


Veeva eTMF significantly enhances efficiency in clinical trials by:
This article illustrates how its automation and centralized platform facilitate:
Ultimately, Veeva eTMF transforms the clinical trial landscape, leading to improved outcomes that are essential in today’s competitive environment.
Veeva eTMF stands at the forefront of transforming clinical trials, delivering innovative solutions that streamline document management and enhance collaboration among research teams. As the demand for faster and more efficient trials escalates, it becomes crucial for stakeholders in the Medtech and Biopharma sectors to understand how Veeva eTMF can significantly reduce timelines and improve compliance.
What challenges do clinical research teams encounter in maintaining efficiency?
How can Veeva eTMF effectively address these issues to propel studies forward?
Bioaccess leverages Veeva's electronic document management system to accelerate research studies in Latin America, ensuring that all essential documents are systematically organized and readily accessible in real-time. This integration not only facilitates quicker regulatory submissions but also enhances communication among stakeholders, significantly reducing the time from study initiation to patient enrollment.
With Bioaccess's specialized expertise and adaptability in managing:
the implementation of a digital management system upholds a high standard of compliance while expediting the research process. This positions it as an invaluable asset for innovators in the Medtech and Biopharma sectors, empowering them to achieve patient enrollment 50% faster and attain substantial cost savings with FDA-ready data.

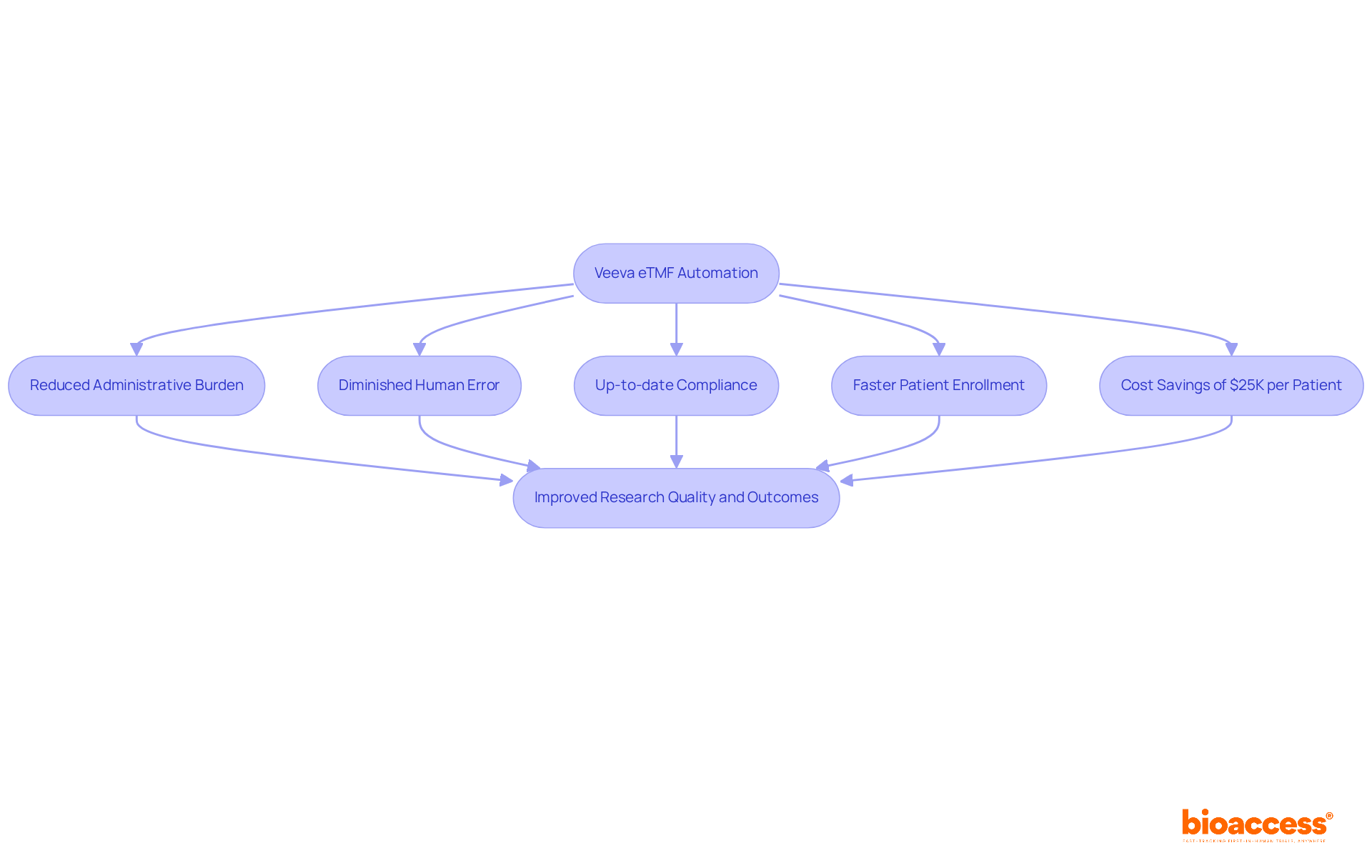
The platform revolutionizes document management in research studies by automating procedures that allow research teams to concentrate on essential tasks rather than administrative burdens. This automation markedly diminishes the risk of human error, guaranteeing that all documents remain up-to-date and compliant with regulatory standards. Coupled with bioaccess®'s ability to enroll treatment-naive cardiology or neurology groups 50% faster than Western locations and achieve $25K savings per patient with FDA-ready data, the process accelerates even further. Consequently, the quality of research and outcomes improves significantly. With 75% of healthcare leaders recognizing the potential of automation to enhance operational efficiency, Veeva's electronic document management system, alongside bioaccess®, stands out as a crucial tool in streamlining workflows and optimizing research management.
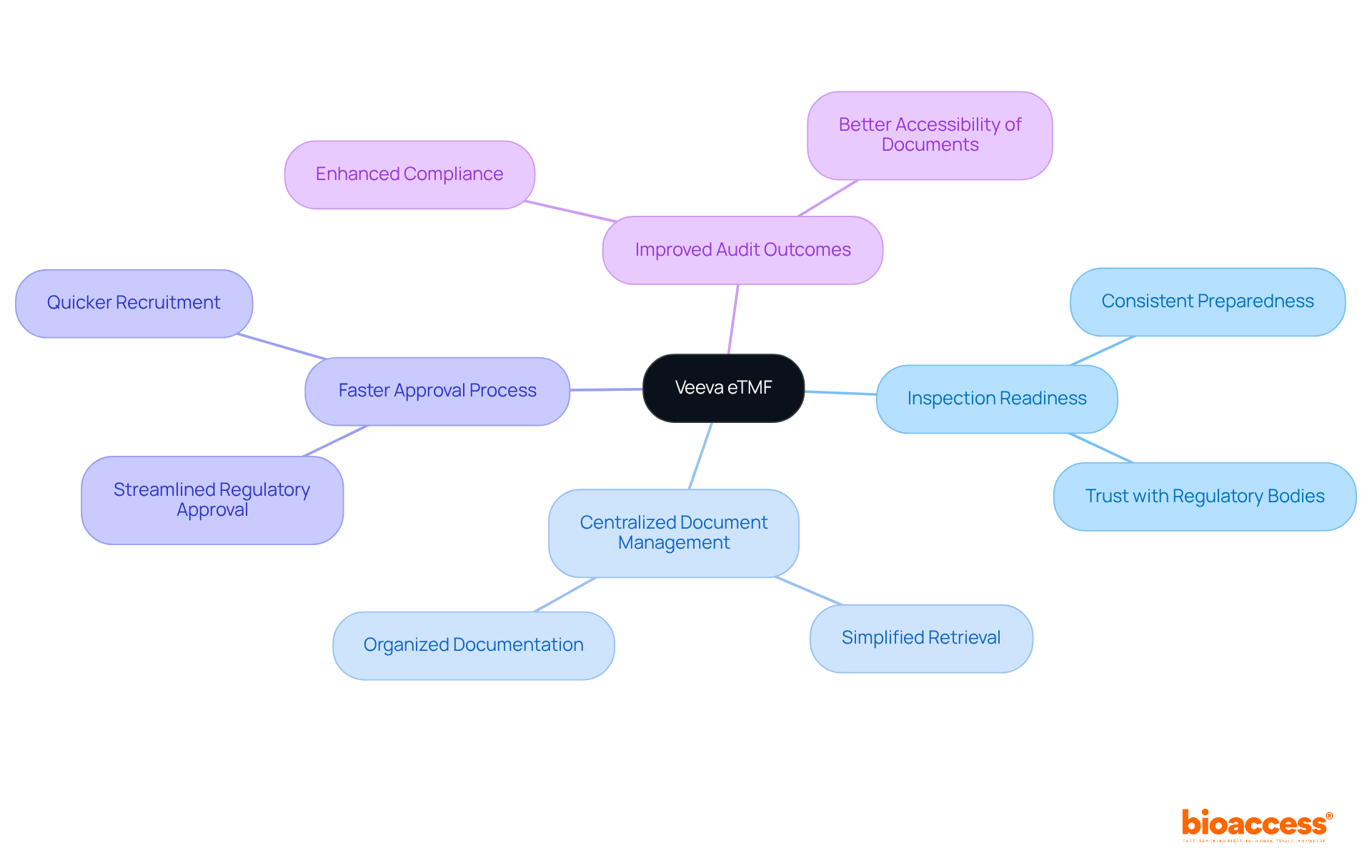
Veeva eTMF clinical trials empower research teams to consistently maintain inspection preparedness. By offering a centralized repository for all essential documents, the platform simplifies the retrieval of information during eTMF clinical trials. This proactive strategy not only builds trust with regulatory bodies but also bolsters the credibility of research findings.
With bioaccess®'s ability to secure regulatory approval in just 6-8 weeks and recruit treatment-naive cardiology or neurology groups 50% faster than Western locations, studies experience a more streamlined approval process. This synergy ultimately expedites the journey to market for innovative therapies.
Moreover, centralized document management significantly improves audit outcomes in eTMF clinical trials, ensuring that all necessary documentation is readily accessible and organized—an essential factor for compliance and efficiency in such healthcare studies.
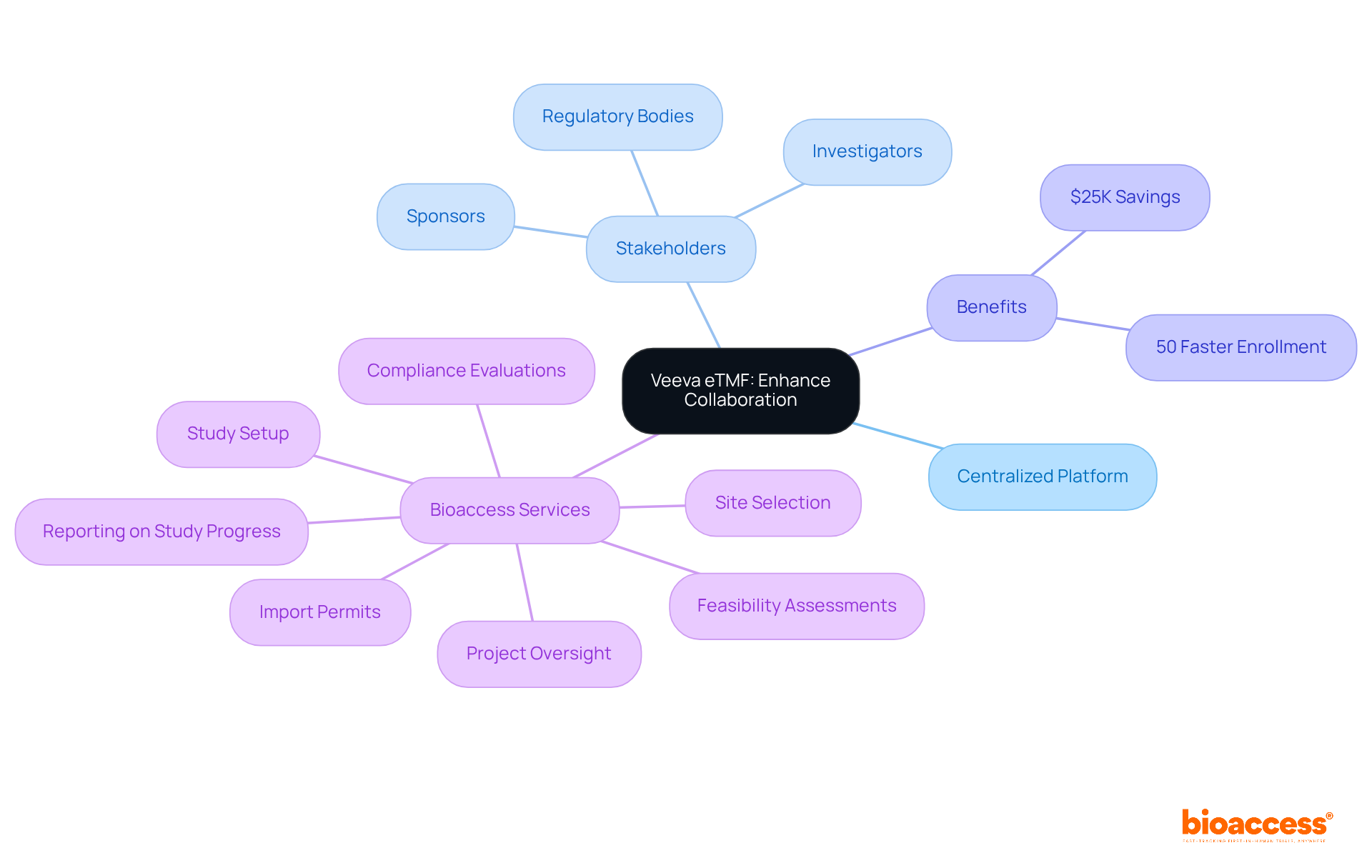
The ETMF clinical trials significantly enhance collaboration among research partners by offering a centralized platform for document access and communication. This functionality allows all stakeholders—including sponsors, investigators, and regulatory bodies—to engage more effectively. Improved teamwork not only simplifies processes but also accelerates decision-making and problem-solving, which are crucial in the dynamic landscape of medical studies.
Organizations utilizing bioaccess's functionalities can achieve patient enrollment 50% faster than traditional methods, resulting in substantial savings of $25K per patient with FDA-ready data—eliminating rework and delays.
Bioaccess's comprehensive research study management services cover:
This integration of shared platforms, such as bioaccess's services, is revolutionizing the operational dynamics of clinical research teams conducting etmf clinical trials, fostering a more agile and responsive environment that ultimately enhances patient outcomes.
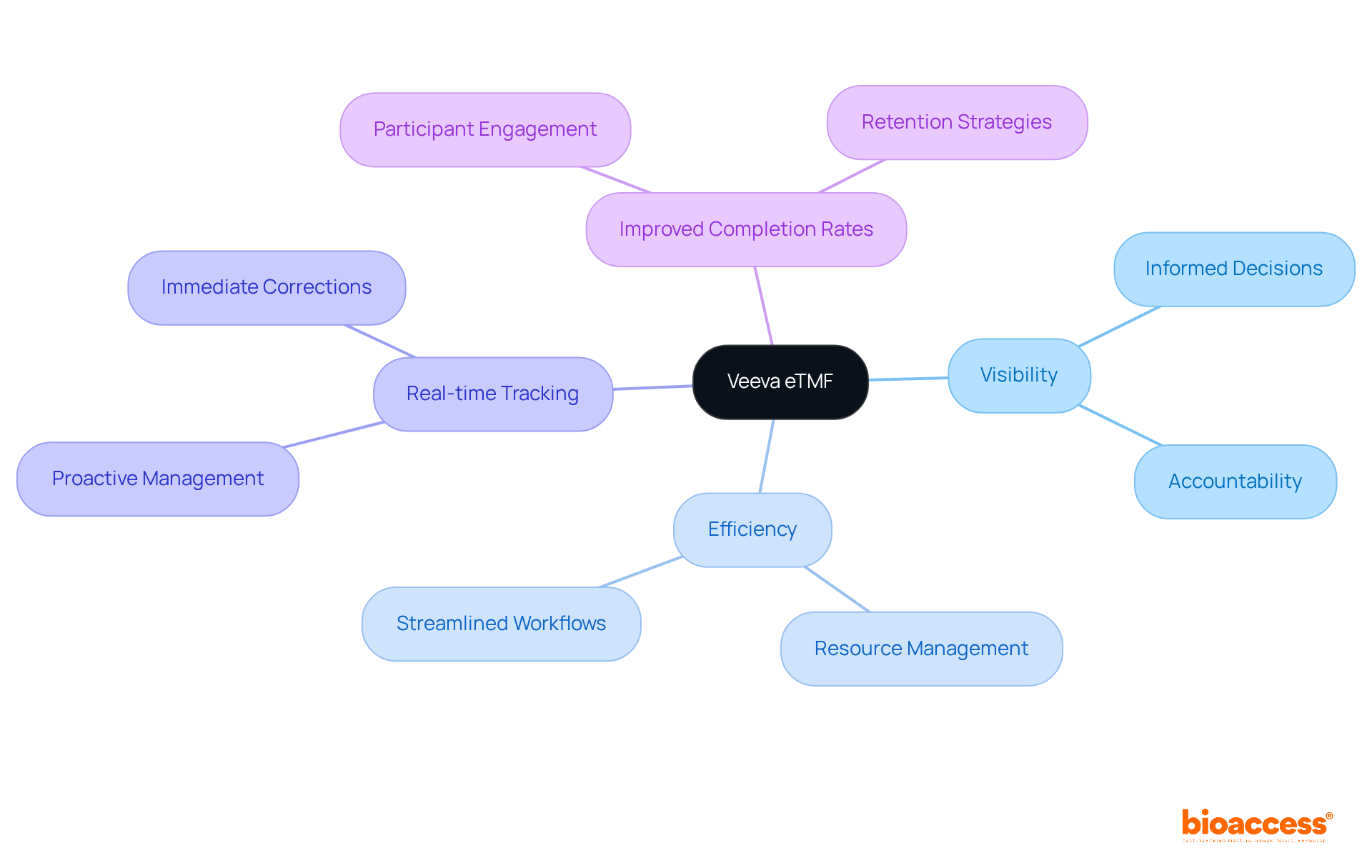
Veeva eTMF provides unparalleled insight into study management, empowering teams to monitor progress and swiftly identify bottlenecks. This real-time tracking capability is crucial for proactive resource and timeline management, ensuring that experiments stay on schedule. With vital information readily accessible, research managers can make informed decisions that significantly enhance overall efficiency.
Research indicates that eTMF clinical trials utilizing advanced tracking systems experience improved completion rates, alongside a notable increase in participant engagement and retention. Expert insights reveal that the integration of such technologies in eTMF clinical trials not only streamlines workflows but also fosters a culture of accountability and transparency among stakeholders, ultimately resulting in elevated success rates in clinical studies.
Veeva eTMF is essential in ensuring quality assurance, providing comprehensive tools for tracking document versions, approvals, and compliance checks. This systematic approach guarantees that all documentation adheres to regulatory standards while upholding the highest quality. By implementing rigorous quality assurance processes, research teams can significantly bolster the reliability of their results, thereby fostering trust among stakeholders. The platform's ability to deliver real-time insights into document compliance not only streamlines workflows but also ensures that all necessary documentation is readily accessible and up-to-date. This accessibility is crucial for maintaining regulatory compliance throughout the entire process lifecycle.
Veeva eTMF revolutionizes document management and collaboration in research studies, leading to significant improvements in timelines and reductions in setbacks. By automating routine administrative tasks, the platform empowers teams to concentrate on essential activities that propel the project forward. This efficiency is crucial, given that nearly 80% of clinical studies fail to meet their initial enrollment targets, frequently due to delays in database management and participant recruitment.
With this platform, organizations can optimize workflows, resulting in expedited site activation and improved data quality. Notably, eTMF clinical trials that utilize an electronic trial master file have reported a 30-36% reduction in administrative workload, enabling faster decision-making and implementation.
Furthermore, the platform's features align with the industry's shift towards interconnected studies, which are increasingly recognized as essential for navigating the complexities of modern research. By enhancing collaboration among sponsors, CROs, and sites, the platform accelerates study progress and contributes to the timely delivery of innovative therapies to patients.
The platform effectively addresses prevalent challenges in etmf clinical trials, including document inconsistencies, communication failures, and compliance issues. By providing a centralized platform for managing all trial-related documents and communications, etmf clinical trials streamline processes and enhance collaboration among teams. For instance, the TMF Transfer feature automates the exchange of completed TMFs between sponsors and CROs, significantly reducing manual work and improving document quality. This capability not only ensures that TMF documents are prepared for inspection but also enhances overall trial readiness for etmf clinical trials.
Furthermore, centralized monitoring systems, akin to those integrated with similar platforms, facilitate real-time data access, enabling proactive identification of discrepancies. This approach has demonstrated effectiveness, with 83% of sites reporting improved data quality scores after addressing risk signals identified through centralized monitoring. By focusing on high-value activities and automating routine tasks, Veeva empowers research teams to concentrate on delivering high-quality studies in etmf clinical trials without unnecessary distractions.
Expert opinions underscore the importance of utilizing centralized platforms to mitigate document discrepancies in medical research. By implementing organized tools for risk evaluations and monitoring essential risk indicators, organizations can prioritize risks to patient safety and data integrity, ultimately enhancing the efficiency and reliability of research studies.
Veeva eTMF employs advanced technologies, such as cloud storage and mobile access, to significantly enhance study efficiency. This platform empowers research teams to access essential documents from any location, facilitating real-time collaboration and improving communication among stakeholders. The integration of AI-driven analytics enables teams to analyze data effectively, leading to informed decision-making. Furthermore, cloud tools allow contract research organizations (CROs) to oversee multiple clients' studies with improved supervision and quicker reporting, thereby enhancing operational efficiency. By embracing these innovative solutions, the platform simplifies research processes and boosts overall operational efficiency, enabling faster adjustments and better results in etmf clinical trials.
Notably, bioaccess® accelerates patient enrollment by 50%, resulting in substantial savings of $25K per patient with FDA-ready data—eliminating rework and delays. This capability addresses common obstacles faced by medtech and biopharma startups during initial-phase studies. To maximize the benefits of bioaccess®, research directors should prioritize the establishment of robust cybersecurity protocols to ensure compliance with regulations such as GDPR and HIPAA, safeguarding sensitive information throughout the research process.
The platform is revolutionizing research studies by delivering a comprehensive solution that significantly enhances efficiency across all phases. This innovation streamlines document management, fosters collaboration, and ensures compliance, effectively addressing the critical needs of clinical research teams.
By implementing the electronic master file system, organizations can refine their research processes, leading to expedited study initiations and improved data accuracy. For example, studies indicate that automating essential processes, such as the collection of site-specific documents, can elevate trial quality and speed, with 61% of users reporting favorable outcomes.
Moreover, the integration of AI within the platform enhances real-time document management, boosting visibility and ensuring a constant state of inspection readiness. As the industry pivots towards digital solutions, the adoption of Veeva eTMF in etmf clinical trials empowers organizations to propel medical knowledge forward and significantly enhance patient care.

Veeva eTMF stands as a transformative tool in clinical trials, fundamentally enhancing efficiency and streamlining processes across various stages of research. By integrating advanced document management capabilities, this platform empowers organizations to maintain compliance, foster collaboration, and ultimately accelerate the journey from study initiation to patient enrollment.
Key insights from the discussion underscore the significant benefits of Veeva eTMF, such as:
Collectively, these features contribute to a more agile research environment, enabling teams to concentrate on high-value activities while minimizing delays and ensuring quality assurance throughout the trial lifecycle.
As the clinical research landscape continues to evolve, embracing innovative solutions like Veeva eTMF is essential for organizations striving to optimize their operations. By leveraging these advancements, research teams can enhance their efficiency and drive better patient outcomes, paving the way for the successful delivery of groundbreaking therapies. The adoption of such technologies transcends mere competitive advantage; it represents a crucial step towards advancing medical knowledge and improving healthcare delivery on a global scale.
What is Bioaccess and how does it enhance clinical trials?
Bioaccess leverages Veeva's electronic document management system to accelerate research studies in Latin America by systematically organizing and providing real-time access to essential documents. This integration facilitates quicker regulatory submissions and enhances communication among stakeholders, reducing the time from study initiation to patient enrollment.
What types of studies does Bioaccess specialize in managing?
Bioaccess specializes in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
How does the integration of Veeva eTMF improve document management in research studies?
Veeva eTMF automates document management procedures, allowing research teams to focus on essential tasks rather than administrative burdens. This automation reduces human error and ensures that all documents remain up-to-date and compliant with regulatory standards.
What are the benefits of using Bioaccess and Veeva eTMF together?
The combination allows for 50% faster enrollment of treatment-naive cardiology or neurology groups compared to Western locations and achieves significant cost savings, approximately $25K per patient, with FDA-ready data. This accelerates the research process and improves the quality of research outcomes.
How does Veeva eTMF ensure inspection readiness throughout clinical trials?
Veeva eTMF provides a centralized repository for all essential documents, simplifying the retrieval of information during clinical trials. This proactive strategy builds trust with regulatory bodies and improves the credibility of research findings.
What is the impact of centralized document management on audit outcomes in clinical trials?
Centralized document management significantly improves audit outcomes by ensuring that all necessary documentation is readily accessible and organized, which is crucial for compliance and efficiency in healthcare studies.
How quickly can Bioaccess secure regulatory approval for studies?
Bioaccess can secure regulatory approval in just 6-8 weeks, facilitating a more streamlined approval process for studies.