing these designs.
Furthermore, the application of statistically based algorithms within clinical trial IRT systems ensures impartial participant distribution, thereby enhancing the reliability of study outcomes. As medical experiments become increasingly complex, with an average of 3.6 million information points gathered in Phase III studies, the role of clinical trial IRT in managing these intricacies is paramount, ensuring that studies remain effective and adaptable to real-time insights.
Additionally, automated processes within IRT systems mitigate the risk of human error in randomization, further bolstering the reliability of study outcomes.
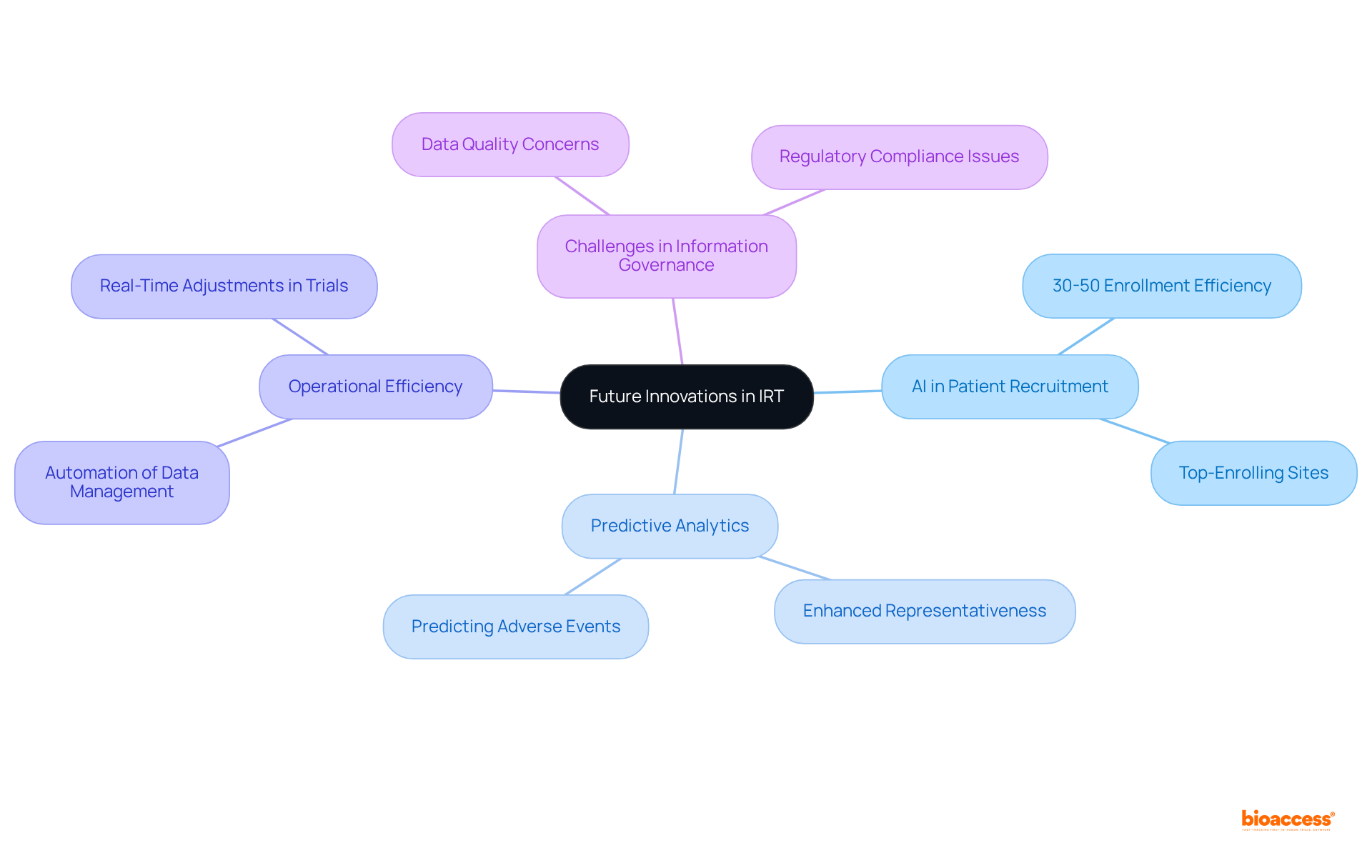
The future of Interactive Response Technology (IRT) stands at the threshold of a transformative shift, propelled by the integration of artificial intelligence (AI) and machine learning (ML). These technologies are set to revolutionize research management by enhancing analytical capabilities, optimizing patient recruitment strategies, and streamlining operational processes. For example, AI models can accurately predict patient recruitment rates and pinpoint top-enrolling sites, thereby improving enrollment efficiency by 30 to 50 percent across diverse therapeutic areas, as evidenced by recent case studies.
With the healthcare information management market projected to grow from $3.11 billion in 2024 to $8.13 billion by 2033, the demand for innovative solutions is unmistakable. AI not only elevates the quality of information but also reduces timelines and costs, facilitating informed decision-making in research studies. By 2025, the application of AI and ML in study management is anticipated to involve advanced predictive analytics, which can enhance representativeness in study populations by balancing groups with specific patient characteristics. Notably, 63% of healthcare information managers consider ensuring information quality their primary concern, underscoring the critical role of AI in addressing this issue.
Furthermore, AI-driven adaptive trial designs enable real-time adjustments based on emerging safety and efficacy signals, significantly enhancing trial responsiveness. This capability is crucial, as 81% of healthcare information managers recognize information governance as a primary challenge in achieving regulatory compliance. By leveraging AI, organizations can effectively address these challenges, ensuring seamless information integration and adherence to regulatory standards. As Anton Lukianchenko aptly noted, "AI has arrived to assist and become a solution for effective management of medical information."
In conclusion, the integration of AI and ML into clinical trial IRT represents not merely a passing trend; it signifies a fundamental evolution in how studies are conducted, promising greater efficiency and improved outcomes for patients and stakeholders alike. To begin harnessing AI technologies in medical studies, research professionals should consider launching pilot projects focused on specific areas such as information management or patient recruitment, gradually expanding as they gain experience and confidence.
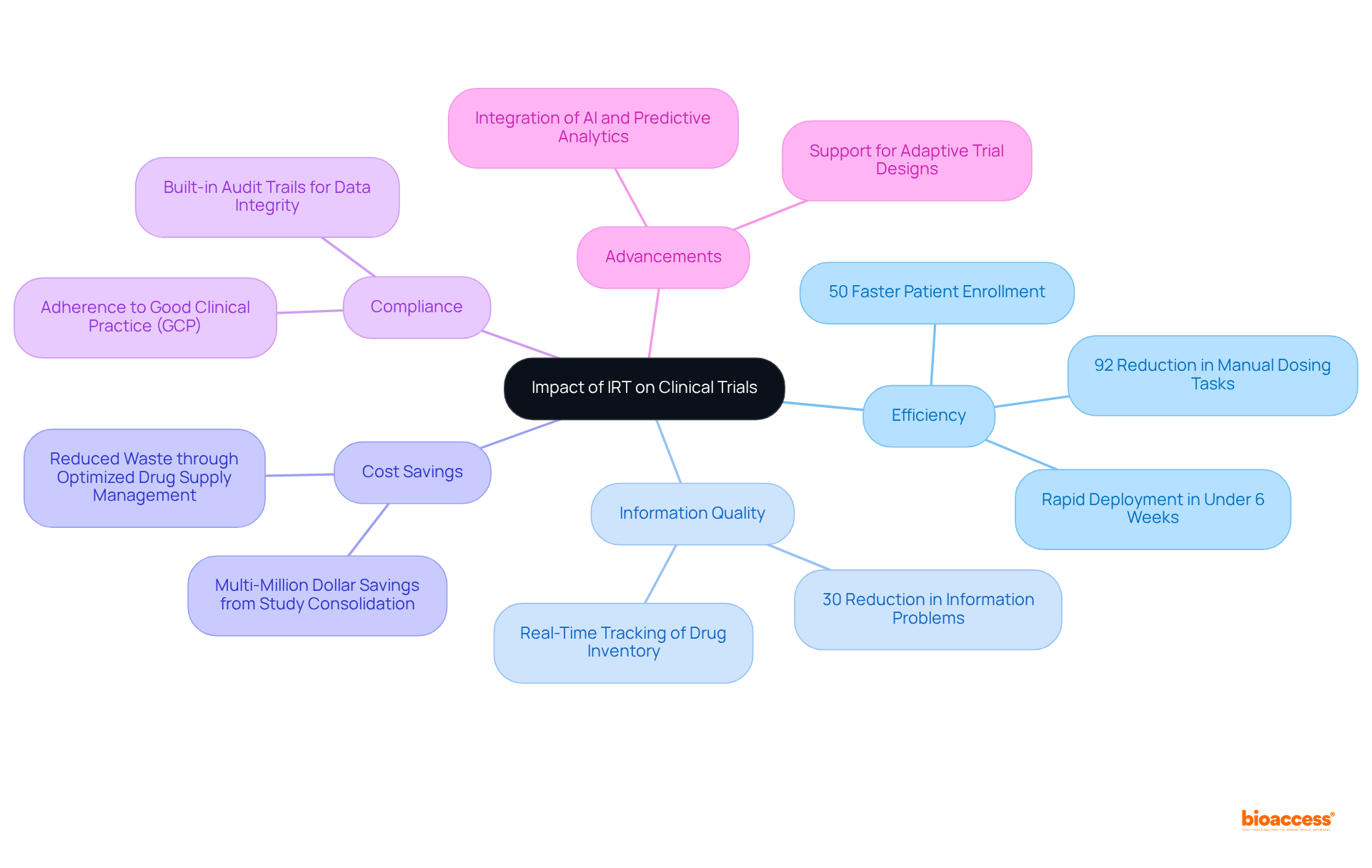
The incorporation of Interactive Response Technology (IRT) in clinical trial IRT has significantly transformed the clinical research environment, enhancing efficiency, improving information quality, and ensuring compliance with regulatory standards. By automating essential processes such as patient randomization, drug supply management, and information gathering, IRT systems empower sponsors to conduct studies with greater efficiency and assurance. Notably, studies employing IRT have demonstrated a 30% reduction in information problems compared to those lacking this technology, underscoring its critical role in enhancing information integrity.
Moreover, IRT's capacity for real-time tracking of drug inventory and patient enrollment has resulted in substantial cost savings, with some sponsors estimating multi-million dollar savings from study consolidation using RTSM. As the clinical research landscape continues to evolve, the clinical trial IRT system stands out as an indispensable tool, fostering a more efficient, cost-effective, and patient-centered approach to clinical studies.
The ongoing advancements in IRT technology, including the integration of AI and predictive analytics, promise to further improve research outcomes in 2025 and beyond. Additionally, IRT enhances compliance with regulatory standards such as Good Clinical Practice (GCP) and 21 CFR Part 11, ensuring that trial data remains secure and accurate.
bioaccess® plays a pivotal role in this landscape by facilitating faster ethical approvals within 4-6 weeks and accelerating patient enrollment by 50% compared to traditional markets, thereby supporting the rapid advancement of medical devices from first-in-human studies to commercialization.
The integration of Interactive Response Technology (IRT) in clinical trials marks a pivotal advancement in enhancing the efficiency and effectiveness of research processes. By automating critical functions such as patient randomization, drug supply management, and real-time data access, IRT not only accelerates patient enrollment but also improves the quality of medical data collected. This technological evolution is essential for Medtech innovators navigating the complexities of modern clinical research, ultimately facilitating expedited approvals and faster access to innovative treatments.
Throughout this article, we have highlighted the key benefits of clinical trial IRT, including:
The adaptability of IRT systems to diverse trial designs further underscores their versatility and significance in achieving successful research outcomes. As the clinical trial landscape evolves, the role of IRT in fostering operational efficiency and regulatory adherence remains paramount.
Looking ahead, the future of IRT is poised for further innovation, particularly with the integration of artificial intelligence and machine learning, which promise to revolutionize research management and optimize patient recruitment strategies. Embracing these advancements will not only enhance the efficiency of clinical trials but also improve patient outcomes and stakeholder satisfaction. As the demand for more effective and patient-centered research methodologies grows, leveraging IRT will be crucial for organizations striving to maintain a leading position in clinical research excellence.
What is bioaccess and how does it utilize Interactive Response Technology (IRT)?
bioaccess leverages Interactive Response Technology (IRT) to revolutionize clinical trial research processes, halving patient enrollment durations compared to traditional methods and enhancing information management through automation of critical functions such as randomization and drug supply management.
What are the benefits of using IRT in clinical trials?
The use of IRT in clinical trials results in efficient execution, expedited approvals within 4-6 weeks, and faster access to groundbreaking treatments. It also boosts operational efficiency, elevates the quality of medical data collected, and ensures studies adhere to high standards of integrity and reliability.
What is the projected market growth for clinical trial IRT?
The clinical trial IRT market is projected to reach approximately USD 41.92 billion by 2033, indicating its increasing importance for Medtech innovators navigating contemporary clinical research complexities.
How does IRT enhance patient randomization in clinical trials?
IRT automates the patient randomization process, ensuring participants are assigned to treatment groups according to predefined protocols, which reduces human error and bias. It also facilitates adaptive randomization, allowing for real-time adjustments based on ongoing data analysis, thereby improving testing efficiency and patient outcomes.
Can you provide an example of how IRT improves patient outcomes?
Real-time adaptive randomization (RTAR) in cardiovascular studies has shown to save lives by assigning more patients to superior treatment groups, thus reducing mortality rates. This demonstrates how IRT enhances patient results and narrows confidence intervals for superior treatment arms.
What role does real-time data access play in clinical trials?
Real-time data access through clinical trial IRT allows sponsors and researchers to track study progress and make informed decisions quickly. It enables the prompt identification of issues, such as patient dropouts or discrepancies, facilitating timely interventions to keep the study on track.
How does the integration of AI and analytics benefit clinical trial IRT?
The integration of AI and analytics streamlines operations within clinical trial IRT, enhancing overall responsiveness. It helps address challenges posed by dispersed information and allows sponsors to leverage automated visualizations and analytics, ultimately improving patient retention and study outcomes.
How much faster is patient enrollment with IRT compared to traditional methods?
Enrollment through clinical trial IRT is 50% faster than in traditional markets, highlighting its effectiveness in accelerating the clinical trial process.