


IRT software revolutionizes clinical trials for researchers by significantly boosting efficiency in participant enrollment, data management, and regulatory compliance. This innovative technology captures attention by demonstrating its potential to transform the clinical research landscape. With automation and real-time data access, IRT software accelerates patient recruitment by an impressive 50%, while also reducing overall trial costs. This ultimately leads to faster market readiness for new medical products, addressing a critical need in the Medtech industry.
As the Medtech landscape evolves, the role of IRT software becomes increasingly vital in overcoming key challenges faced by researchers. By streamlining processes and enhancing data management, IRT software not only improves operational efficiency but also ensures compliance with regulatory standards. This dual benefit fosters a more effective research environment, allowing teams to focus on what truly matters: advancing medical innovation.
In conclusion, the importance of collaboration in leveraging IRT software cannot be overstated. By embracing these technological advancements, researchers can navigate the complexities of clinical trials more effectively. The next steps involve integrating IRT solutions into research strategies, ensuring that the benefits of enhanced efficiency and reduced costs are fully realized.
The landscape of clinical trials is rapidly evolving, driven by the need for efficiency, accuracy, and compliance. At the forefront of this transformation is IRT (Interactive Response Technology) software, which offers researchers innovative tools that streamline processes and enhance participant engagement. As organizations strive to navigate the complexities of modern clinical research, a crucial question arises: how can IRT software not only accelerate trial timelines but also ensure high-quality outcomes in an increasingly competitive environment?
bioaccess® leverages advanced IRT software to revolutionize clinical study processes, significantly shortening timelines for ethical approvals and patient enrollment. This integration of IRT software allows bioaccess® to effectively manage complex trial logistics, ensuring studies are conducted with exceptional efficiency. Not only does this approach accelerate the path to market for innovative medical products, but it also enhances information integrity and compliance with regulatory standards.
With IRT, participant enrollment occurs 50% faster than traditional methods, and the automation of critical functions minimizes human error, leading to improved study outcomes. Recent advancements in IRT software, including real-time data access and adaptive randomization, empower researchers to make swift, informed decisions, further optimizing study efficiency. Industry leaders emphasize that implementing IRT is becoming essential for modern clinical trials, with numerous organizations reporting significant cost savings and enhanced participant engagement.
In summary, bioaccess®'s commitment to utilizing IRT software underscores its dedication to advancing healthcare solutions and improving patient outcomes. As the Medtech landscape evolves, collaboration and innovation will be key in addressing the challenges faced in clinical research.

Contemporary IRT software is essential in clinical research, designed with sophisticated structures that facilitate seamless integration and management of information. These systems provide real-time updates and centralized access to trial details, which are vital for maintaining information integrity and ensuring compliance with regulatory standards. The shift to cloud-based solutions empowers researchers to access critical information from anywhere, significantly enhancing collaboration and informed decision-making.
Industry specialists highlight that utilizing real-time information access in clinical research can lead to a remarkable 30% reduction in research timelines compared to traditional methods. Furthermore, implementing best practices such as standardized information collection protocols and regular compliance training can bolster the efficiency and reliability of IRT software systems. As organizations increasingly prioritize regulatory compliance, adopting innovative information management strategies becomes essential for achieving impactful research outcomes.
With bioaccess's comprehensive clinical study management services—including feasibility assessments, site selection, compliance evaluations, study setup, and project oversight—organizations can accelerate their clinical studies. This approach not only achieves participant enrollment 50% faster but also realizes savings of $25K per individual with FDA-ready information. As you consider your own challenges in clinical research, reflect on how these advancements can transform your processes and outcomes.

IRT software transforms participant recruitment by automating the identification and enrollment of qualified individuals. This innovative approach employs sophisticated algorithms and analytics, enabling rapid connections between individuals and suitable studies based on their medical backgrounds and demographics. As a result, the recruitment process accelerates, achieving patient enrollment 50% faster than traditional methods. This efficiency translates into significant cost savings of $25K per patient, all while ensuring FDA-compliant information that eliminates the need for rework and delays.
Such advancements not only streamline the recruitment process but also enhance the quality of participant selection. This leads to more reliable study outcomes, effectively addressing the recruitment challenges faced by Medtech and biopharma startups. In a landscape where time and resources are critical, organizations aiming to optimize their clinical research efforts can find that leveraging IRT software is a game-changer.

Instant access to information is revolutionizing decision-making in clinical studies through IRT software. Researchers can continuously monitor individual progress, track adverse events, and analyze data trends as they emerge. This immediate access to critical information allows for proactive adjustments to trial protocols, ensuring studies remain on course and comply with regulatory standards.
With bioaccess®, treatment-naive cardiology or neurology groups can be enrolled 50% faster than conventional Western sites, resulting in substantial savings of $25K per individual with FDA-ready data—no rework, no delays. As Barry Lake, President and Co-founder of RealTime-Devana, notes, "Sponsors and CROs finally have access to the appropriate information at the right moment—patient screening and enrollment trends, feasibility turnaround times, contract execution speeds, and more." This access not only enhances patient screening and enrollment but also facilitates informed decision-making throughout the study process.
By utilizing impartial information from top-performing research locations, the IRT software empowers researchers to make knowledgeable choices, ultimately improving the efficiency and effectiveness of clinical studies. The landscape of Medtech is evolving, and collaboration is key to navigating these challenges effectively.

Ensuring regulatory compliance in the clinical research process is crucial for startups navigating a complex regulatory landscape, which is where IRT software plays a vital role. By automating documentation and reporting, and providing real-time access to information, these systems maintain accurate records that meet regulatory standards. They also offer essential audit trails and compliance checks to demonstrate adherence to Good Clinical Practice (GCP) guidelines.
With features like automated alerts for compliance deadlines and customizable reporting tools, IRT software empowers startups to accelerate their approval processes. This capability allows them to effectively compete against established life sciences companies. As the Medtech landscape evolves, leveraging such technology becomes not just beneficial but necessary for success.

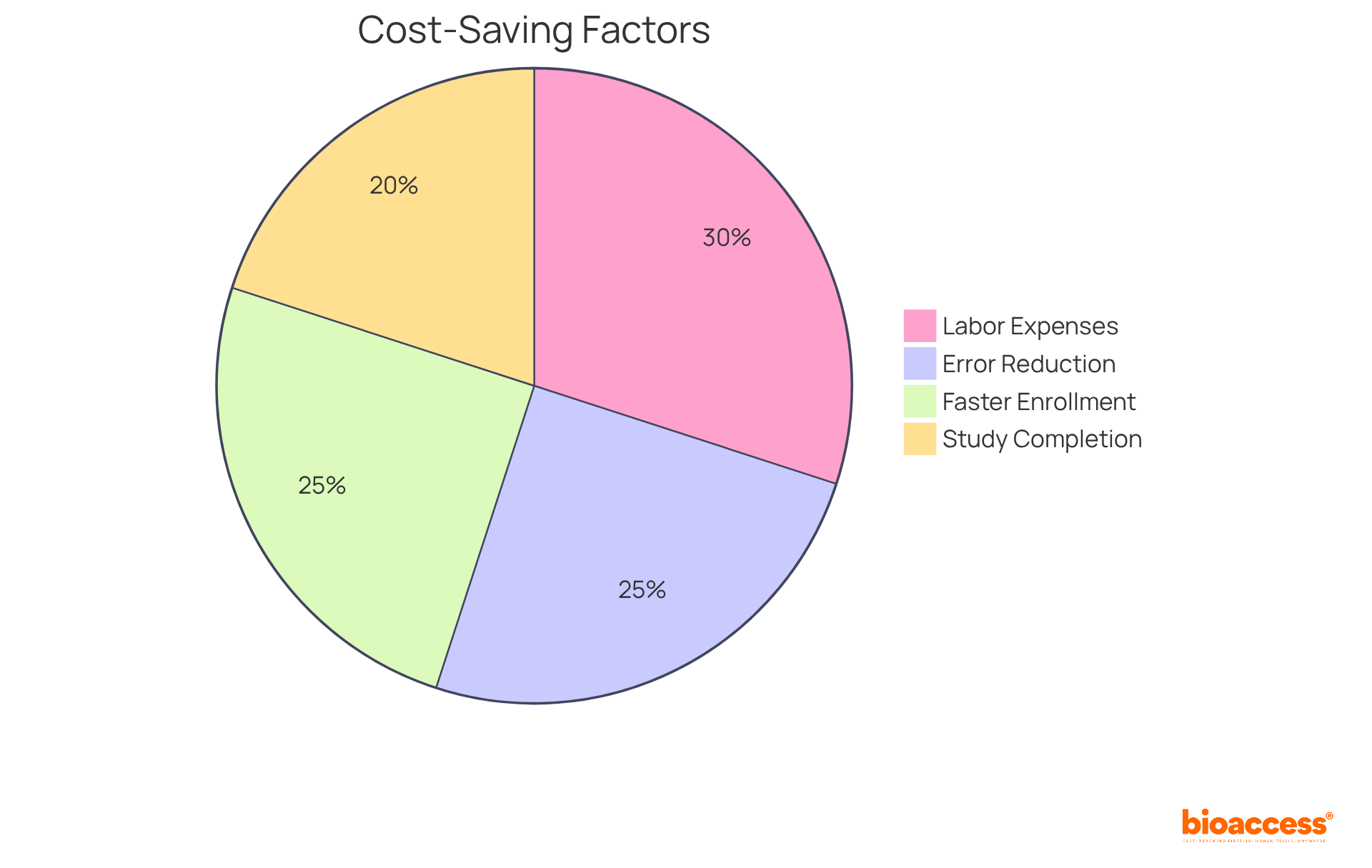
Implementing IRT software from bioaccess® can lead to substantial cost savings in clinical trials. By automating processes like tracking individuals, collecting information, and reporting, researchers can significantly reduce labor expenses and minimize the risk of errors that could result in costly delays. Notably, bioaccess® allows treatment-naive cardiology or neurology cohorts to be enrolled 50% faster than Western sites, translating to $25K savings per patient with FDA-ready data—no rework, no delays.
Furthermore, the efficiency achieved through bioaccess®'s IRT software accelerates study completion, ultimately lowering overall research costs and expediting PMA data submissions by as much as 11 months. This capability not only streamlines operations but also enhances the overall effectiveness of clinical research, making bioaccess® a vital partner in navigating the complexities of the Medtech landscape.
IRT solutions are inherently scalable, effectively addressing the diverse needs of various clinical studies, from small pilot research to extensive multi-site investigations. This adaptability empowers researchers to customize the software according to their specific requirements, significantly enhancing operational efficiency and optimizing resource allocation throughout the study. Notably, bioaccess® enables treatment-naive cardiology or neurology groups to be enrolled 50% faster than their Western counterparts, leading to substantial savings of $25K per individual with FDA-ready data—no rework, no delays.
Clinical study managers have underscored that this flexibility not only streamlines workflows but also enhances data precision and participant involvement. As Naveen Rao pointed out, healthcare systems are increasingly seeking technology that can evolve alongside their needs, underscoring the critical importance of integrating adaptable IRT software. Furthermore, the demand for such solutions is amplified by the growing complexity of clinical studies, necessitating a flexible approach to meet various research requirements.

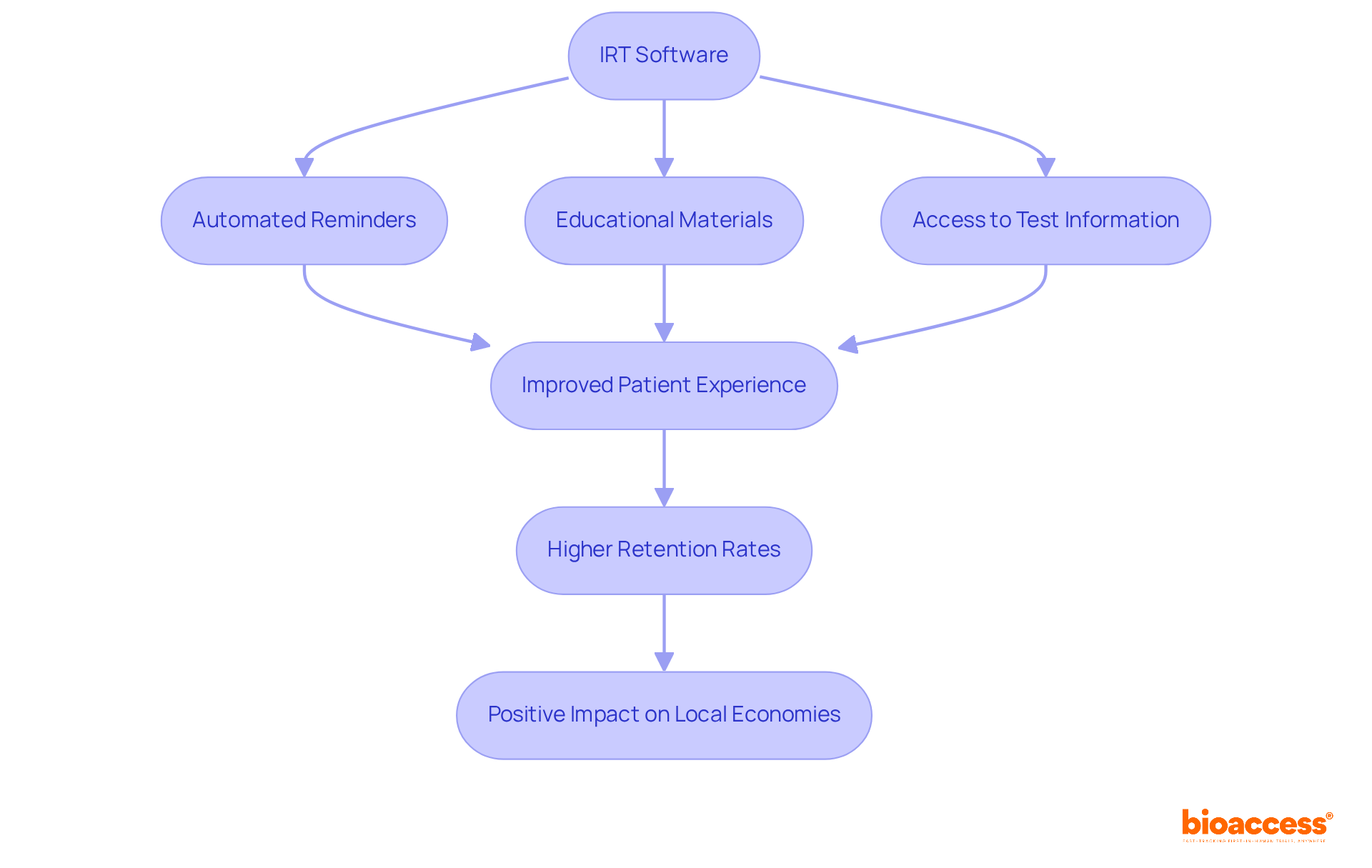
IRT software significantly enhances patient involvement by offering essential tools that facilitate communication and support throughout the study process. Automated reminders, educational materials, and easy access to test information keep participants informed and motivated. Additionally, comprehensive clinical study management services—such as feasibility assessments, compliance evaluations, site selection, and project oversight—ensure that studies are conducted efficiently and in line with local regulations.
This holistic approach not only improves patient experiences but also contributes to higher retention rates, which are vital for the validity of study outcomes. By creating a positive experience and ensuring robust management of the study, IRT software solutions play an instrumental role in the success of clinical investigations. Ultimately, this success has a ripple effect, positively impacting local economies through job creation and advancements in healthcare.
The incorporation of artificial intelligence within IRT software is revolutionizing clinical study procedures through advanced predictive analytics and enhanced information processing capabilities. AI algorithms excel at analyzing extensive datasets, uncovering trends, predicting outcomes, and refining study designs. This technological advancement streamlines clinical trial efficiency and significantly improves information quality, leading to more reliable and actionable outcomes.
For instance, bioaccess®'s integrated IRT information platform enables treatment-naive cardiology or neurology groups to enroll participants 50% faster than conventional Western sites, achieving substantial cost savings of $25K per patient with FDA-ready information—no rework, no delays. This innovation addresses a critical issue in clinical research, as nearly a third of all phase III studies fail due to enrollment challenges. As Hitesh Chopra, PhD, notes, "AI’s ability to sift through vast amounts of information, identify trends, and make accurate predictions has the potential to accelerate the creation of new treatments."
While AI-driven predictive analytics enhance experimental outcomes, it is essential to address challenges such as information quality and ethical considerations to ensure successful implementation. By leveraging real-world applications, the potential of bioaccess®'s integrated IRT software information platform to improve success rates in studies and expedite the journey to market for new therapies becomes increasingly evident.

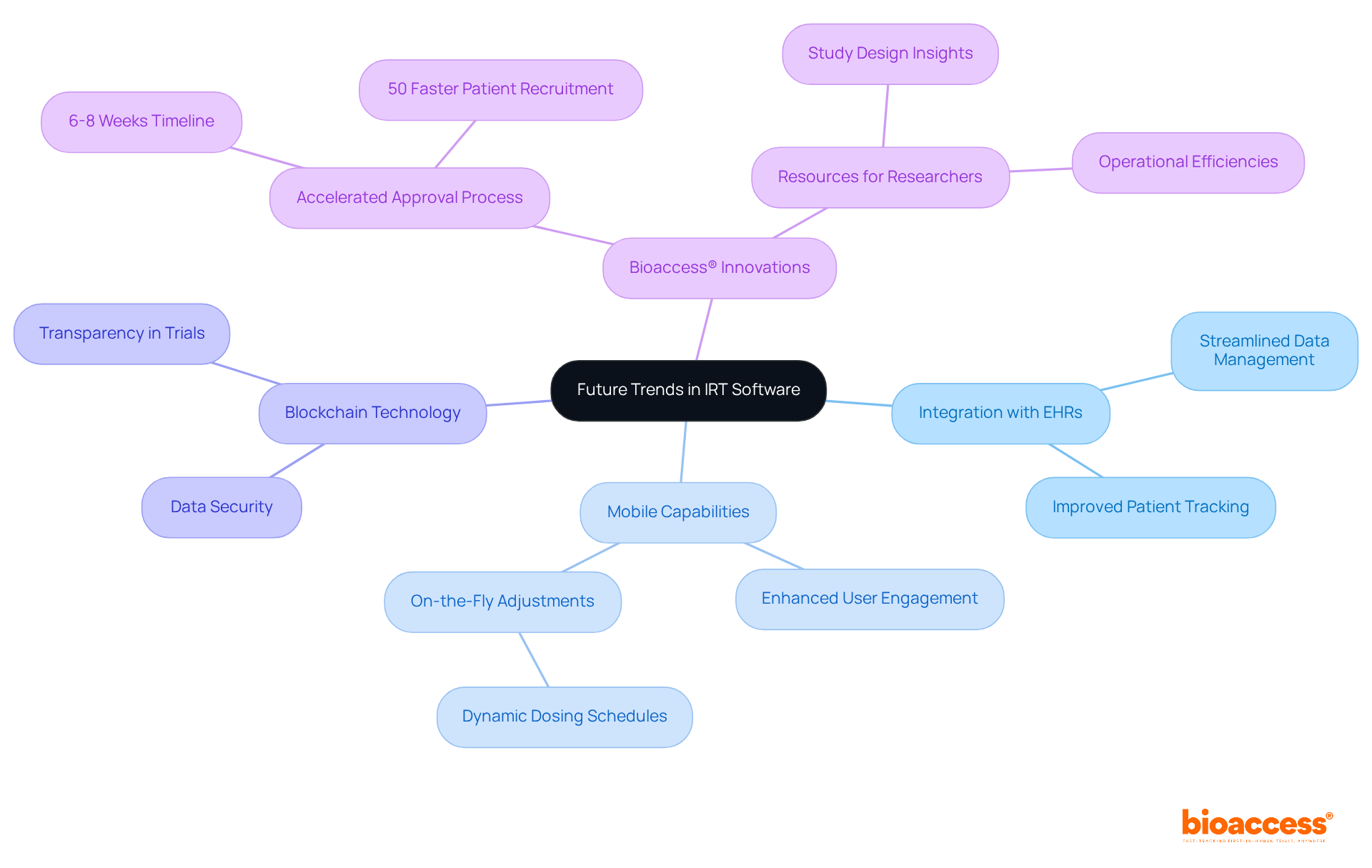
As the clinical research environment evolves, several trends are shaping the future of IRT software. These trends include:
By embracing these innovations, researchers can optimize study processes and enhance overall outcomes, ensuring that clinical research remains at the forefront of medical advancement.
Notably, bioaccess® stands out with its regulatory approval process, achieving results in just 6-8 weeks compared to the typical 6-12 months in the US/EU. This expedited timeline allows for the inclusion of treatment-naive cardiology or neurology groups 50% faster than conventional Western locations, effectively addressing patient recruitment challenges that often hinder early-phase clinical studies. Furthermore, bioaccess® provides targeted resources on study design, data collection, and operational efficiencies, offering valuable insights for researchers navigating this complex landscape.
As Jon Paras, Senior Vice President of IRT Strategy, points out, "Clinical studies are becoming more intricate, necessitating quicker development cycles and tougher regulatory compliance." This underscores the need for modern IRT software that empowers clinical research teams with user-friendly interfaces, enabling on-the-fly adjustments to patient dosing schedules. By leveraging these advancements, including bioaccess®'s FDA-ready data solutions, researchers can optimize their clinical trials, ultimately enhancing the efficiency and effectiveness of their studies.
The integration of IRT software marks a significant evolution in the realm of clinical trials, empowering researchers to streamline processes, enhance data management, and boost patient engagement. By harnessing advanced technology, IRT software not only speeds up participant enrollment but also guarantees regulatory compliance and cost-effectiveness, fundamentally changing how clinical studies are executed.
Key benefits of IRT software are evident throughout this discussion:
As the clinical research landscape evolves, the necessity of adopting innovative IRT software becomes increasingly clear. Embracing these advancements positions organizations to adeptly navigate the complexities of modern trials while enhancing overall healthcare solutions. By investing in IRT technology, researchers can refine their study processes, achieve substantial cost savings, and ultimately deliver improved patient outcomes, paving the way for the future of clinical research.
What is bioaccess® and how does it utilize IRT software?
bioaccess® leverages advanced IRT software to revolutionize clinical study processes, significantly shortening timelines for ethical approvals and patient enrollment while managing complex trial logistics efficiently.
How does IRT software improve participant enrollment in clinical trials?
IRT software enables participant enrollment to occur 50% faster than traditional methods and automates critical functions, minimizing human error and leading to improved study outcomes.
What recent advancements in IRT software enhance study efficiency?
Recent advancements include real-time data access and adaptive randomization, which empower researchers to make swift, informed decisions and optimize study efficiency.
What are the benefits of using IRT software in clinical trials?
Benefits include significant cost savings, enhanced participant engagement, and improved compliance with regulatory standards, with many organizations reporting a 30% reduction in research timelines.
How does modern software architecture enhance data management in IRT solutions?
Contemporary IRT software is designed with sophisticated structures that facilitate seamless integration and management of information, providing real-time updates and centralized access to trial details.
What impact does cloud-based IRT software have on clinical research?
Cloud-based solutions empower researchers to access critical information from anywhere, enhancing collaboration and informed decision-making in clinical research.
What comprehensive services does bioaccess® provide for clinical study management?
bioaccess® offers services including feasibility assessments, site selection, compliance evaluations, study setup, and project oversight to accelerate clinical studies.
How does IRT software streamline patient recruitment for clinical trials?
IRT software automates the identification and enrollment of qualified individuals using sophisticated algorithms and analytics, achieving patient enrollment 50% faster than traditional methods.
What cost savings can organizations expect by using IRT software for patient recruitment?
Organizations can realize significant cost savings of $25K per patient while ensuring FDA-compliant information that eliminates the need for rework and delays.
Why is implementing IRT software becoming essential for modern clinical trials?
Implementing IRT software is becoming essential due to its ability to enhance study efficiency, reduce timelines, and improve participant selection, addressing recruitment challenges faced by Medtech and biopharma startups.