


Navigating the complex landscape of clinical labeling compliance is essential for organizations committed to ensuring patient safety and regulatory adherence. With guidelines from regulatory bodies like the FDA and EMA constantly evolving, understanding these requirements is not just beneficial; it’s crucial for mitigating risks and enhancing operational efficiency. As the stakes rise, organizations must ask: how can they effectively implement best practices to streamline their labeling processes while keeping pace with technological advancements? This article explores four key strategies that empower teams to achieve compliance, boost accuracy, and foster a culture of continuous improvement in clinical labeling.
To achieve adherence to clinical guidelines, understanding the regulations set by organizations like the FDA and EMA is crucial. The FDA's 21 CFR Part 312 lays out essential requirements for investigational new drugs, while the EU MDR specifies necessary information for medical device markings. Familiarity with these regulations not only ensures legal compliance but also enhances patient safety and product traceability. For example, the EU MDR requires labels to include a Unique Device Identifier (UDI), which bolsters traceability and oversight, with compliance deadlines varying by device category.
Regularly reviewing updates from regulatory agencies and utilizing resources such as the FDA's guidance documents can help keep practices current and compliant. The FDA's guidance on the Clinical Studies Section of packaging, for instance, clarifies which studies should be included, ensuring that all vital information is effectively communicated. Additionally, the use of standardized symbols under the EN ISO 15223-1:2021 standard enhances clarity and efficiency in signage, addressing space constraints and multilingual needs.
By focusing on these compliance aspects, producers can mitigate risks associated with clinical labelling errors, which can cost between $150,000 and $2.5 million if detected before distribution. Furthermore, losing market access due to identification issues can drain millions from your business, underscoring the critical need for accurate and compliant identification practices. With the expertise of Ana Criado, Director of Compliance at bioaccess, who has extensive oversight experience in Colombia, and Katherine Ruiz, a specialist in Oversight for Medical Devices and In Vitro Diagnostics in Colombia, organizations can navigate these complex requirements more effectively.

To implement streamlined tagging processes, organizations must adopt standardized protocols that clearly define each step of the tagging workflow. This approach is crucial for clinical research, as clinical labelling ensures compliance and efficiency. Developing reusable templates for labels that meet regulatory requirements can be applied across various studies, enhancing consistency and reliability.
Employing project management tools effectively monitors the progress of tagging tasks, ensuring deadlines are consistently met. For instance, integrating a centralized management system for tags allows for real-time updates and approvals, significantly reducing the time allocated for revisions. Regular audits of the marking process are essential; they help identify bottlenecks and areas for improvement, ensuring that the marking workflow remains both efficient and compliant.
Statistics indicate that adopting standardized identification processes can lead to substantial time savings, enhancing overall operational efficiency. The Data Collection and Tagging Market was valued at USD 3.0 Billion in 2023 and is anticipated to reach USD 29.2 Billion by 2032, reflecting the increasing significance of efficient tagging strategies. Moreover, the growing need for multilingual identification is vital for worldwide reach and adherence, guaranteeing that instructions and safety information are accessible to diverse populations.
Non-compliance with clinical labelling regulations poses significant risks, including health hazards and costly product withdrawals. This underscores the necessity of maintaining rigorous marking standards. As organizations navigate these challenges, the importance of collaboration and adherence to established protocols cannot be overstated.
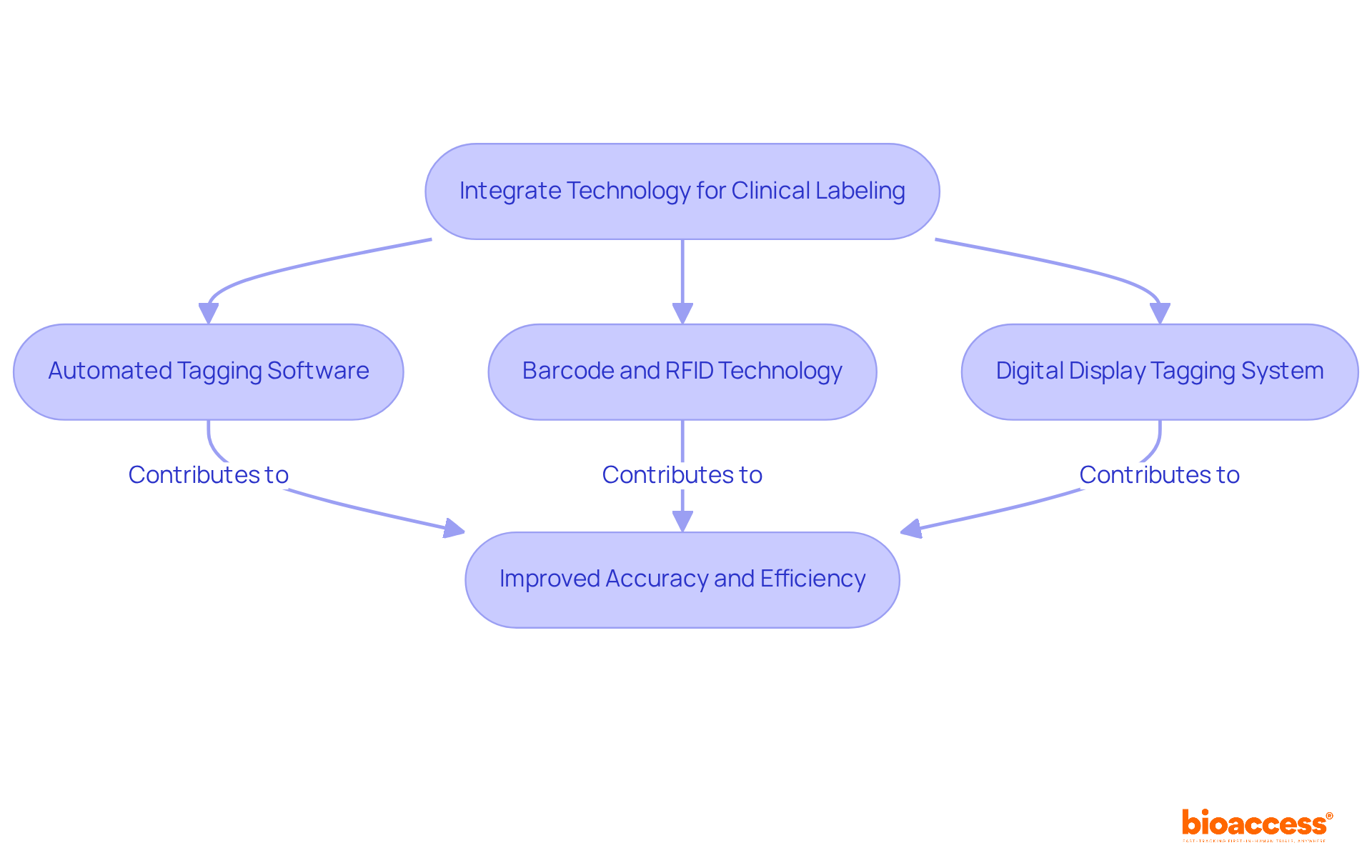
Incorporating technology into clinical labelling significantly improves both accuracy and efficiency. Automated tagging software, for instance, minimizes manual input errors by generating tags based on predefined templates and data entries. Furthermore, the use of barcode and RFID technology streamlines tracking and inventory management of clinical supplies, ensuring that the correct tags are applied to the appropriate products.
Consider the implementation of a digital display tagging system, which allows for real-time updates to tag content. This capability is particularly beneficial in dynamic clinical environments where changes occur frequently. By embracing these technologies, organizations can not only improve adherence but also simplify their processes for clinical labelling, ultimately leading to more efficient clinical operations.
The integration of such technologies is not merely an enhancement; it is a necessity in today’s fast-paced landscape of clinical labelling. Organizations that adopt these innovations position themselves at the forefront of clinical research, ready to tackle the challenges of accuracy and efficiency head-on.
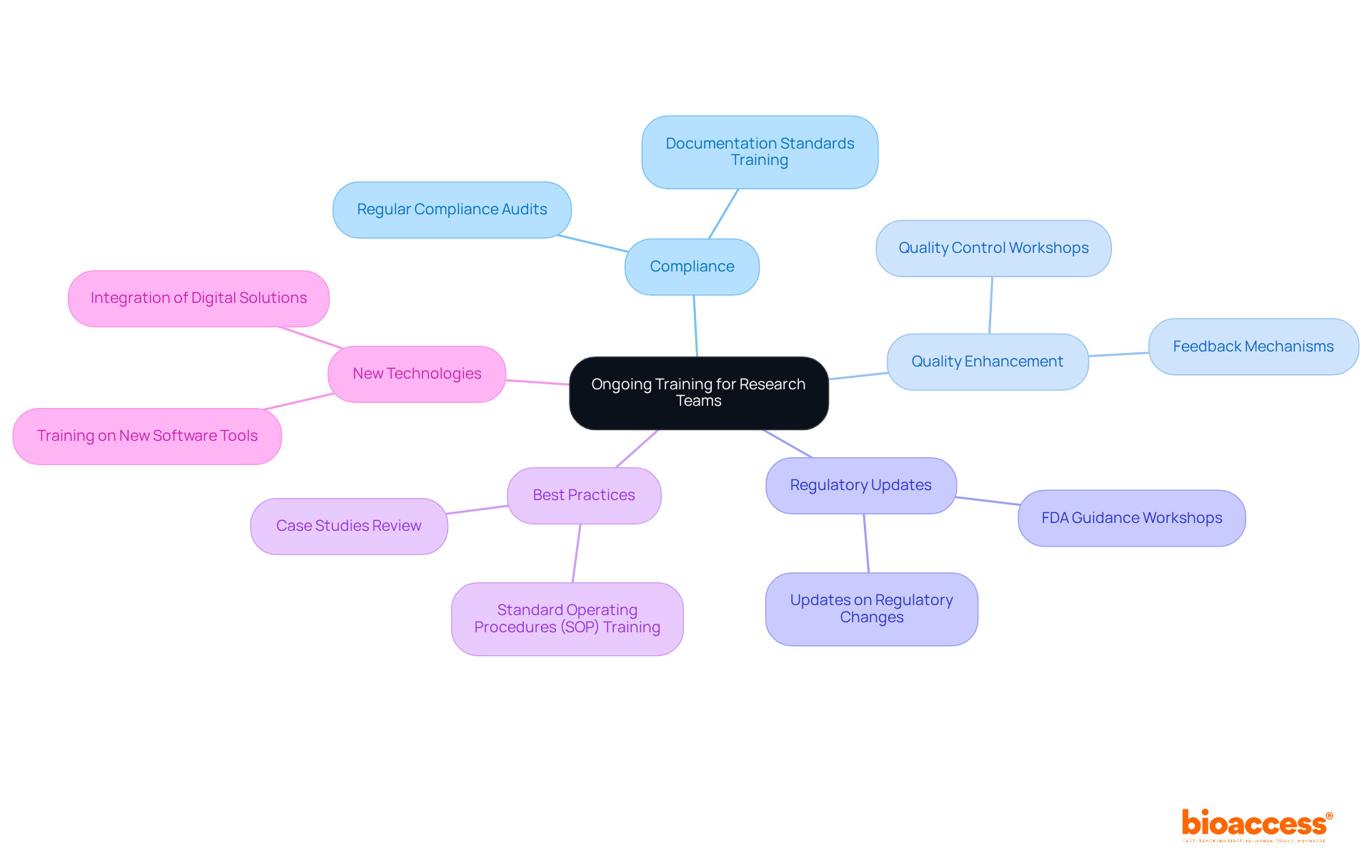
Investing in continuous education for research teams is not just beneficial; it’s essential for maintaining compliance and enhancing the quality of clinical documentation. Regular training sessions must focus on the latest regulatory updates, best practices for identification, and the integration of new technologies. For example, workshops that delve into the implications of the latest FDA guidance can empower teams to adapt their packaging strategies effectively.
Moreover, cultivating a culture of continuous learning through online courses and certifications enables team members to stay abreast of industry trends and innovations. This proactive approach not only equips teams with the necessary knowledge and skills but also fosters a sense of empowerment and engagement. By prioritizing training, organizations can ensure their teams are well-prepared to execute labeling tasks both effectively and compliantly.
Understanding and adhering to clinical labeling compliance is not merely a regulatory obligation; it is a crucial element in ensuring patient safety and operational efficiency. This article underscores the necessity of being well-versed in the regulatory landscape, implementing streamlined processes, leveraging technology, and investing in ongoing training for research teams. By prioritizing these best practices, organizations can significantly enhance their compliance efforts and mitigate the risks associated with clinical labeling errors.
Key insights discussed include:
Ultimately, the significance of clinical labeling compliance transcends mere adherence to regulations; it fosters a culture of excellence within clinical research. Organizations are encouraged to take proactive steps toward implementing these best practices, ensuring they not only meet current standards but also position themselves for future advancements in clinical labeling. Embracing these strategies will enhance compliance and contribute to the overall success and integrity of clinical trials.
Why is it important to understand regulatory requirements for clinical labeling?
Understanding regulatory requirements is crucial for ensuring legal compliance, enhancing patient safety, and improving product traceability in clinical guidelines.
What regulations do the FDA and EMA set for clinical labeling?
The FDA's 21 CFR Part 312 outlines essential requirements for investigational new drugs, while the EU MDR specifies necessary information for medical device markings.
What is the Unique Device Identifier (UDI) and why is it important?
The UDI is a requirement under the EU MDR that enhances traceability and oversight of medical devices. It is crucial for ensuring compliance and can vary by device category.
How can organizations stay updated on regulatory requirements?
Organizations can stay updated by regularly reviewing updates from regulatory agencies and utilizing resources such as the FDA's guidance documents.
What information should be included in the Clinical Studies Section of packaging?
The FDA's guidance provides clarity on which studies should be included in the Clinical Studies Section to ensure all vital information is communicated effectively.
How does the EN ISO 15223-1:2021 standard contribute to clinical labeling?
This standard promotes the use of standardized symbols, enhancing clarity and efficiency in signage while addressing space constraints and multilingual needs.
What are the potential costs associated with clinical labeling errors?
Clinical labeling errors can cost between $150,000 and $2.5 million if detected before distribution, highlighting the importance of compliance.
What risks do organizations face due to inaccurate identification practices?
Organizations risk losing market access due to identification issues, which can result in significant financial losses.
Who can help organizations navigate complex regulatory requirements?
Experts like Ana Criado, Director of Compliance at bioaccess, and Katherine Ruiz, a specialist in Oversight for Medical Devices and In Vitro Diagnostics, can provide guidance in navigating these requirements.