


The best practices for GMP (Good Manufacturing Practices) in medical device compliance are crucial for ensuring product quality and safety. Establishing a robust Quality Management System (QMS) is foundational, as it sets the framework for maintaining thorough documentation. Furthermore, ensuring personnel training is essential, as it equips the workforce with the necessary skills to adhere to compliance standards. Effective labeling and packaging controls must also be implemented to meet regulatory requirements and mitigate the risks associated with non-compliance. Organizations that embrace these practices not only enhance their operational efficacy but also significantly reduce the risk of severe penalties.
Good Manufacturing Practices (GMP) serve as the backbone of quality assurance in the medical device industry, establishing the standards that ensure safety and efficacy from design to distribution. As regulatory scrutiny intensifies, organizations face a pressing need to not only comprehend these practices but also to implement them effectively, thereby avoiding costly penalties and maintaining market access.
What strategies can be adopted to navigate the complexities of GMP compliance? Furthermore, how can companies cultivate a culture of excellence that safeguards both their products and their reputation?
Good Manufacturing Practices (GMP) for medical devices encompass a comprehensive set of regulations and guidelines that ensure their quality and safety throughout their lifecycle. Key principles include:
Grasping these fundamentals is essential for any organization involved in the manufacturing of medical devices, as they establish the foundation for GMP for medical devices and the assurance of product safety and efficacy. The market size for GMP Testing Services is projected to be substantial, highlighting the imperative of adhering to these regulations to avoid penalties and ensure market access.
For instance, the FDA's GMP for medical devices delineates minimum requirements for the methods, facilities, and controls utilized in the manufacturing, processing, and packaging of medical devices. Non-compliance with GMP for medical devices can lead to severe repercussions, including fines and potential imprisonment for manufacturers, highlighting the necessity of adhering to GMP for medical devices.
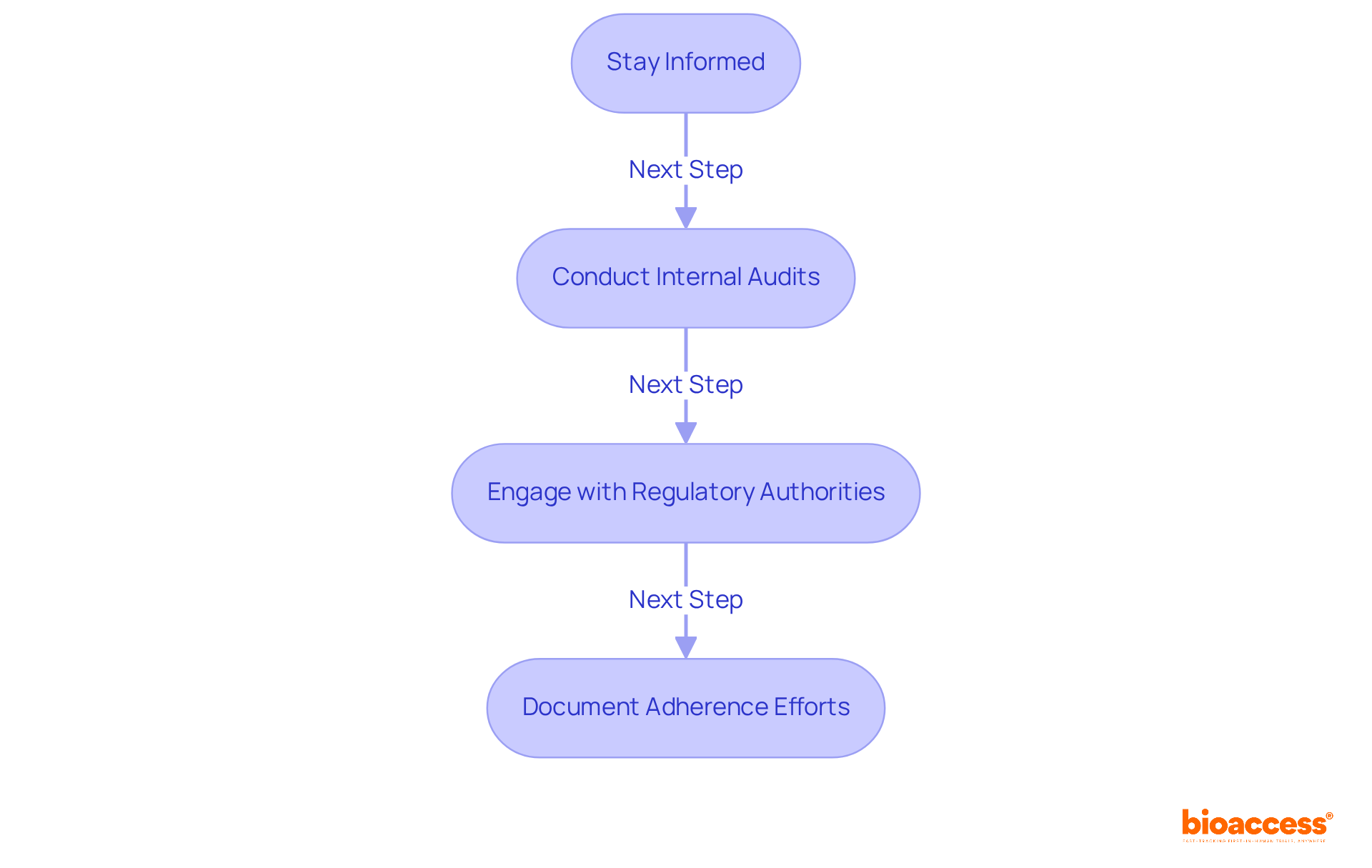
To ensure regulatory compliance in GMP for medical devices practices, organizations must take decisive steps.
Stay Informed: It is essential to regularly review updates from regulatory bodies such as the FDA and ISO to remain compliant with the latest standards and guidelines. The FDA's new Quality Management System Regulation, effective February 2, 2026, highlights the importance of GMP for medical devices and staying current with evolving regulations.
Conducting internal audits is crucial for evaluating adherence to GMP for medical devices regulations and identifying areas for improvement. These audits proactively address deficiencies, often revealing issues such as incomplete documentation and inadequate training records. Regular internal audits have demonstrated their effectiveness in strengthening documentation integrity and enhancing employee readiness for inspections, significantly reducing the risk of regulatory penalties.
Engage with Regulatory Authorities: Building connections with regulatory agencies enhances communication and provides insights into adherence expectations. This engagement assists organizations in managing the complexities of regulatory requirements and refining their adherence strategies.
Document Adherence Efforts: Maintaining detailed records of adherence activities—such as audits, training sessions, and corrective actions—is essential. Precise documentation showcases adherence to regulatory demands and is a key focus during FDA inspections.
For instance, the FDA's recent amendments to the GMP for medical devices requirements highlight the necessity for manufacturers to adapt their practices to align with evolving regulations. Businesses that proactively address regulatory concerns are better positioned to avoid costly fines and product recalls, ultimately enhancing their market standing. In fiscal year 2024, a significant rise in Warning Letters issued to medical device manufacturers emphasizes the critical need for robust adherence practices.
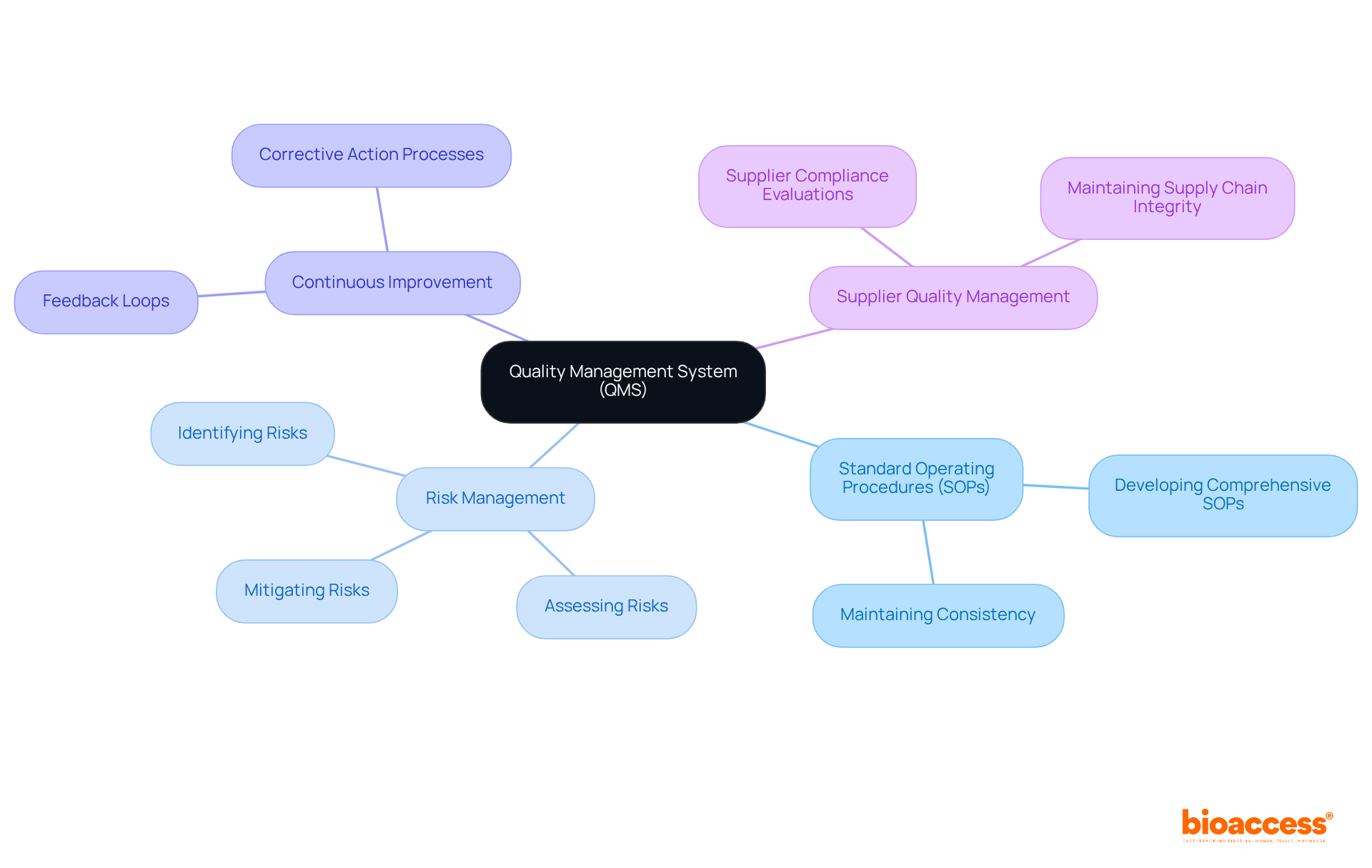
Implementing a robust Quality Management System (QMS) is essential for guaranteeing adherence to gmp for medical devices in the industry. Key components include:
Organizations can utilize Corrective and Preventive Action (CAPA) systems to document and address performance issues effectively. A well-executed QMS not only improves product standards but also simplifies adherence efforts, enabling easier navigation through regulatory inspections and audits. Additionally, companies implementing a QMS can reduce quality-related costs by an average of 15%-20%, highlighting the financial benefits of robust quality management. It is crucial to include essential elements such as document control, change control, and supplier qualification within the QMS framework to guarantee thorough adherence to FDA 21 CFR Parts 210 and 211, which outline the minimum requirements for gmp for medical devices.
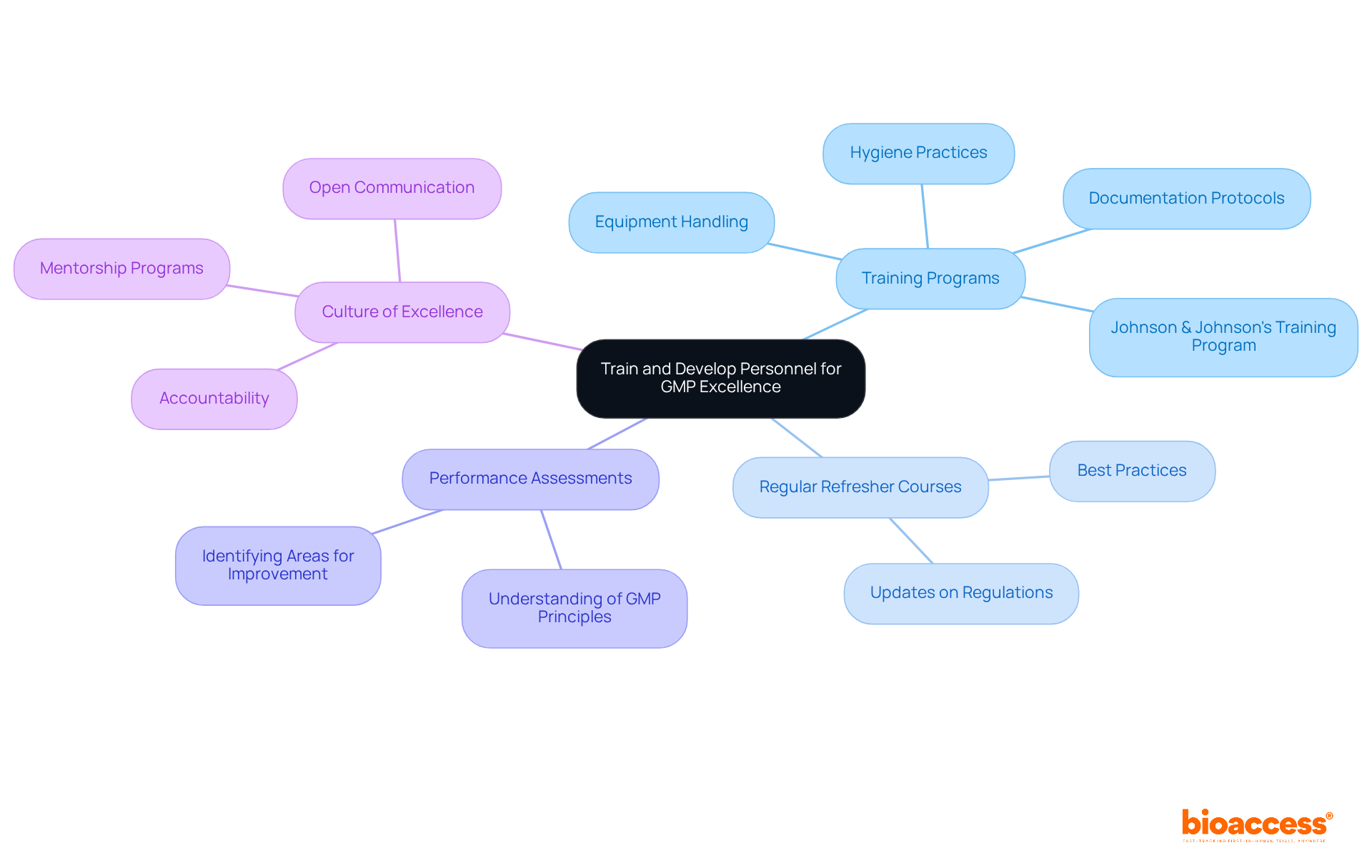
To achieve excellence in GMP for medical devices, organizations must prioritize the training and development of their personnel. Comprehensive training programs are essential; they should encompass all facets of GMP for medical devices, including:
Such initiatives ensure that employees are well-versed in the necessary standards and practices related to GMP for medical devices. Organizations with well-implemented quality and regulatory training experience a 30% reduction in product recalls, underscoring the importance of investing in comprehensive training.
Regular refresher courses are vital to keep staff informed about the latest regulations and best practices related to GMP for medical devices. Ongoing education strengthens principles and helps staff adjust to regulatory changes, thus fostering a culture of adherence to GMP for medical devices. Performance assessments should be conducted regularly to gauge employees' understanding of GMP for medical devices principles and identify areas needing improvement. This proactive strategy aids in upholding elevated criteria and ensures that staff are prepared to manage compliance tasks efficiently with respect to GMP for medical devices.
Fostering a culture of excellence is crucial. Encouraging open communication and accountability among team members enhances this culture. As Mark Bridges notes, these training programs play an instrumental role in building a culture of accountability and vigilance, which is critical for adhering to GMP for medical devices, leading to safer, higher-quality products and less regulatory risk.
For instance, organizations can implement mentorship programs where seasoned staff guide new employees through GMP practices. This knowledge transfer is essential for ensuring compliance with quality criteria, including GMP for medical devices, and nurturing a sense of responsibility among all team members. Johnson & Johnson's comprehensive training program on GMP for medical devices serves as a relevant case study, demonstrating the effectiveness of such initiatives in enhancing operational efficiency and maintaining high standards. By investing in personnel training, companies can significantly enhance compliance with GMP for medical devices, reduce errors, and ultimately improve patient safety. Notably, only about 40% of surveyed individuals rated the training courses as good, highlighting the need for improvement in training measures.
Good Manufacturing Practices (GMP) are essential for ensuring the quality and safety of medical devices. Adhering to the principles of GMP not only helps organizations maintain compliance with regulatory requirements but also fosters a culture of excellence that enhances product integrity. By implementing robust Quality Management Systems (QMS), maintaining thorough documentation, and prioritizing personnel training, manufacturers can significantly mitigate risks associated with non-compliance and ensure their products meet the highest standards.
Key insights from the article emphasize the importance of understanding GMP fundamentals, staying informed about regulatory changes, and engaging with regulatory authorities. Regular internal audits, comprehensive training programs, and a focus on continuous improvement are critical components that contribute to a successful GMP framework. These practices not only facilitate compliance but also position organizations to respond effectively to evolving industry challenges.
Ultimately, investing in GMP compliance is not merely a regulatory obligation; it is a commitment to patient safety and product excellence. Organizations are encouraged to adopt these best practices proactively, ensuring they remain competitive in the medical device market while safeguarding the health and well-being of those who rely on their products. Embracing a culture of quality and accountability will lead to sustainable success and reduced regulatory risks in the ever-evolving landscape of medical device manufacturing.
What are Good Manufacturing Practices (GMP) for medical devices?
Good Manufacturing Practices (GMP) for medical devices are a set of regulations and guidelines designed to ensure the quality and safety of medical devices throughout their lifecycle.
What is the importance of Quality Management in GMP?
Quality Management involves establishing a quality management system (QMS) that integrates all aspects of production, from design to distribution. It is crucial for compliance, as indicated by the FDA's identification of 'Design Controls' and 'CAPA' as common shortcomings in Warning Letters.
Why is documentation essential in GMP?
Documentation is vital for maintaining detailed records of manufacturing procedures and regulatory compliance, which aids in traceability and facilitates inspections by regulatory authorities like the FDA.
How does personnel training impact GMP compliance?
Thorough training of employees in GMP principles is critical for maintaining standards. Organizations that prioritize training often experience fewer regulatory issues, as knowledgeable staff can better identify and mitigate risks.
What role do facility and equipment play in GMP?
Facilities and equipment must be designed and maintained to prevent contamination and ensure consistent product quality. Regular assessments are necessary to meet current standards and avoid regulatory challenges due to outdated systems.
What are the labeling and packaging controls in GMP?
Accurate labeling and packaging are crucial to prevent misbranding and ensure compliance with safety requirements. Organizations must ensure that labels are clear, correctly applied, and stored appropriately.
What are the consequences of non-compliance with GMP for medical devices?
Non-compliance with GMP can lead to severe repercussions, including fines and potential imprisonment for manufacturers, emphasizing the necessity of adhering to these regulations.
What is the projected market size for GMP Testing Services?
The market size for GMP Testing Services is projected to be substantial, highlighting the importance of compliance with GMP regulations to avoid penalties and ensure market access.