


The four best practices for achieving success in pharma clinical trials encompass:
These practices are supported by a detailed examination of how:
can significantly improve trial efficiency, participant engagement, and overall research integrity.
In the rapidly evolving landscape of pharmaceutical research, the success of clinical trials hinges on strategic planning and execution. By adopting best practices in:
stakeholders can significantly enhance the effectiveness and efficiency of their studies.
However, with the myriad of challenges faced in these areas, how can researchers ensure that their clinical trials not only meet regulatory standards but also engage participants and yield meaningful results? This article delves into four essential practices that can pave the way for successful pharma clinical trials, offering insights that could transform research outcomes.
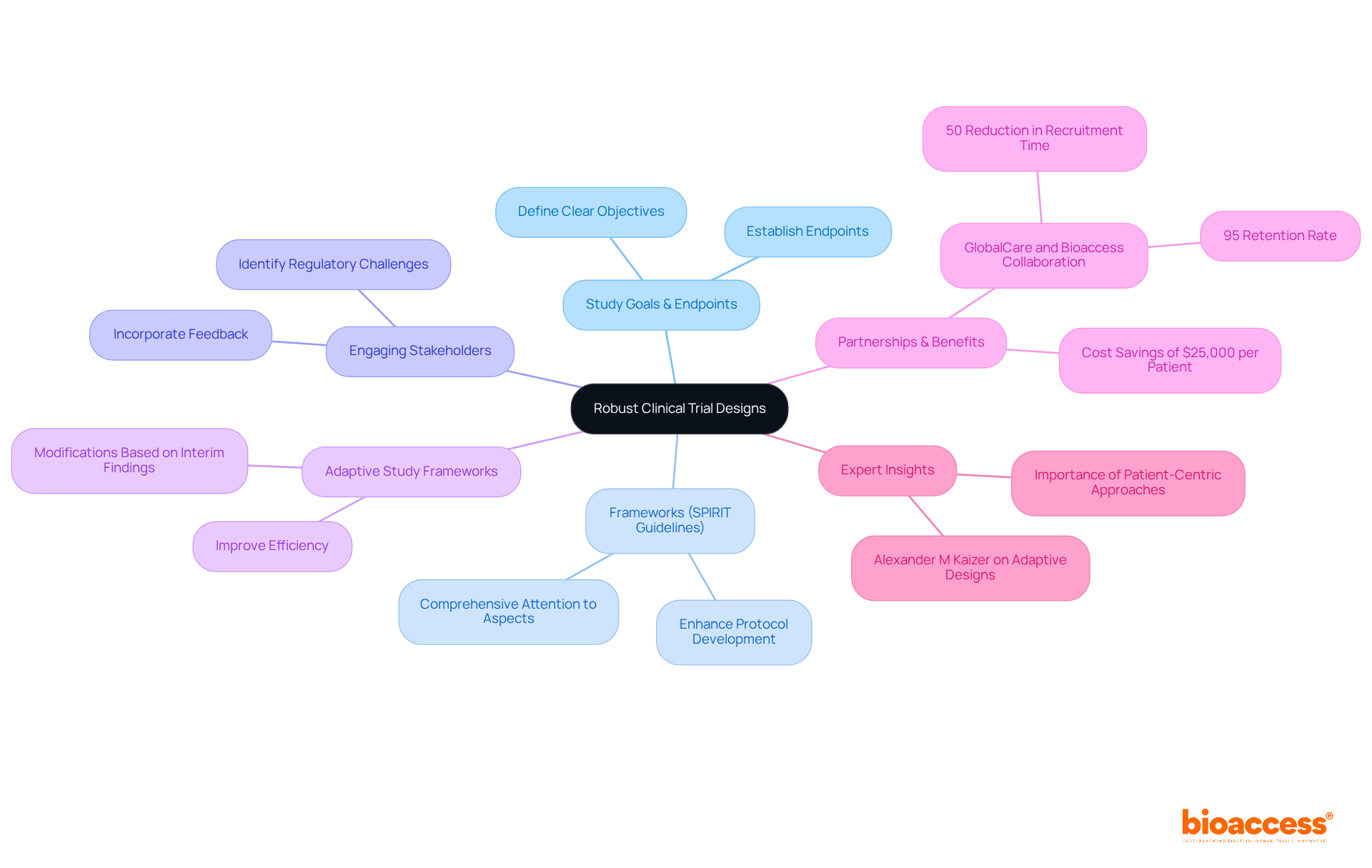
To establish robust pharma clinical research frameworks, it is essential to start with a precise definition of the study's goals and endpoints. Leveraging established frameworks, such as the SPIRIT guidelines, can significantly enhance protocol development by ensuring comprehensive attention to all critical aspects. Engaging stakeholders early in the pharma clinical planning process is crucial for identifying potential regulatory challenges and incorporating their feedback into the framework.
Furthermore, considering adaptive study frameworks in pharma clinical research allows for modifications based on interim findings, thereby improving efficiency and optimizing resource allocation. For instance, the implementation of adaptive methodologies in oncology trials has demonstrated that flexibility can yield quicker and more relevant outcomes, ultimately enhancing the success of clinical research initiatives.
Notably, partnerships like that of GlobalCare with bioaccess® in pharma clinical trials have achieved over a 50% reduction in recruitment time and a remarkable 95% retention rate, underscoring the tangible benefits of flexible strategies. Additionally, bioaccess®'s innovative approach enables the enrollment of treatment-naive cardiology and neurology cohorts at a pace 50% faster than traditional methods, resulting in significant cost savings of $25,000 per patient with FDA-ready data.
Integrating insights from specialists, such as Alexander M Kaizer, who emphasizes that adaptive designs can accelerate development timelines, further underscores the importance of these pharma clinical methodologies in medical research.
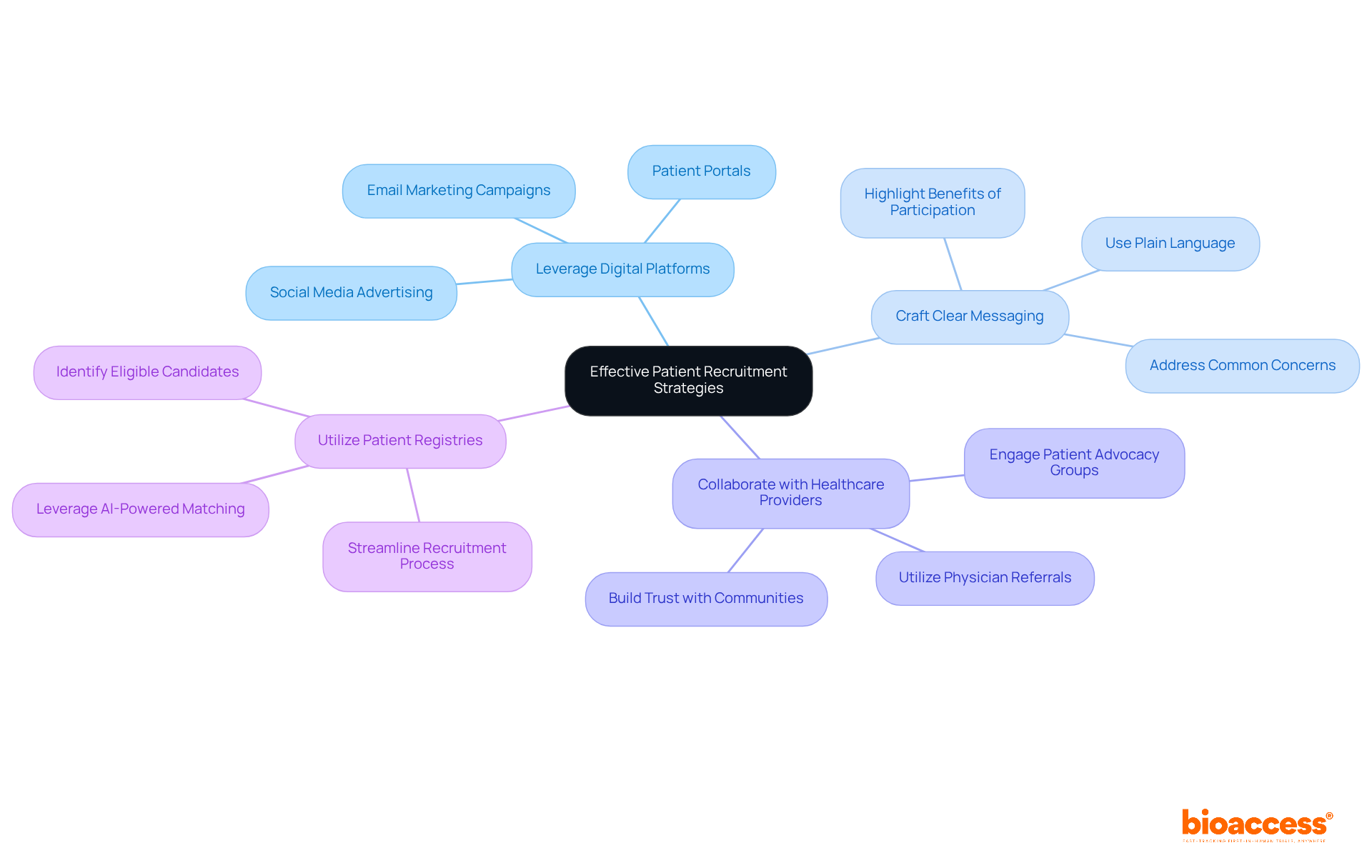
To implement effective patient recruitment strategies, leveraging digital platforms and social media is essential for connecting with potential participants. Crafting clear and compelling messaging that highlights the benefits of participation is crucial, as it addresses common concerns and misconceptions. Collaborating with healthcare providers and patient advocacy groups enhances outreach efforts by tapping into existing networks, fostering trust and engagement. Additionally, utilizing patient registries and databases allows for the identification of eligible candidates, streamlining the recruitment process.
For instance, a biopharma company successfully employed targeted online campaigns in its pharma clinical activities, achieving a remarkable 30% increase in enrollment speed compared to traditional methods. This situation illustrates how digital approaches can significantly improve patient recruitment effectiveness in research studies.
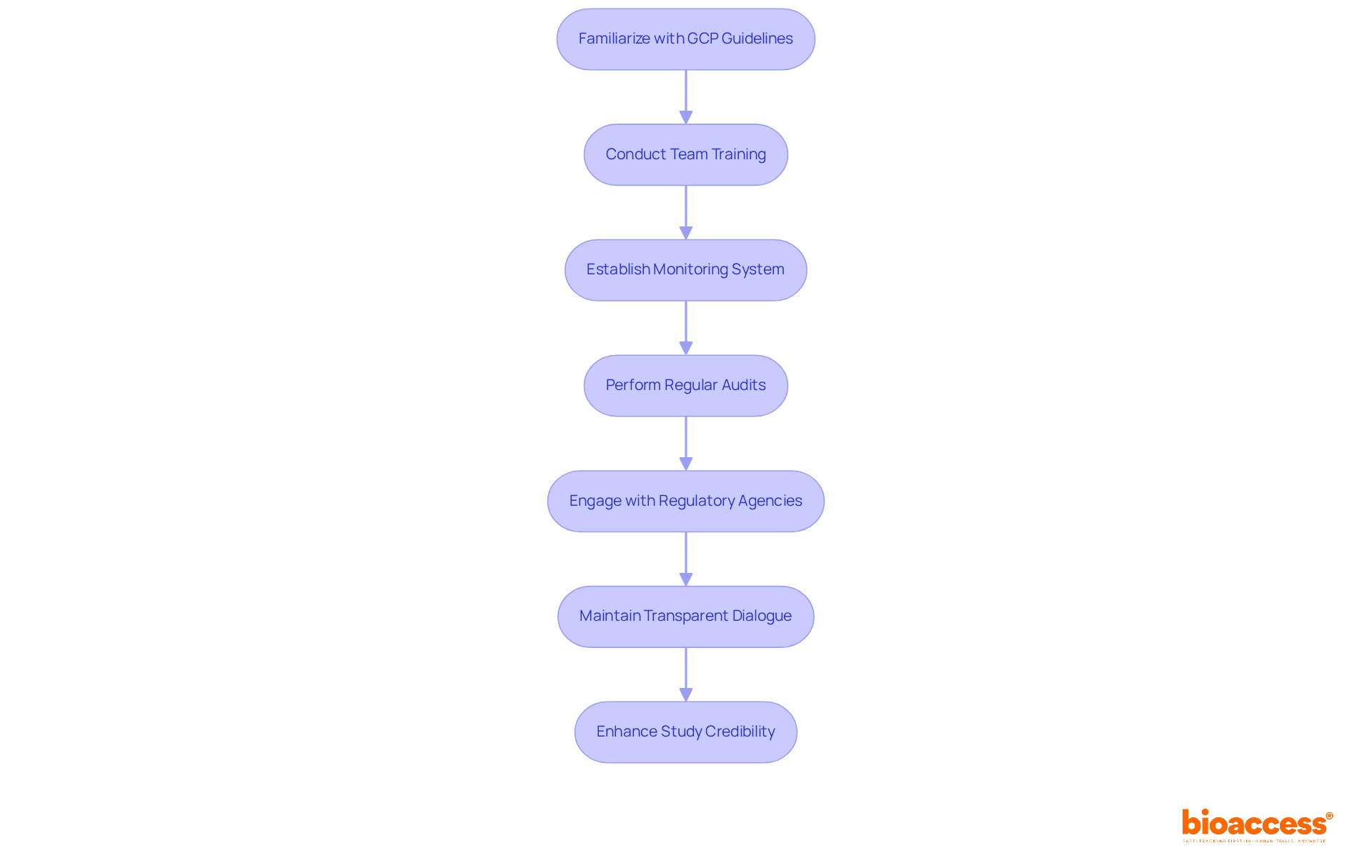
To maintain regulatory compliance and uphold ethical standards in research studies, it is imperative to familiarize oneself with Good Clinical Practice (GCP) guidelines and local regulations. Comprehensive training for all team members regarding compliance requirements and ethical considerations is essential.
Establishing a robust monitoring system for compliance, which encompasses regular audits and reviews, can significantly bolster integrity. Engaging with regulatory agencies early in the process clarifies expectations and allows for proactive addressing of potential concerns.
For instance, a research study that adeptly navigated complex regulatory landscapes secured prompt approvals by maintaining transparent dialogue with ethics boards, fostering participant confidence and ensuring adherence to ethical norms. This proactive approach not only streamlines the approval process but also enhances the overall credibility of the study.
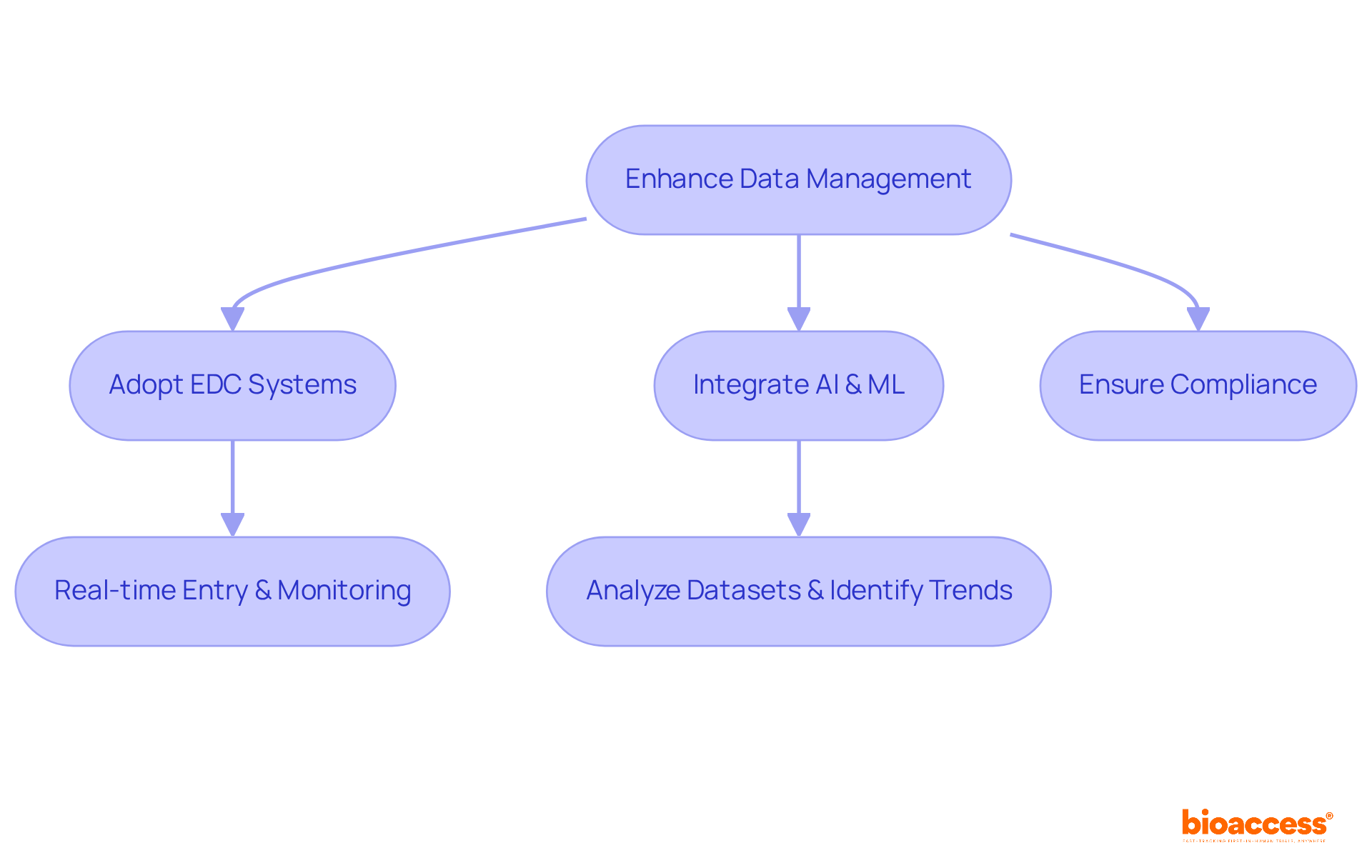
To enhance information management in pharma clinical studies, the adoption of electronic information capture (EDC) systems is imperative. These pharma clinical systems facilitate real-time information entry and monitoring, significantly improving accuracy and operational efficiency. The integration of artificial intelligence (AI) and machine learning (ML) can further optimize information management by analyzing extensive datasets to identify trends and inform critical modifications.
A recent study that implemented a cloud-based information management system demonstrated a remarkable 40% reduction in entry errors, coupled with improved access to real-time insights for informed decision-making.
It is essential that these data management systems adhere to regulatory standards and integrate seamlessly with other platforms used in the trial, thereby ensuring a cohesive and efficient research process.
Establishing successful pharma clinical trials relies on the implementation of best practices that enhance design, recruitment, compliance, and data management. By concentrating on robust clinical trial designs, organizations can set clear objectives and adapt methodologies in response to emerging findings, ultimately driving efficiency and success. The integration of innovative recruitment strategies, particularly through digital platforms, ensures that trials swiftly attract the right participants, while adherence to regulatory compliance and ethical standards fortifies the integrity of the research process.
Key insights from the article underscore the importance of:
The ability to streamline patient recruitment and maintain rigorous compliance not only accelerates trial timelines but also enhances the credibility and reliability of the findings. Moreover, the use of electronic data capture systems and AI-driven analytics exemplifies how technology can transform data management, leading to more informed decision-making and reduced errors.
In conclusion, the landscape of pharma clinical trials is rapidly evolving, necessitating a commitment to best practices that prioritize efficiency, ethical standards, and innovative approaches. Emphasizing these strategies not only improves the likelihood of successful outcomes but also contributes to the advancement of medical research as a whole. As the industry progresses, embracing these principles will be crucial for achieving excellence in clinical trials and ultimately delivering better healthcare solutions.
What is the first step in establishing robust pharma clinical research frameworks?
The first step is to start with a precise definition of the study's goals and endpoints.
How can established frameworks enhance protocol development in clinical trials?
Leveraging established frameworks, such as the SPIRIT guidelines, ensures comprehensive attention to all critical aspects of protocol development.
Why is engaging stakeholders early in the clinical planning process important?
Engaging stakeholders early is crucial for identifying potential regulatory challenges and incorporating their feedback into the research framework.
What are adaptive study frameworks, and how do they benefit clinical research?
Adaptive study frameworks allow for modifications based on interim findings, improving efficiency and optimizing resource allocation in clinical research.
Can you provide an example of the benefits of adaptive methodologies in clinical trials?
In oncology trials, the implementation of adaptive methodologies has demonstrated that flexibility can yield quicker and more relevant outcomes, enhancing the success of clinical research initiatives.
What achievements have partnerships like GlobalCare with bioaccess® made in pharma clinical trials?
They have achieved over a 50% reduction in recruitment time and a 95% retention rate, highlighting the benefits of flexible strategies in clinical trials.
How does bioaccess®'s approach impact the enrollment of specific cohorts?
Bioaccess®'s innovative approach enables the enrollment of treatment-naive cardiology and neurology cohorts at a pace 50% faster than traditional methods.
What are the cost savings associated with bioaccess®'s methods?
Their methods result in significant cost savings of $25,000 per patient while providing FDA-ready data.
Who emphasizes the importance of adaptive designs in accelerating development timelines?
Specialist Alexander M Kaizer emphasizes that adaptive designs can accelerate development timelines in medical research.