


Ensuring clinical compliance is not merely a regulatory necessity; it stands as a cornerstone of ethical research that safeguards participant safety and upholds data integrity. As organizations prepare for the evolving landscape of clinical trials, grasping and implementing best practices in compliance becomes essential for success.
But what are the most effective strategies to navigate the complexities of clinical research compliance, especially as the industry encounters new challenges and technologies? Delving into these critical practices unveils how organizations can bolster their compliance efforts and achieve superior outcomes in their clinical trials.
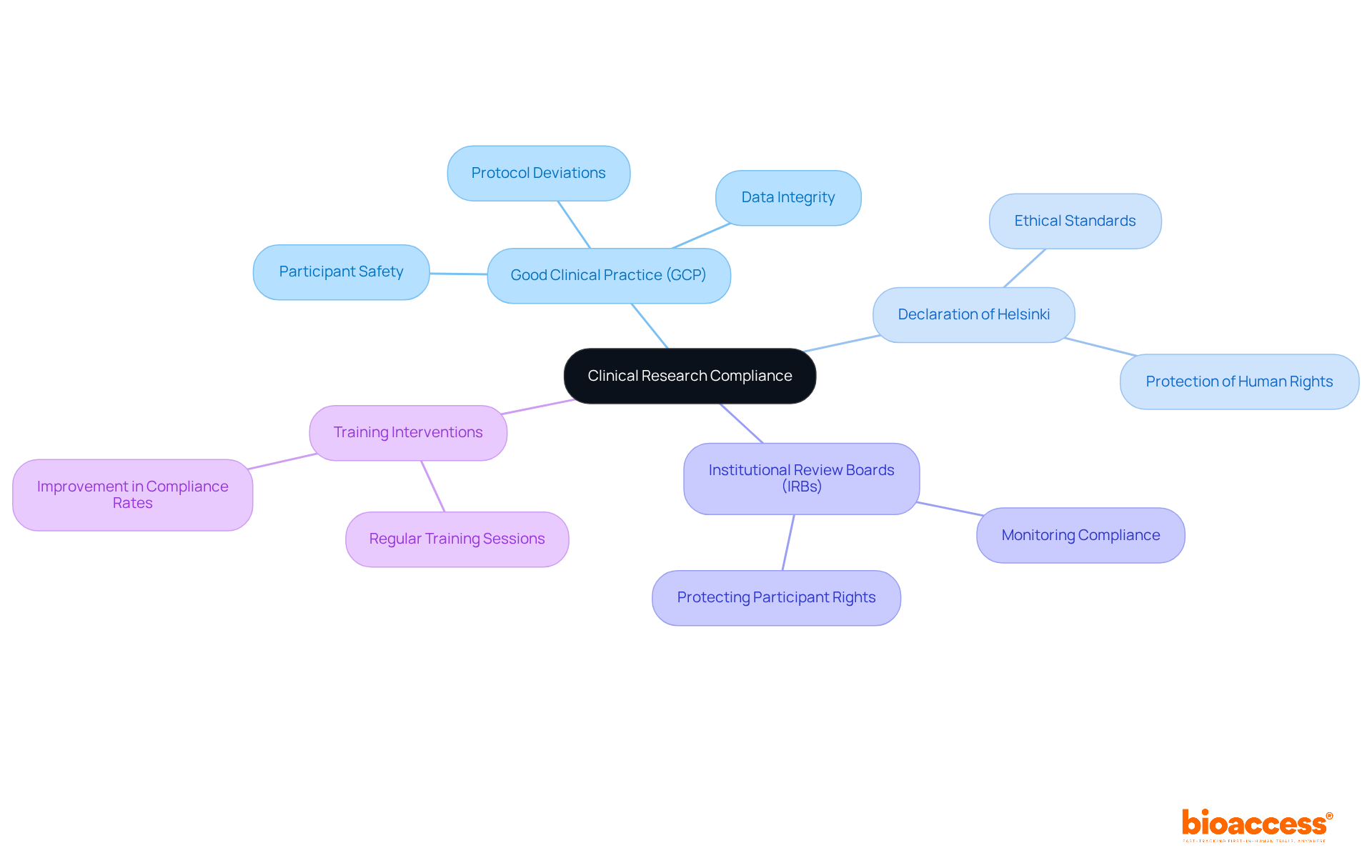
Clinical compliance in clinical research adherence is governed by a range of regulations and guidelines, with Good Clinical Practice (GCP) guidelines being paramount. These guidelines, in conjunction with the Declaration of Helsinki and local regulatory requirements, are essential for ensuring clinical compliance, safeguarding participant safety, ensuring data integrity, and maintaining ethical standards throughout the research process. Organizations must recognize the functions of Institutional Review Boards (IRBs) and regulatory entities, which play a crucial role in monitoring clinical compliance and protecting participant rights. Consistent education and updates on these regulations foster a culture of clinical compliance among research teams.
For instance, a clinical research site that conducts regular training sessions on clinical compliance has reported a significant reduction in protocol deviations. This proactive approach not only streamlines testing operations but also enhances participant safety. As we move into 2025, the focus on clinical compliance with GCP guidelines has intensified, with organizations acknowledging their substantial influence on outcomes. Compliance rates have improved, with studies indicating that tailored training interventions can lead to a 20% increase in assessment scores post-training. Such improvements underscore the necessity of clinical compliance with GCP adherence to achieve successful clinical trial execution.
To ensure compliance success, organizations must prioritize several essential factors:
Clear Documentation: Maintaining thorough and accurate documentation of all trial-related activities-including protocols, consent forms, and regulatory submissions-is vital. This practice not only supports clinical compliance but also facilitates audits and inspections, ensuring that all data is readily accessible and verifiable.
Comprehensive Clinical Trial Management Services: Engaging in feasibility assessments and site selection is crucial for determining the most appropriate research locations and principal investigators (PIs). This foundational step ensures that assessments are arranged effectively and adhere to regulatory requirements. Additionally, reviewing and providing feedback on study documents, along with acquiring import permits, are vital elements that enhance adherence.
Regular Audits: Conducting internal audits is essential for assessing adherence to protocols and regulations. These audits help identify potential issues before they escalate, thereby ensuring clinical compliance and fostering a culture of continuous improvement. For instance, a biopharma firm that implemented regular internal audits noted a 30% reduction in compliance-related problems, which accelerated approval processes and strengthened connections with regulatory agencies.
Stakeholder Engagement: Open communication with all stakeholders-including sponsors, regulatory bodies, and participants-is critical. Involving stakeholders facilitates the swift and efficient resolution of regulatory issues, thereby improving overall study integrity.
Risk Management: Establishing a robust risk management framework is essential for identifying, evaluating, and mitigating regulatory risks throughout the study lifecycle. This proactive approach not only safeguards participant safety but also reinforces the credibility of the research. Moreover, effective project management and oversight are crucial to ensure that all elements of the study achieve clinical compliance with regulatory standards.
The impact of these practices on clinical study adherence rates is significant. Research indicates that organizations with robust internal audit procedures experience higher adherence levels and fewer documentation errors. By implementing these best practices, organizations can enhance their operational efficiency and ensure successful clinical trial outcomes.

Creating thorough educational programs for researchers is essential for promoting a culture of clinical compliance in clinical research. The following key components are critical for an effective training program:
For instance, a CRO that redesigned its training program to incorporate interactive learning and frequent updates reported a 40% increase in regulatory knowledge among staff, leading to enhanced study outcomes. This example underscores the importance of investing in comprehensive training to achieve clinical compliance and foster a knowledgeable workforce.

Utilizing technology for regulatory oversight significantly enhances the efficiency and effectiveness of clinical trials, particularly through bioaccess's specialized services. Key technologies include:
Electronic Data Capture (EDC): Implementing EDC systems streamlines data collection and facilitates real-time monitoring of adherence to research protocols. These systems can reduce operational costs by up to 30% and enhance data integrity by minimizing manual entry errors, achieving error rates as low as 0.14% with double-data entry methods. bioaccess® utilizes EDC systems to support various study types, including Early-Feasibility and First-In-Human studies, ensuring robust data management.
Compliance Management Software: Specialized software for tracking compliance metrics, managing documentation, and facilitating audits automates many compliance-related tasks, thereby reducing the risk of human error. Compliance officers report substantial time savings, allowing them to focus on strategic initiatives rather than manual data entry. bioaccess® incorporates such software into its clinical management services to ensure clinical compliance with regulatory standards.
Remote Monitoring Tools: Remote monitoring technologies allow supervision of study sites, ensuring compliance with protocols without the necessity for regular on-site visits. This method is especially advantageous in decentralized studies, where upholding regulations can be difficult due to geographical dispersion. bioaccess® employs these tools to enhance oversight in its clinical studies across Latin America.
Artificial Intelligence (AI): AI-powered solutions for predictive analytics can pinpoint potential regulatory risks before they develop into major concerns, improving proactive management of research protocols. bioaccess® utilizes AI technologies to enhance regulatory monitoring and improve study results.
For instance, a Medtech firm that implemented both an EDC system and regulatory oversight software reported an impressive 50% decrease in errors related to regulations. This led to faster trial completion and improved data integrity, highlighting the substantial benefits of integrating technology into clinical compliance monitoring. Additionally, a case study by AVS Life Sciences demonstrated how modernizing a biotechnology GMP facility with EDC software improved quality assurance and regulatory adherence, providing a practical example of these technologies' real-world advantages.
However, it is essential to acknowledge that healthcare professionals in emerging regions may prefer traditional paper methods, which can pose challenges in the implementation of EDC systems. Addressing these potential pitfalls is crucial for a balanced view of the technology's adoption.

Ensuring clinical compliance success is a multifaceted endeavor that hinges on understanding fundamental regulations, implementing effective strategies, and leveraging technology. A proactive approach to clinical compliance not only safeguards participant safety but also enhances the integrity and efficiency of clinical trials.
Key arguments highlight the importance of:
as foundational practices that contribute to compliance success. Furthermore, comprehensive training programs tailored to the specific roles of researchers are essential. Integrating advanced technologies, such as electronic data capture and compliance management software, streamlines processes and minimizes errors.
Organizations involved in clinical research must adopt these best practices to foster a culture of compliance. By prioritizing education, embracing innovative technologies, and maintaining open lines of communication among all stakeholders, the path to successful clinical trials becomes clearer. This commitment to clinical compliance not only enhances research outcomes but ultimately serves the greater goal of advancing healthcare and improving patient safety.
What is clinical research compliance?
Clinical research compliance refers to adherence to a range of regulations and guidelines that govern clinical research, primarily the Good Clinical Practice (GCP) guidelines, the Declaration of Helsinki, and local regulatory requirements.
Why are GCP guidelines important in clinical research?
GCP guidelines are essential for ensuring clinical compliance, safeguarding participant safety, ensuring data integrity, and maintaining ethical standards throughout the research process.
What role do Institutional Review Boards (IRBs) play in clinical research compliance?
IRBs are crucial in monitoring clinical compliance and protecting participant rights, ensuring that research is conducted ethically and in accordance with regulatory standards.
How can organizations promote a culture of clinical compliance?
Organizations can promote a culture of clinical compliance by providing consistent education and updates on regulations, as well as conducting regular training sessions for research teams.
What impact does regular training on clinical compliance have?
Regular training on clinical compliance has been shown to significantly reduce protocol deviations and enhance participant safety, streamlining testing operations.
How has the focus on clinical compliance evolved as we approach 2025?
The focus on clinical compliance with GCP guidelines has intensified, with organizations recognizing their substantial influence on outcomes and improving compliance rates.
What are the benefits of tailored training interventions in clinical research?
Tailored training interventions can lead to a notable increase in assessment scores, with studies indicating a 20% improvement post-training, highlighting the necessity of GCP adherence for successful clinical trial execution.