


Decentralized trials drive cost savings in clinical research by significantly reducing operational expenses and accelerating patient recruitment through innovative technologies and flexible participation options. These trials can lower costs by 10-25%, attributed to fewer physical locations and enhanced recruitment strategies. This ultimately leads to faster enrollment and improved data integrity—critical elements for efficient clinical research. As such, the adoption of decentralized trials is not merely a trend but a strategic imperative for the future of clinical research.
Decentralized trials are reshaping the landscape of clinical research, offering a revolutionary approach that not only enhances operational efficiency but also significantly reduces costs. By leveraging innovative technologies and flexible methodologies, these trials present an opportunity for substantial savings while improving patient recruitment and engagement. However, as the industry embraces this transformation, a pressing question arises: how can stakeholders fully capitalize on the benefits of decentralized trials while ensuring compliance and maintaining data integrity?
bioaccess® excels in delivering innovative solutions that accelerate decentralized studies, leveraging its profound understanding of regulatory processes and patient recruitment strategies. By harnessing local insights and resources, bioaccess® ensures cost-effectiveness and enhances operational efficiency, leading to quicker approvals and streamlined study management through its comprehensive clinical study management services, which include:
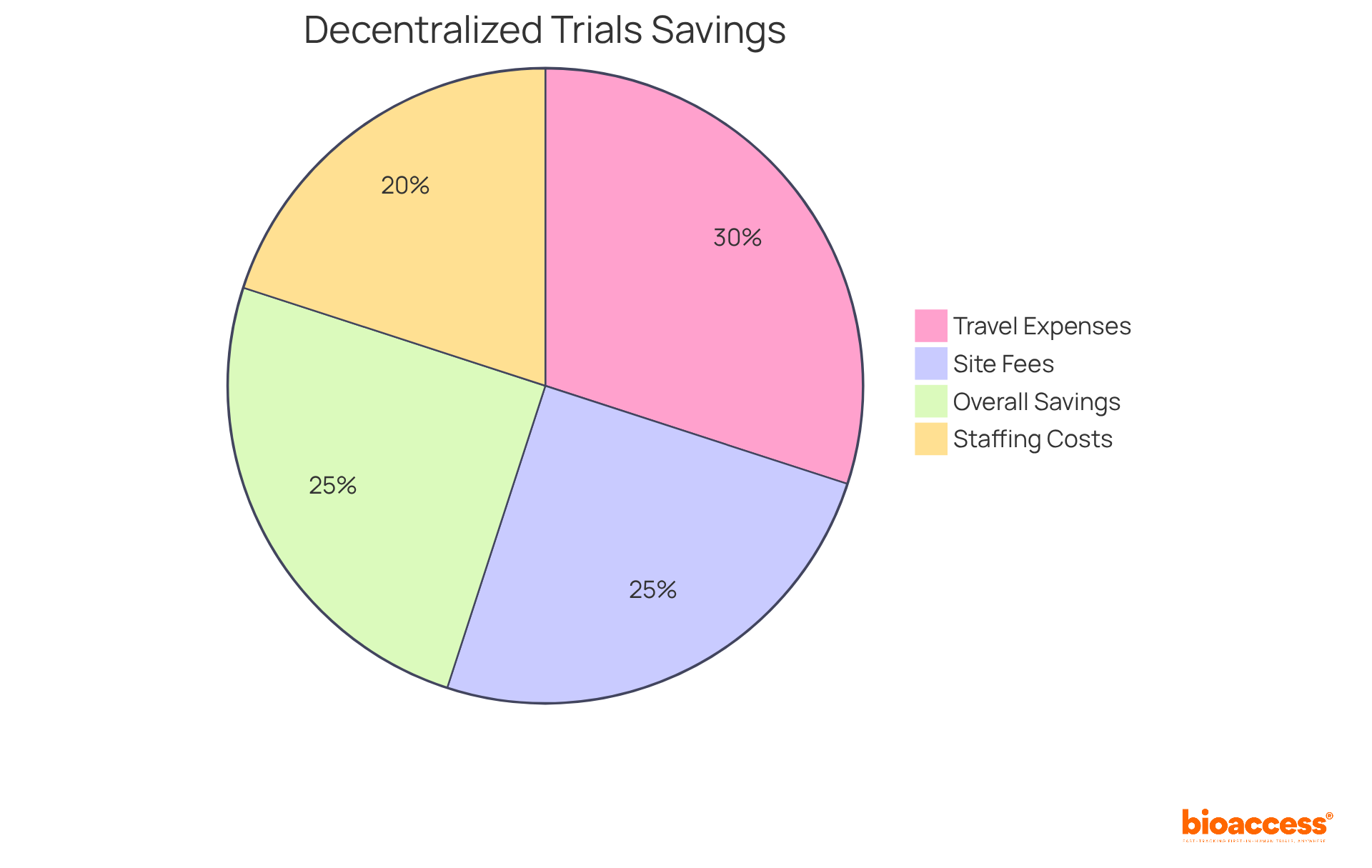
This strategic approach empowers Medtech, Biopharma, and Radiopharma innovators to achieve decentralized trials cost savings while adhering to rigorous quality standards. Notably, the decentralized trials cost savings can decrease operational expenses by 10-25%, primarily through fewer locations and reduced travel costs for participants.
Furthermore, the integration of remote technologies has demonstrated an ability to improve patient recruitment and retention rates, achieving 50% faster patient enrollment and contributing to decentralized trials cost savings of $25K with FDA-ready data—no rework, no delays.
As the decentralized medical research market is projected to grow from $8.5 billion in 2023 to $13.3 billion by 2030, bioaccess® positions itself as a pivotal contributor in this evolving landscape, facilitating decentralized trials cost savings through the effective implementation of economical solutions for its clients.
Decentralized studies are revolutionizing the landscape of clinical research by offering decentralized trials cost savings through significantly reducing operational expenses. By minimizing reliance on physical locations, sponsors can eliminate considerable overheads associated with traditional study frameworks. This paradigm shift facilitates remote monitoring and virtual visits, leading to decentralized trials cost savings through reduced travel expenses, site fees, and staffing costs. Studies suggest that the decentralized trials cost savings can lower overall expenses by 10-25%, driven by fewer locations and reduced researcher fees.
With bioaccess®, sponsors can enroll treatment-naive cardiology or neurology cohorts 50% faster than conventional Western sites, achieving $25K savings per patient through FDA-ready data—effectively eliminating rework and delays. The integration of telehealth and digital tools not only streamlines processes but also enhances compliance and data integrity. Remote monitoring has proven effective, with significant savings reported from these methodologies. The PREVENTABLE study, which employed direct-to-participant telehealth methods, showcased how home delivery of study medication and remote follow-ups can sustain participant engagement while providing decentralized trials cost savings.
Industry leaders underscore the significance of these innovations. One specialist noted that distributed studies are uniquely positioned to engage a diverse range of participants by removing geographic and temporal barriers, ultimately leading to more effective and economical research outcomes. As the adoption of distributed methodologies continues to rise—evidenced by a 93% increase in distributed studies from 2020 to 2022—the potential for decentralized trials cost savings in medical research becomes increasingly evident. It remains crucial to maintain investigator oversight and safety monitoring in decentralized studies to ensure participant safety and data integrity. Furthermore, bioaccess offers comprehensive clinical study management services, including:
ensuring a holistic approach to clinical research.
Decentralized trials significantly enhance participant recruitment by eliminating geographical barriers, enabling individuals to engage from the comfort of their homes. This convenience not only boosts enrollment rates but also fosters involvement among those who might otherwise be deterred by the need for extensive travel. Research indicates that traditional recruitment methods can lead to a staggering 90% decline in qualified individuals, underscoring the importance of accessibility.
By leveraging targeted outreach strategies, bioaccess™ effectively engages diverse patient populations, achieving recruitment timelines that are 50% faster than conventional methods. Successful examples, such as the TREAT Now study, demonstrate how digital platforms and community networks can streamline the identification of eligible participants, ultimately accelerating enrollment in research.
Furthermore, the collaboration between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading hub for medical research in Latin America, supported by Colombia's Minister of Health. This initiative not only enhances recruitment strategies but also emphasizes the significance of comprehensive clinical study management services, including:
Ensuring participant safety and supervision in distributed studies is paramount, as highlighted by industry professionals, guaranteeing that the integrity of the research process is maintained while improving accessibility.
Decentralized studies significantly enhance participant involvement by offering flexible participation options and tailored communication. Patients can engage with study teams through AI-driven platforms and decentralized research networks, receive timely updates, and access educational resources customized to their specific needs. This proactive engagement fosters a sense of ownership and commitment among participants, leading to retention rates that can improve by up to 30%. Notably, as much as 30% of individuals withdraw from clinical studies due to inadequate communication, highlighting the necessity for efficient engagement strategies.
Research indicates that when individuals are actively involved, they are more likely to adhere to study protocols, resulting in improved data quality and overall study success. Furthermore, the integration of mobile applications enhances communication, enabling features like eConsent, medication reminders, and secure messaging, which are crucial for maintaining participant involvement.
As Clinvigilant Research emphasizes, "Enhancing participant involvement is no longer optional—it’s crucial for study efficiency, adherence, and success." Organizations that prioritize personalized communication and digital engagement strategies are better positioned to navigate the complexities of clinical research, ultimately achieving decentralized trials cost savings while improving outcomes. Additionally, it is essential to consider the legal and ethical implications of participant involvement to uphold trust and integrity throughout the study process.
Decentralized studies enhance adaptability by enabling individuals to engage in ways that align with their lifestyles. Options such as remote monitoring, virtual consultations, and home-based assessments cater to individual schedules and preferences. These accommodations not only expand the participant pool but also yield more diverse and representative data.
The shift towards distributed medical studies, accelerated by the pandemic, has fundamentally transformed participant recruitment strategies. For instance, technologies like telemedicine and electronic health outcome assessments have proven effective in maintaining individual engagement during lockdowns, demonstrating that high-quality data can be collected remotely.
As a result, decentralized studies have become essential to contemporary clinical research, reflecting real-world disease distribution and addressing challenges faced by individuals with long commutes or mobility issues. This patient-focused approach is vital for fostering ongoing involvement and improving overall study outcomes.
Decentralized studies empower researchers to connect with a wider geographic audience, particularly those in remote or underserved areas. By leveraging digital tools and collaborating with local healthcare providers, bioaccess™ effectively engages diverse patient populations that traditional studies frequently overlook. This strategy not only enriches the data collected but also guarantees that the findings are pertinent across various demographics.
For instance, the collaboration between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading site for clinical studies in Latin America, a move supported by Colombia's Minister of Health. Such initiatives are expected to contribute to decentralized trials cost savings by dramatically decreasing recruitment times, with GlobalCare Clinical Trials partnering with bioaccess™ to achieve over a 50% reduction in recruitment duration and a remarkable 95% retention rate.
These efforts exemplify the potential of decentralized studies to bridge participation gaps, ultimately leading to more representative and relevant research outcomes.
The effectiveness of decentralized studies is fundamentally rooted in the strategic use of technology, encompassing telemedicine platforms, electronic data capture systems, and mobile health applications. These digital tools facilitate real-time data gathering and remote participant monitoring, ensuring seamless communication between study teams and subjects.
For instance, studies employing telemedicine have demonstrated an average enrollment rate of 4.5 individuals per month, significantly higher than the 1.25 individuals per month typical in entirely in-person studies. This integration not only enhances operational efficiency but also improves data accuracy, leading to more reliable outcomes.
Furthermore, research indicates that 89% of patients who opted for decentralized methods completed their studies, compared to just 60% in conventional settings. By incorporating these technologies into study design, bioaccess® is positioned to enhance clinical research processes. Their comprehensive services include:
This strategic approach results in decentralized trials cost savings by promoting cost savings and accelerating enrollment, achieving results 50% faster and saving $25K per individual with FDA-ready data. To fully leverage the advantages of decentralized studies, it is essential for stakeholders to collaborate effectively and address any patient concerns regarding technology.

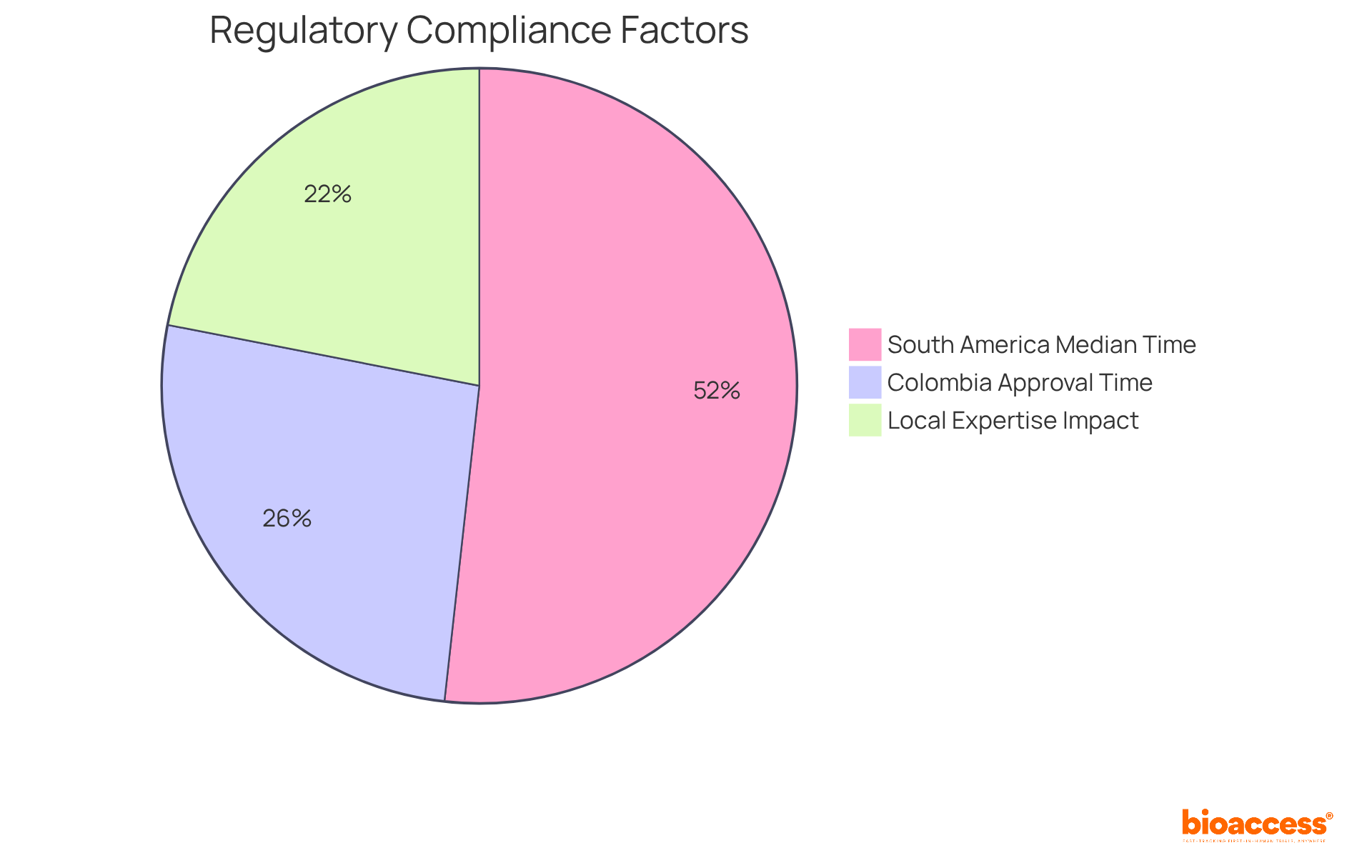
Decentralized studies significantly enhance regulatory compliance by leveraging local expertise and fostering established connections with regulatory authorities. At bioaccess®, we prioritize adherence to local regulations while upholding global standards, ensuring that all testing activities remain compliant. This dual approach expedites the regulatory authority (RA) approval process and mitigates the risk of non-compliance, instilling confidence among stakeholders.
In Colombia, the total IRB/EC and MoH (INVIMA) review takes only 90-120 days, a remarkable improvement compared to the median RA approval time of 236 days often observed in South America. Furthermore, the incorporation of local knowledge into the regulatory structure is essential, as it directly influences the effectiveness of medical studies and enhances the overall success of innovative treatments.
With a population exceeding 50 million and 95% coverage under universal healthcare, Colombia offers a robust recruitment environment. By optimizing compliance through local insights, bioaccess® effectively reduces timelines and facilitates quicker participant recruitment and study activation, which ultimately contributes to decentralized trials cost savings and efficiency in clinical research.
Decentralized studies significantly enhance data gathering by leveraging digital tools that facilitate real-time observation and reporting. By employing mobile applications and wearables to gather data directly from individuals, bioaccess® improves both accuracy and completeness. This method minimizes data entry errors and allows for prompt analysis, which is critical for improving test results.
Research indicates that the integration of decentralized trials cost savings has resulted in a 10-25% reduction in costs, primarily due to fewer locations and decreased patient visit expenses. Additionally, AI-driven applications have demonstrated an ability to enhance the quality of collected data, ensuring that medical research is not only efficient but also reliable.
As the healthcare landscape evolves, the role of digital tools in medical studies becomes increasingly vital, underscoring the importance of precise data in achieving successful research outcomes.
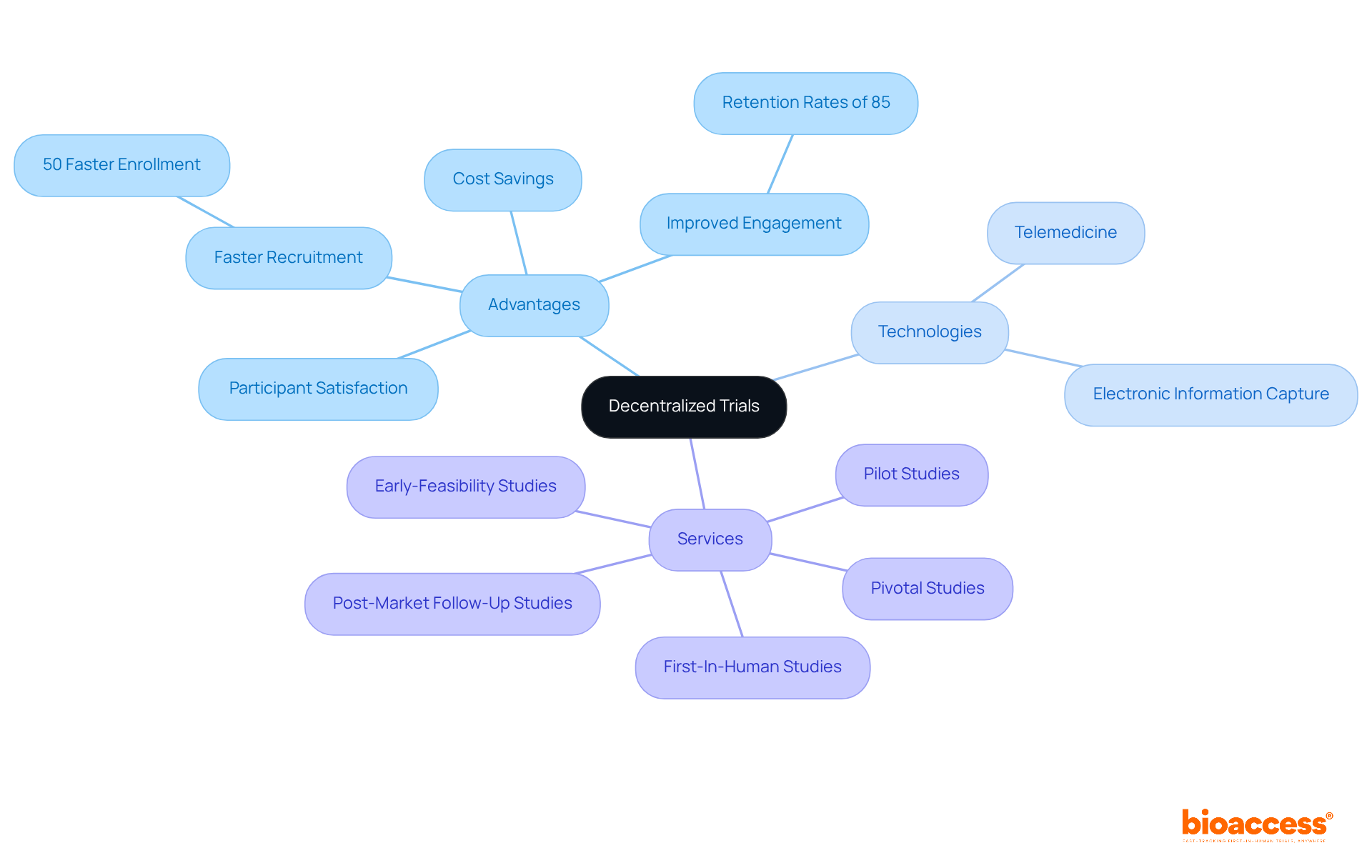
Decentralized trials are revolutionizing medical research by providing innovative solutions that effectively tackle traditional challenges. These methodologies lead to substantial decentralized trials cost savings while accelerating recruitment processes, achieving enrollment rates that are 50% faster than conventional methods. Improved engagement of participants stands out as another crucial advantage, with research indicating that individual-centered approaches can attain retention rates as high as 85%, compared to only 60% in conventional settings.
Furthermore, the incorporation of advanced technologies, such as telemedicine and electronic information capture, enhances data quality and participant satisfaction. As the medical research landscape evolves, adopting decentralized methodologies becomes essential for advancing medical knowledge and improving patient outcomes. This shift towards innovative practices is underscored by the fact that over 80% of potential participants actively seek research studies online, emphasizing the necessity for organizations to bolster their digital outreach.
bioaccess® offers comprehensive trial management services, including:
Ensuring that trials are executed efficiently and effectively. Embracing these changes is not merely advantageous; it is imperative for the future of clinical research.
Decentralized trials are fundamentally reshaping the clinical research landscape by introducing innovative methodologies that significantly reduce costs and enhance operational efficiency. By prioritizing patient-centric approaches and leveraging technology, these trials present a transformative solution to traditional research challenges, ultimately leading to a more accessible and effective clinical research environment.
This article highlights several key advantages of decentralized trials:
Furthermore, the integration of advanced technologies has proven to enhance data collection quality and regulatory compliance, further solidifying the benefits of this approach.
As the field of medical research continues to evolve, it is essential for organizations to embrace decentralized methodologies to remain competitive and relevant. This shift not only promises substantial cost savings but also fosters a more inclusive and efficient research process. Stakeholders are encouraged to explore and adopt these innovative practices, ensuring that clinical trials are not only cost-effective but also aligned with the needs and preferences of diverse patient populations.
What is bioaccess® and what services does it provide?
bioaccess® specializes in accelerating decentralized trials by offering innovative solutions that include early-feasibility studies, first-in-human studies, and post-market clinical follow-up studies. They leverage local insights and resources to enhance operational efficiency and ensure cost-effectiveness.
How do decentralized trials lead to cost savings?
Decentralized trials can reduce operational expenses by 10-25% by minimizing reliance on physical locations, which lowers travel costs, site fees, and staffing expenses. This cost-saving is achieved through fewer locations and reduced researcher fees.
What impact does bioaccess® have on patient recruitment in decentralized trials?
bioaccess® enhances patient recruitment by eliminating geographical barriers, allowing participants to engage from home. Their strategies enable recruitment timelines that are 50% faster than conventional methods, significantly increasing enrollment rates.
How much can sponsors save per patient using bioaccess®?
Sponsors can achieve savings of $25K per patient through FDA-ready data, effectively eliminating rework and delays associated with traditional study methods.
What technologies does bioaccess® integrate to improve decentralized trials?
bioaccess® integrates telehealth and digital tools to streamline processes, enhance compliance, and improve data integrity, allowing for effective remote monitoring and virtual visits.
What is the projected growth of the decentralized medical research market?
The decentralized medical research market is expected to grow from $8.5 billion in 2023 to $13.3 billion by 2030, indicating a significant shift towards decentralized trial methodologies.
What are some examples of the comprehensive clinical study management services provided by bioaccess®?
bioaccess® offers a range of services including feasibility studies, site selection, compliance reviews, study setup, project management, and reporting to ensure a holistic approach to clinical research.
How do decentralized trials improve participant safety and data integrity?
While decentralized trials enhance accessibility and convenience, maintaining investigator oversight and safety monitoring is crucial to ensure participant safety and the integrity of the research process.