


The article highlights the transformative potential of electronic lab notebooks (ELNs) in enhancing clinical research. By improving efficiency, fostering collaboration, bolstering data security, and ensuring regulatory compliance, ELNs are positioned as essential tools in the research landscape. Notably, they save researchers significant time on documentation, facilitate real-time collaboration, and uphold data integrity. Collectively, these advantages streamline the research process and expedite the development of medical innovations, underscoring the critical role of ELNs in advancing clinical research.
The landscape of clinical research is rapidly evolving, driven by the need for efficiency, accuracy, and collaboration. As researchers strive to streamline their processes and enhance data integrity, electronic lab notebooks (ELNs) have emerged as a pivotal tool in achieving these goals. This article explores ten transformative ways that ELNs can significantly improve clinical research workflows, from fostering collaboration among research teams to ensuring robust data security and regulatory compliance.
However, as organizations navigate the transition from traditional paper notebooks to digital solutions, they face challenges that raise important questions:
bioaccess® leverages the capabilities of electronic lab notebooks (ELNs) to significantly enhance lab notebook utilization in clinical research workflows, facilitating faster information collection and analysis. By transitioning to digital documentation, researchers gain immediate access to up-to-date information, which drastically reduces the time from study initiation to results. This increased agility is crucial in the fast-paced environments of Medtech and Biopharma, where timely information is essential for informed decision-making and accelerating regulatory submissions.
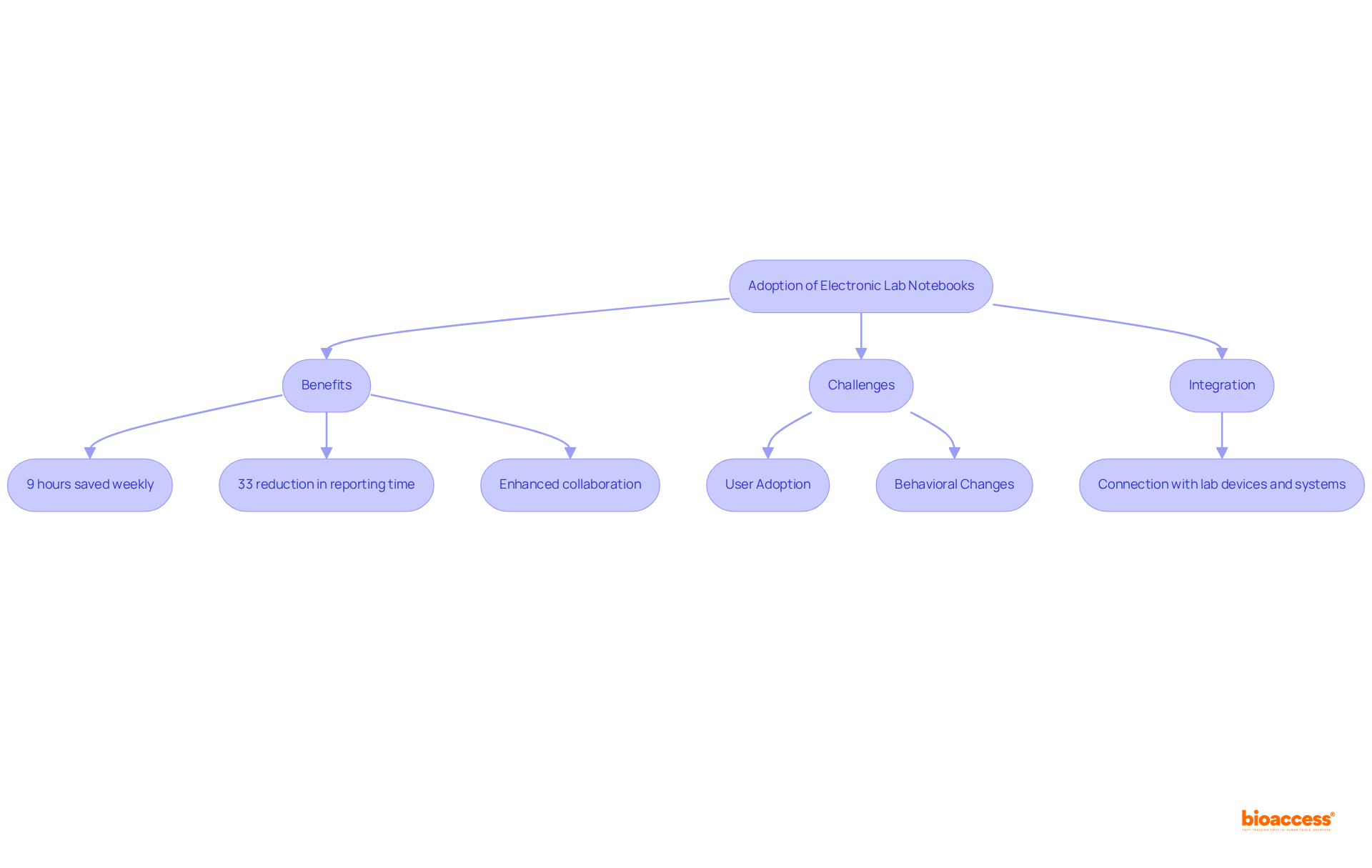
Current statistics reveal that researchers using lab notebooks electronically save an average of 9 hours weekly, translating to a 33% reduction in time spent on reporting tasks, and they can expect a return on investment within 3-4 months. Furthermore, the adoption of lab notebooks in electronic format has been shown to foster collaboration among research groups, enabling seamless exchange of information and insights—an imperative for driving innovation in clinical trials.
However, the transition from paper notebooks to electronic lab notebooks presents challenges, including user adoption and the necessity for organizations to manage behavioral changes among scientists. Additionally, electronic lab notebooks can integrate with laboratory devices and interconnect with various systems, enhancing efficiency and the quality of information.
As the industry advances, the integration of electronic lab notebooks is highlighted as a transformative approach to improving study efficiency and accelerating the journey to market for groundbreaking medical solutions.
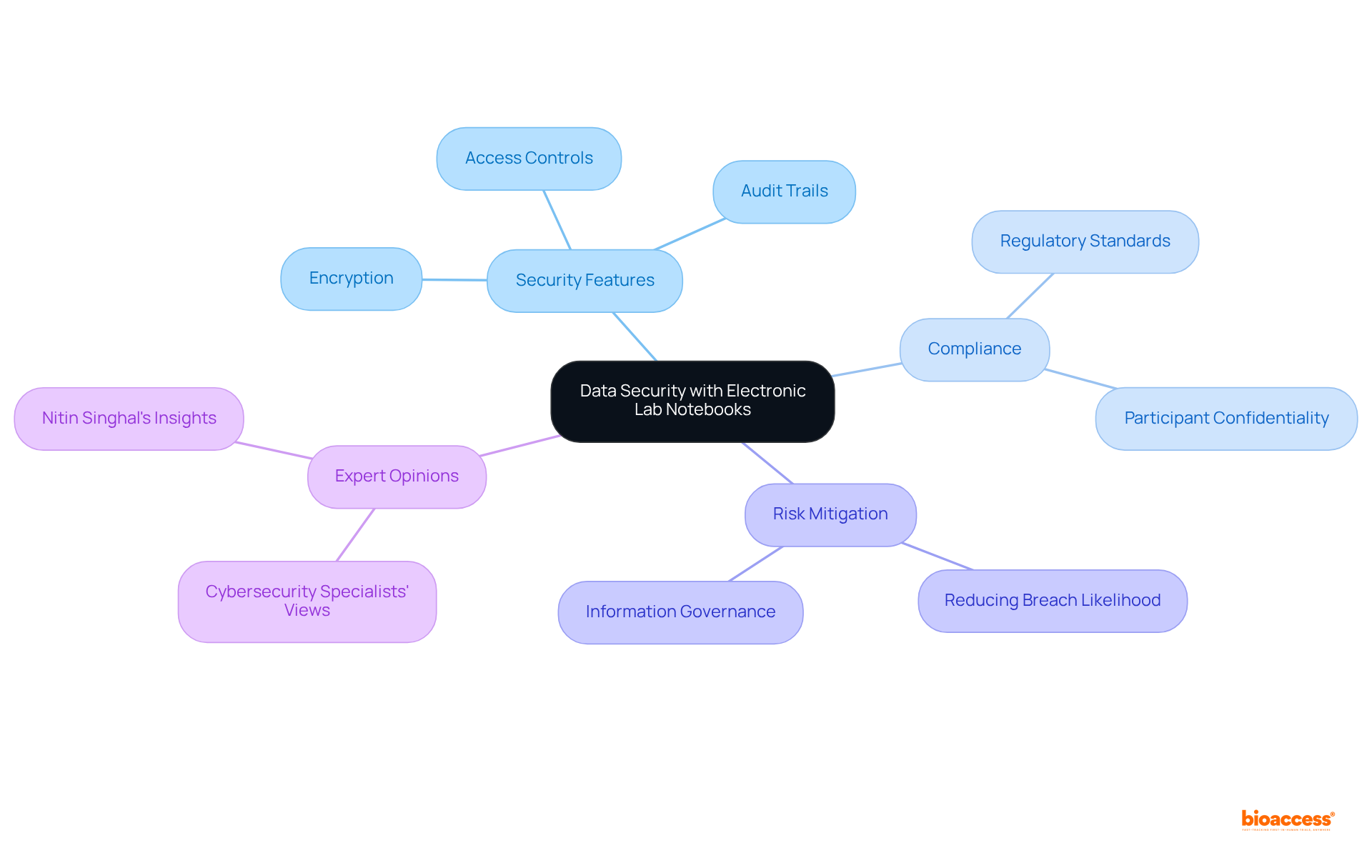
Electronic laboratory notebooks (ELNs) are vital instruments that ensure information security in medical studies, featuring sophisticated security attributes such as encryption, access controls, and thorough audit trails. These functionalities are essential for safeguarding sensitive patient information and research results from unauthorized access and potential breaches.
By implementing electronic lab notebooks, bioaccess® not only enhances the security of trial information but also ensures compliance with evolving regulatory standards, thereby protecting the integrity of trials and preserving participant confidentiality.
As cybersecurity specialists emphasize, safeguarding trial information is crucial, with robust information governance being a primary strategy in mitigating risks associated with breaches. Organizations that adopt ELNs can significantly reduce the likelihood of information exposure, aligning with best practices in information management and security. This proactive approach fosters trust among participants and strengthens the overall framework of clinical research.
A recent report highlights that 31% of organizations reported experiencing a breach this year, underscoring the critical need for effective information protection measures. Additionally, Nitin Singhal, VP of Engineering, asserts, "Security isn’t a final checkpoint; it’s the foundation of product philosophy. Integrating security from the outset is crucial to mitigate costly reputational damage post-launch."
Furthermore, with 95% of security breaches originating from human errors, the adoption of electronic lab notebooks can significantly minimize these risks through enhanced training and governance.
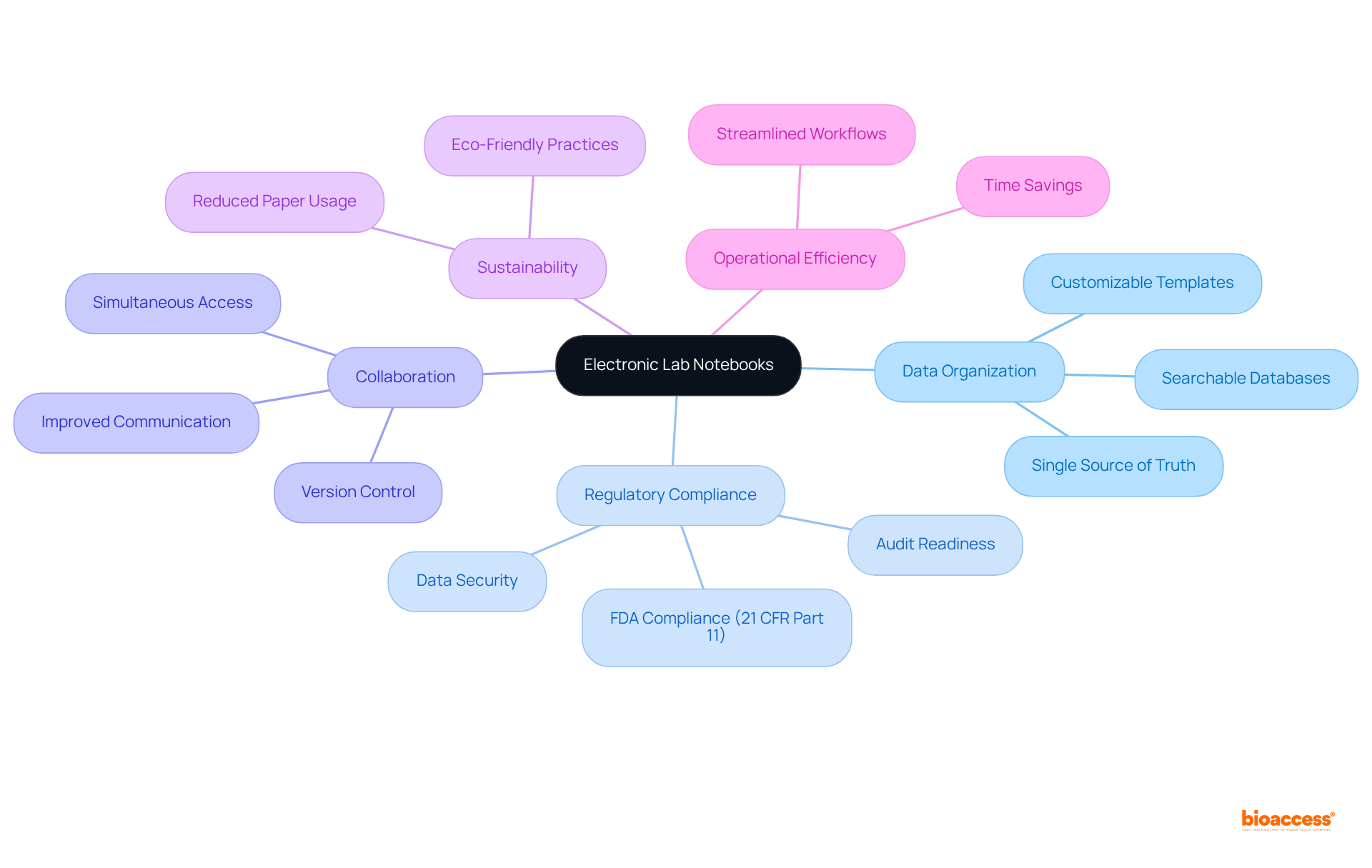
Lab notebooks, specifically Electronic Lab Notebooks (ELNs), revolutionize information organization by enabling researchers to systematically categorize and label their entries. This structured methodology not only facilitates rapid information retrieval but is also essential during audits and regulatory reviews.
With features such as searchable databases and customizable templates, researchers at bioaccess® can adeptly manage large volumes of information, ensuring that crucial details remain easily accessible. As medical trials become increasingly complex—evidenced by a 61% rise in eligibility criteria from 2001 to 2015 and 56% of locations reporting heightened complexity compared to three years prior—effective information management becomes paramount.
ELNs address this need by streamlining workflows and enhancing collaboration among team members, allowing simultaneous access to documents without the risk of version conflicts. This capability is particularly beneficial in a landscape where, as of May 2023, 53% of all registered trials are conducted outside the US, underscoring the necessity for robust data accessibility across diverse investigative environments.
By adopting electronic lab notebooks, organizations can significantly enhance their operational efficiency, compliance, and ultimately, the success of their clinical study initiatives. As Dr. Morgana Moretti, an active scientist and freelance medical writer, notes, 'Many electronic lab notebooks also ensure compliance with FDA 21 CFR Part 11,' highlighting their critical role in maintaining regulatory standards.
Furthermore, the use of electronic lab notebooks promotes sustainability by minimizing unnecessary paper consumption, which aligns with the growing emphasis on eco-friendly practices in research.
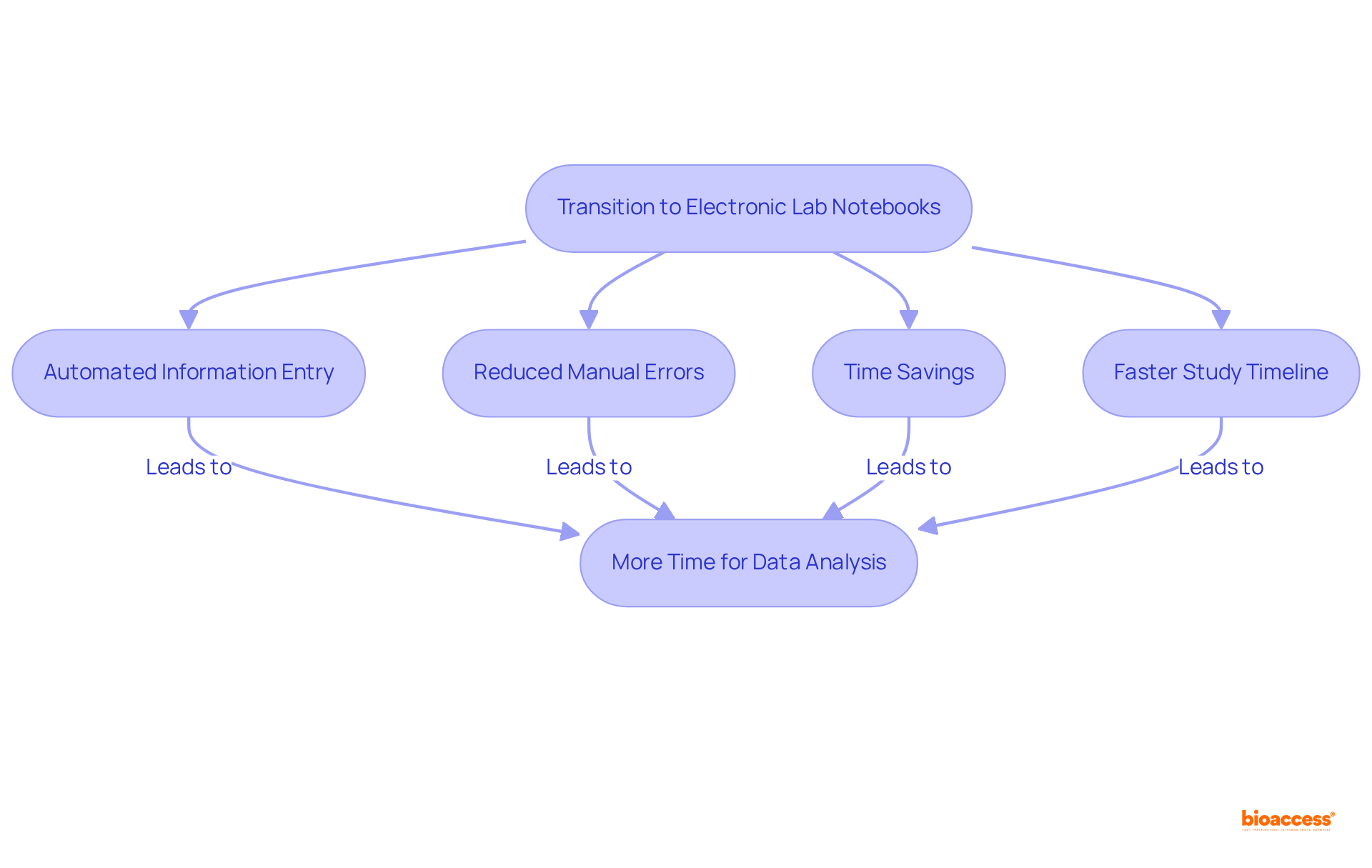
The application of lab notebooks significantly streamlines documentation procedures, yielding substantial time savings for researchers. By automating information entry tasks such as timestamping and formatting, and utilizing templates, a lab notebook minimizes the likelihood of manual errors and expedites the documentation process.
Studies reveal that researchers can save an average of nine hours per week following the implementation of an ELN, with some reporting savings of up to 17 hours. Dr. Arianna Ferrini, a biomedical scientist, emphasizes, 'Lab notebooks in electronic format offer researchers simpler access to precise data,' enabling bioaccess® researchers to devote more time to data analysis and interpretation rather than being burdened by administrative tasks.
Consequently, the overall study timeline is accelerated, facilitating faster progress in clinical studies and trials.
Electronic laboratory notebooks significantly enhance collaboration by enabling multiple researchers to access and contribute to the same notebook in real time. This functionality cultivates a dynamic environment where team members can discuss findings, share feedback, and make informed decisions with agility.
Studies indicate that teams employing SciNote experience a 16% reduction in time spent on scheduling and planning, allowing for increased focus on critical activities. For example, research demonstrates that scientists saved an average of 9 hours weekly by utilizing SciNote, translating into more time devoted to innovative solutions. Furthermore, 6 out of 7 users reported considerable time savings after transitioning to SciNote, reinforcing its effectiveness.
The return on investment for integrating electronic lab notebooks is typically realized within 3-4 months, establishing it as an invaluable asset for scientific teams. At bioaccess®, this collaborative approach not only enhances the quality of research but also accelerates the development of groundbreaking medical technologies.
Groups report that the implementation of electronic lab notebooks promotes seamless communication, enabling them to manage projects and exchange information efficiently, irrespective of geographical barriers. As one study group noted, the transition to an electronic lab notebook has transformed their workflow, enhancing collaboration and ultimately leading to better patient outcomes.
By leveraging the capabilities of electronic lab notebooks, bioaccess® empowers researchers to collaborate more effectively, driving progress in clinical research and innovation.
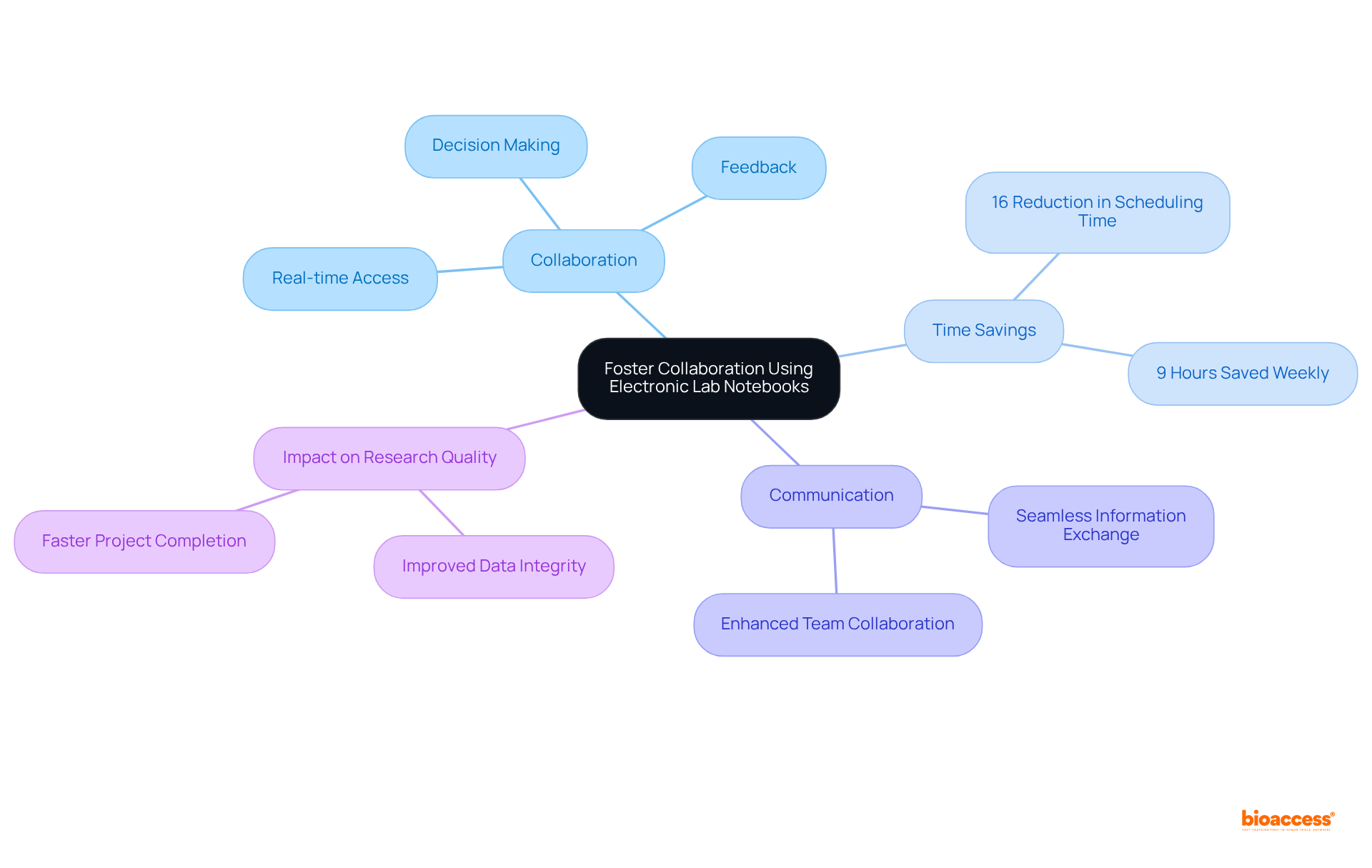
Lab notebooks are essential for ensuring regulatory compliance, incorporating features that enhance information integrity and traceability. They automatically generate audit trails, document changes, and maintain version control—critical components for meeting stringent regulatory requirements.
For instance, lab notebooks that are electronic facilitate adherence to FDA regulations, including 21 CFR Part 11, by providing electronic signatures and secure information storage. By utilizing ELNs, bioaccess® can significantly streamline compliance processes, simplifying the task of meeting guidelines established by regulatory bodies.
This capability not only bolsters trial integrity but also improves the overall efficiency of documentation, enabling quicker and more reliable data management. With the electronic lab notebook market projected to grow at a CAGR of 10%, adopting these lab notebooks is increasingly crucial for organizations aiming to uphold high standards of regulatory compliance in medical studies.
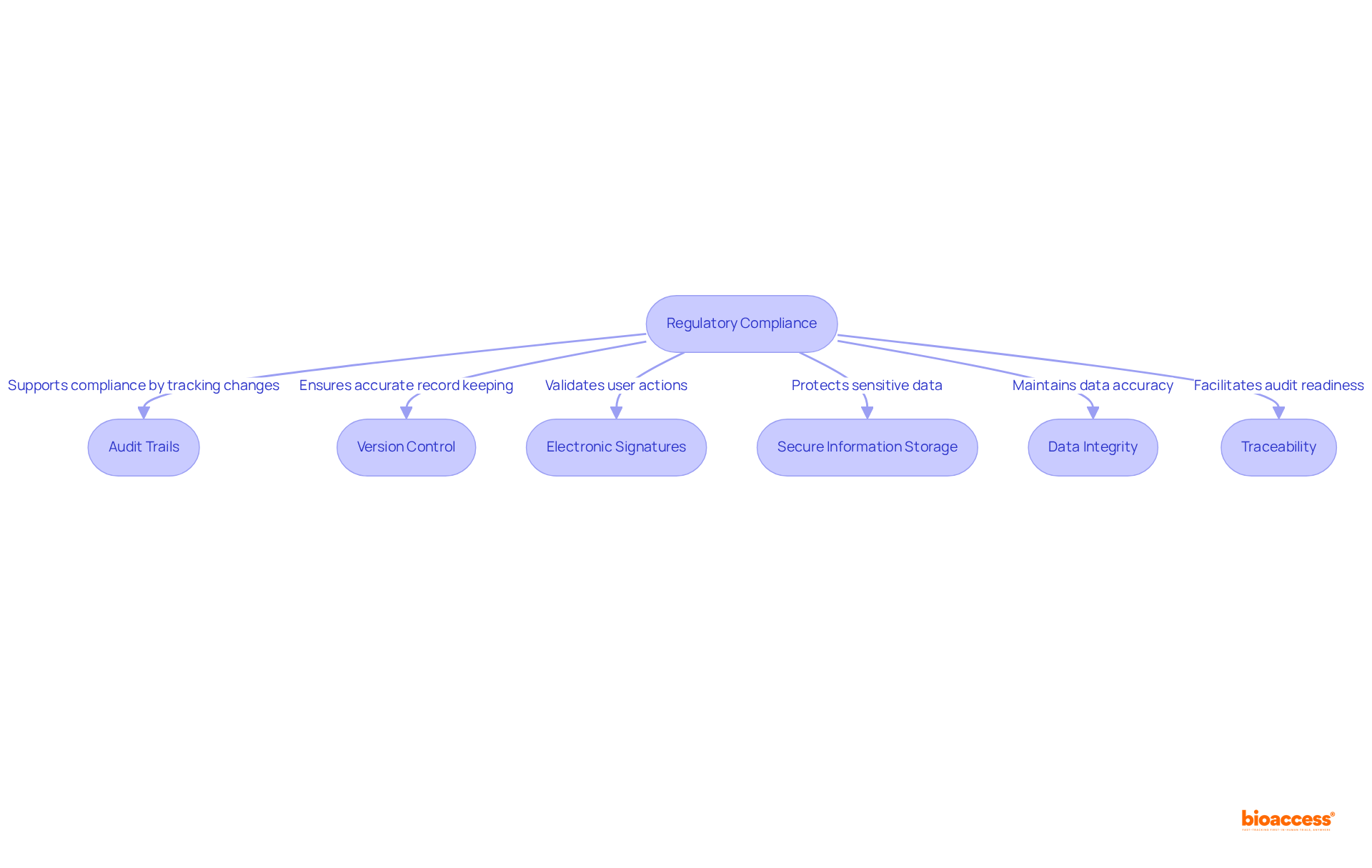
Electronic laboratory notebooks (ELNs) are increasingly recognized for their adaptability to diverse research needs, establishing themselves as essential tools in medical trials. Researchers have the ability to customize templates, workflows, and data entry formats to align with specific study requirements, thereby enhancing the overall efficiency of data management. This level of adaptability is particularly beneficial for organizations like bioaccess®, which can tailor electronic lab notebooks to the unique demands of each research trial, ensuring that the tools employed are optimized for varying project specifications.
Current trends indicate that 49% of R&D laboratories in medicine and biotech view the implementation of lab notebooks as a crucial technological enhancement. This shift underscores the growing recognition of lab notebooks, specifically electronic lab notebooks, as versatile instruments capable of evolving alongside the dynamic landscape of medical studies. For example, platforms such as Indigo ELN and Labnote.co provide customizable features that empower researchers to create experiment-specific templates, thereby streamlining documentation processes. Indigo ELN, in particular, allows users to develop templates for different experiment types without altering the underlying code, exemplifying its inherent flexibility.
As emphasized by industry experts, the ability to customize ELNs to meet specific research requirements not only improves data management but also fosters collaboration among research teams. This flexibility is vital in healthcare settings where various types of information must be integrated securely and effectively. Furthermore, the development cost of a lab notebook in electronic format ranges from $120,000 to $600,000, depending on the feature set, with implementation time for custom ELN software spanning from 6 to over 16 months. By leveraging these customizable capabilities, researchers can refine their workflows, ultimately leading to more successful clinical trial outcomes.
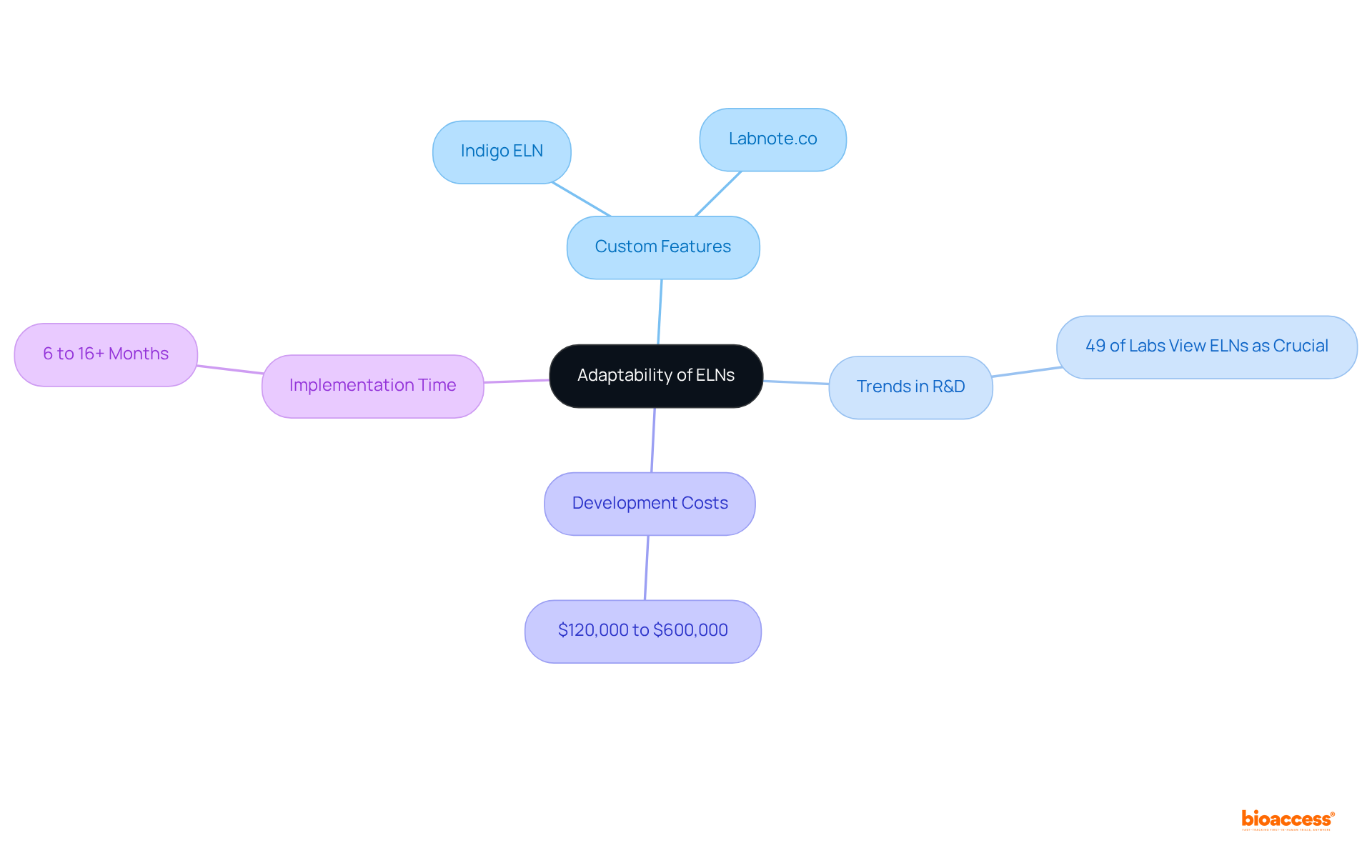
Electronic laboratory notebooks (ELNs) significantly elevate information analysis by integrating advanced analytical tools and streamlining export processes. Researchers can quickly visualize trends, generate comprehensive reports, and perform statistical analyses directly within the ELN platform. This functionality not only enhances the analytical workflow but also expedites the decision-making process at bioaccess®, enabling teams to derive actionable insights more efficiently.
Laboratories utilizing lab notebooks electronically have reported improved information exchange and record management, which are crucial for timely information interpretation. Consequently, the ability to access and scrutinize information effortlessly leads to better patient outcomes and accelerates the advancement of medical innovations.
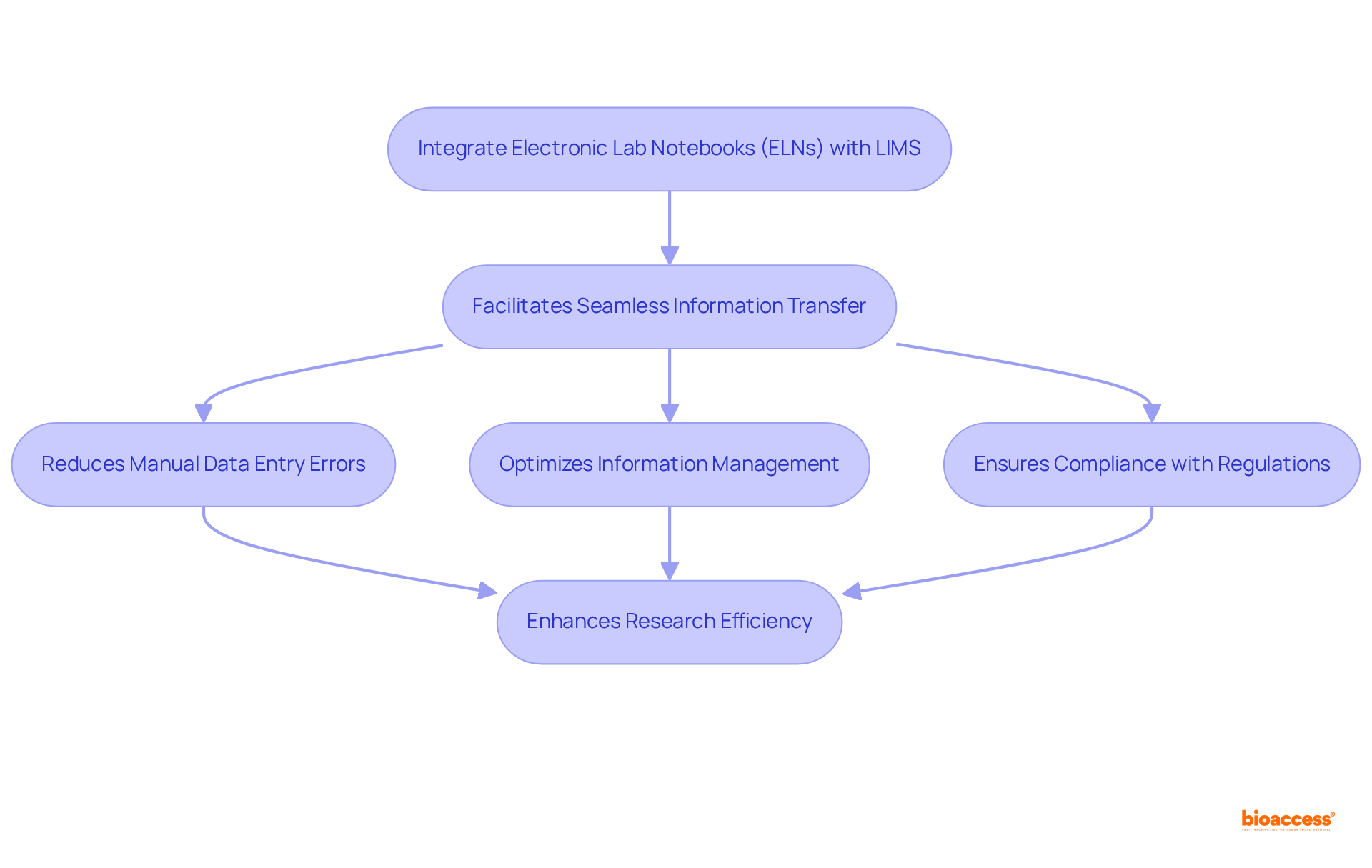
Integrating lab notebooks, specifically electronic lab notebooks (ELNs), with laboratory information management systems (LIMS) and other research tools is essential for fostering a cohesive research environment, significantly enhancing clinical research efficiency. This integration facilitates seamless information transfer, thereby reducing the risk of errors associated with manual data entry. For instance, platforms like SciSure’s Digital Lab Platform (DLP) exemplify this synergy by merging ELN and LIMS functionalities, enabling automated workflows and comprehensive sample tracking. Such systems not only optimize information management but also ensure compliance with industry regulations, thereby enhancing overall information integrity.
Notably, BlueLot ELN+ includes built-in LIMS features, further illustrating the advantages of integration in modern laboratories. At bioaccess®, with over 15 years of industry experience, this interconnectedness is pivotal in accelerating research studies, ensuring that all data remains consistent and easily accessible. The ability to generate comprehensive reports for compliance and audits further supports regulatory adherence, facilitating innovation for development teams. For example, Pilatus Biosciences successfully utilized SciSure’s DLP to streamline their operations, demonstrating the effectiveness of such integrations.
As the landscape of laboratory management evolves, the incorporation of lab notebooks and laboratory information management systems is becoming vital for achieving operational excellence in clinical trials. This evolution ultimately results in quicker and more reliable findings, underscoring the importance of collaboration and the next steps in advancing clinical research.
The adoption of lab notebooks, specifically electronic lab notebooks (ELNs), presents immediate benefits while also laying the groundwork for long-term success in clinical research. By ensuring data integrity, facilitating collaboration, and streamlining compliance, ELNs empower bioaccess® to establish a solid foundation for future research initiatives. This forward-thinking approach is crucial for driving innovation and enhancing medical knowledge within the Medtech and Biopharma industries. As we navigate the complexities of clinical research, the role of the lab notebook becomes increasingly vital, encouraging collaboration and addressing key challenges effectively.
The integration of electronic lab notebooks (ELNs) into clinical research signifies a transformative shift that enhances efficiency, security, and collaboration among researchers. By adopting ELNs, organizations can streamline documentation processes, improve data accessibility, and ensure compliance with regulatory standards, ultimately accelerating the pace of medical innovation. This digital transition not only saves valuable time but also fosters a collaborative environment essential for driving advancements in clinical trials.
Key insights from the article highlight the numerous advantages of utilizing ELNs, including:
The ability to customize ELNs to meet specific research needs further underscores their versatility, allowing researchers to adapt workflows and templates to suit diverse project requirements. Additionally, the integration of ELNs with other research tools amplifies their effectiveness, creating a cohesive ecosystem that enhances overall research outcomes.
In conclusion, the adoption of electronic lab notebooks is not merely a trend but a crucial step toward achieving long-term success in clinical research. By prioritizing the use of ELNs, organizations can ensure data integrity, foster collaboration, and navigate the complexities of regulatory compliance with ease. Embracing this technology is essential for researchers aiming to make significant contributions to the Medtech and Biopharma industries, ultimately leading to improved patient outcomes and groundbreaking medical solutions.
What is bioaccess® and how does it enhance clinical research?
bioaccess® leverages electronic lab notebooks (ELNs) to improve lab notebook utilization in clinical research workflows, facilitating faster information collection and analysis. This transition to digital documentation allows researchers immediate access to up-to-date information, reducing the time from study initiation to results.
How much time can researchers save by using electronic lab notebooks?
Researchers using electronic lab notebooks typically save an average of 9 hours weekly, which translates to a 33% reduction in time spent on reporting tasks.
What is the expected return on investment for adopting electronic lab notebooks?
Organizations can expect a return on investment within 3-4 months after adopting electronic lab notebooks.
What challenges are associated with transitioning from paper notebooks to electronic lab notebooks?
Challenges include user adoption and managing behavioral changes among scientists as they transition to using electronic lab notebooks.
How do electronic lab notebooks contribute to data security in clinical research?
Electronic lab notebooks feature security attributes such as encryption, access controls, and audit trails, which are essential for safeguarding sensitive patient information and ensuring compliance with regulatory standards.
What recent statistics highlight the importance of data security in clinical trials?
A recent report indicated that 31% of organizations experienced a data breach this year, emphasizing the critical need for effective information protection measures.
How do electronic lab notebooks improve data organization and accessibility?
ELNs enable researchers to systematically categorize and label their entries, facilitating rapid information retrieval and ensuring crucial details are easily accessible, which is essential during audits and regulatory reviews.
What features do electronic lab notebooks offer to manage large volumes of information?
Features include searchable databases and customizable templates that help researchers manage large amounts of information effectively.
How do electronic lab notebooks support collaboration among research teams?
ELNs allow simultaneous access to documents without the risk of version conflicts, enhancing collaboration among team members.
How do electronic lab notebooks align with sustainability efforts in research?
The use of electronic lab notebooks minimizes unnecessary paper consumption, supporting eco-friendly practices in research.